Back to Journals » Pragmatic and Observational Research » Volume 16
Comprehensive Description of an Automated Drug Dispensing System Database
Authors Wittwer NL , Meier CR , Recknagel MJ, Allemann S , Schneider C
Received 13 September 2024
Accepted for publication 10 March 2025
Published 8 April 2025 Volume 2025:16 Pages 129—134
DOI https://doi.org/10.2147/POR.S488210
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor David Price
Nina L Wittwer,1,2 Christoph R Meier,1,2 Martin J Recknagel,3 Samuel Allemann,1,* Cornelia Schneider1,2,*
1Department of Pharmaceutical Sciences, University of Basel, Basel, Switzerland; 2Hospital Pharmacy, University Hospital Basel, Basel, Switzerland; 3Medifilm AG, Oensingen, Solothurn, Switzerland
*These authors contributed equally to this work
Correspondence: Samuel Allemann, Pharmaceutical Care Research Group, University of Basel, Department of Pharmaceutical Sciences, Klingelbergstrasse 50, Basel, 4056, Switzerland, Email [email protected]
Abstract: Medifilm is a company that blisters drug therapies recorded by pharmacists in the Medifilm software. The Medifilm dataset collates this information and provides details on drug substances, dosages, pharmacotherapy duration, the sequence of therapies, as well as demographic data on the patients. This article aims to provide an overview of the database, to describe the contents, and to demonstrate possibilities for researchers. The database and the recorded information were described. Furthermore, the data coverage was characterized in terms of the number of available pharmacies, patients, and their drug regimens. The database has been recording data since 2013 and has registered 470,801 blistered therapies for 45,594 patients ordered by 441 pharmacies so far. The longitudinal nature of the database allows researchers to study drug utilization, including medication changes, initiations, and discontinuations over time.
Keywords: medication blistering, drug therapies, medication changes, drug utilization, polypharmacy
Introduction
In Switzerland, basic health insurance is mandatory for all individuals.1 The Federal Office of Public Health defines what must be covered in the basic health insurance, and all health insurances providing basic health insurance must cover at least this minimal set.2,3 Basic health insurance covers weekly dispensing systems if a patient has a prescription for it from a medical doctor and takes at least three medications per week. The services can be reimbursed at most once per week.4 In Switzerland, most medications need to be prescribed by a medical doctor.2 Drugs can then - depending on the canton - either be obtained directly from the prescribing doctor (self-dispensing cantons), or patients can redeem the prescription at a pharmacy of their choice to obtain the drug.5
Automated drug dispensing systems (ADDs), including drug blister packaging, have been demonstrated to reduce errors in drug administration.6 These systems are particularly beneficial for populations at higher risk of medication errors, such as patients prescribed multiple medications or those aged 75 years or older, and are therefore a viable option for care homes. By automating the dispensing process, ADDs aim to improve patient safety and support better health outcomes. In Switzerland, medications are repacked and blistered in pharmacies or specialized companies. Since 2007, Medifilm AG has been repackaging the medication of patient-tailored therapies in a continuous soft pouch string. The string (Medifilm®) is rolled up per patient and per order and packed for delivery. The service is primarily used for home residents. Each pouch is imprinted with information on the patient, the intake time, the number of medications, medication details, and usage instructions (Figure 1). Pouches can hold solid medications such as tablets or capsules but are not suitable for liquid or semi-solid formulations. Empty pouches with a comment can be added to the pharmacotherapy as a memory aid for the patient or nursing staff, if medications are not suitable for blistering. Pharmacies can order Medifilm® for a patient using an internet-based software (Figure 2). The patient file containing information on therapies and master data is managed by the pharmacies. Therefore, the Medifilm database contains all patient data registered by the pharmacy using the Medifilm software, including demographics, medications, therapies, and ordered blistered medications.
 |
Figure 1 Medifilm® pouch, adapted with permission from Medifilm AG.7 |
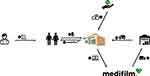 |
Figure 2 Work flow, adapted with permission from Medifilm AG and Servier Medical Art.7,8 The prescription issued by the treating doctor (1) authorises a patient to obtain the medication from a pharmacy (2). Pharmacies can order Medifilm® for a patient using an internet-based software (3). After blister packaging through automated packaging machines, the finished Medifilm® is delivered via pharmaceutical logistic partner (4) to the pharmacy (5) and from there to the patient (6). Health insurances reimburse the service if the pharmacy serves patients who have to take at least three different medications per week according to a doctor’s prescription (7). |
The Medifilm database records detailed information on patients who have ordered Medifilm®, such as information on patient demographics eg, age and sex. In addition, all drugs that can be blistered are added to a patient’s pharmacotherapy list. Any other drug can be selected from the list of known medicines and medicinal products or added as free text. The start and end dates of each pharmacotherapy are also documented, along with each order of the pharmacotherapy as physical blister roll. Information on the medication, dosage, pharmacotherapy duration, and admin time and date are provided. An overview of the main variables and the database architecture are provided in Table 1 and Figure 3. Linkage to other data, like electronic health records or hospital data, is currently not available.
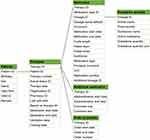 |
Figure 3 Database architecture. |
 |
Table 1 Summary of Data Tables and Fields |
Data Coverage
As of June 2023, the Medifilm database covers 3,582,060 orders. Out of 47,187 patients, 45,594 (96.6%) ordered 470,801 therapies through 441 pharmacies (Table 2). The pharmacies were located in 23 of Switzerland’s 26 cantons (not in Obwalden, Glarus, and Appenzell Innerrhoden) and covered all language regions. The average time between the first and last order of a pharmacotherapy of a patient was 534.7 ± 641.0 days. The average age of patients at the time of their first order was 77.0 ± 17.5 years. On average, each patient received 10.3 ± 13.0 pharmacotherapies, with each pharmacotherapy consisting of an average of 6.8 ± 3.0 drugs. A patient had on average 8.4 ± 4.6 different drugs.
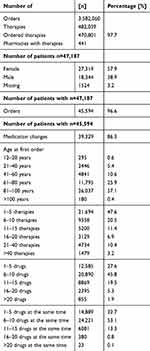 |
Table 2 Medifilm Database Characteristics |
Discussion
To ensure patient safety, it is crucial that the recorded data - relevant to the pharmacotherapy that is blistered - is of high quality. Pharmacists or pharmaceutical technicians enter patient data into Medifilm software. Various checks are performed to ensure the quality of the recorded data and to detect input errors. These include checking for maximum values, avoiding duplicate rows, displaying additional information such as crushability or suspendability, and displaying the drug picture. When validating the pharmacotherapy, additional information such as alternative preparations or drug interaction checks are displayed. All data is validated by an authorized pharmacist. Authorization is granted following an application including verification at the MedReg register of medical professionals.9 It is also possible to compare a patient’s new pharmacotherapy with an old pharmacotherapy.
A strength of the Medifilm database is that it provides detailed information on patient medication, including pharmacotherapy changes such as dosage adjustments, medication initiation, and discontinuation, which are entered by health care professionals. Furthermore, the Medifilm database offers longitudinal data, currently spanning a 10-year period. Compared to other Swiss claims databases, drug information is more frequently updated, providing a more comprehensive picture of a patient’s pharmacotherapy. The patient population in the Medifilm database consists predominantly of elderly individuals with polypharmacy, providing an opportunity to study a multimorbid population.
The quality of the data depends on the accuracy and completeness of information that pharmacies record in the Medifilm software. Data that is not recorded by pharmacies is not captured in the database. The quality of data varies across pharmacies and patients. For example, non-blistered medications, such as liquid medications, patches, suppositories, dermatological preparations, granules, and effervescent tablets might be added as free text in the record of one patient, but not in another. Additionally, the quality of free text data varies. Medifilms® are usually ordered for multimorbid patients, and thus the database is not representative for the general population.
While Medifilm® is a tool designed to support medication adherence, it cannot guarantee that all prescribed medications will be taken.
The Medifilm database has the potential to study medication use and medication changes in patients with polypharmacy and has previously been used to analyze the utilization of splitting tablets.10
Data Resource Access
More information about the Medifilm database can be found at https://www.medifilm.ch/de/ and data access can be requested from Medifilm AG ([email protected]).
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available due to confidentiality requirements issued by Medifilm AG. Analysis codes and datasets can be made available by the corresponding author ([email protected]) upon reasonable request and with permission of Medifilm AG.
Ethics Approval
According to article 22 of the Swiss Federal Law on data protection, ethics approval is not necessary for retrospective and anonymous analyses.11
Consent for Publication
All authors have read the manuscript and given their consent for its publication.
Acknowledgments
Selected artwork (pharmacy) shown in Figure 2 was used from pictures provided by Servier Medical Art (Servier; https://smart.servier.com/), licensed under a Creative Commons Attribution 4.0 Unported License. We used Deepl Write (Deepl SE, Germany) for final language editing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
MJR is employed by Medifilm AG. The authors report no other conflicts of interest in this work.
References
1. Biller-Andorno N, Zeltner T. Individual responsibility and community solidarity — the Swiss Health Care System. N Engl J Med. 2015;373(23):2193–2197. doi:10.1056/NEJMp1508256
2. Federal Office of Public Health (FOPH). List of Specialties (SL); 2023. Available from: http://www.spezialitätenliste.ch/.
3. Federal Office of Public Health (FOPH). Analysis List (AL); 2024. Available from: https://www.bag.admin.ch/bag/de/home/versicherungen/krankenversicherung/krankenversicherung-leistungen-tarife/Analysenliste.html.
4. pharmaSuisse, santésuisse, curafutura. Tarifstruktur-Vertrag LOA IV/1; 2016. Available from: https://pharmasuisse.org/system/files/media/documents/2023-10/Tarifstruktur-Vertrag-LOA-IV-1.pdf.
5. Arslan S. 21.3881 | selbstmedikation Arzneimittel. Wo stehen wir heute? | amtliches Bulletin | das Schweizer Parlament; 2021. Available from: https://www.parlament.ch/de/ratsbetrieb/amtliches-bulletin/amtliches-bulletin-die-verhandlungen?SubjectId=57767.
6. Hänninen K, Ahtiainen HK, Suvikas-Peltonen EM, Tötterman AM. Automated unit dose dispensing systems producing individually packaged and labelled drugs for inpatients: a systematic review. Eur J Hosp Pharm. 2023;30:127–135. doi:10.1136/ejhpharm-2021-003002
7. Medifilm AG. medifilm.ch. Available from: https://www.medifilm.ch/de/.
8. Servier. Servier Medical Art. Available from: https://smart.servier.com.
9. Federal Office of Public Health (FOPH). MedReg register of medical professions. Available from: https://www.bag.admin.ch/bag/en/home/berufe-im-gesundheitswesen/medizinalberufe/medizinalberuferegister-medreg.html.
10. Allemann SS, Bornand D, Hug B, Hersberger KE, Arnet I. Issues around the prescription of half tablets in Northern Switzerland: the irrational case of Quetiapine. Biomed Res Int. 2015;2015:602021. doi:10.1155/2015/602021
11. Fedlex. Federal Act on Data Protection (FADP); 2019. Available from: https://www.fedlex.admin.ch/eli/cc/1993/1945_1945_1945/en.
 © 2025 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a
Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the
original author and source are credited.
© 2025 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a
Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the
original author and source are credited.

