Back to Journals » Drug Design, Development and Therapy » Volume 19
Comprehensive Treatment of Functional Dyspepsia Using Traditional Chinese Medicine: A Review Based on Pathophysiological Perspectives
Received 16 January 2025
Accepted for publication 9 May 2025
Published 24 June 2025 Volume 2025:19 Pages 5349—5367
DOI https://doi.org/10.2147/DDDT.S514042
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Solomon Tadesse Zeleke
Mingming Fan,1 Xiangbin Pan,2 Yingzhe Liu3
1Department of Geriatric, Heilongjiang Academy of Traditional Chinese Medicine, Harbin, Heilongjiang, People’s Republic of China; 2Department of Spleen, Stomach and Hepatobiliary, Second Affiliated Hospital of Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang, People’s Republic of China; 3First Department of Endocrinology, First Affiliated Hospital of Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang, People’s Republic of China
Correspondence: Yingzhe Liu, First Department of Endocrinology, First Affiliated Hospital of Heilongjiang University of Chinese Medicine, 26 heping Road, Harbin, Heilongjiang, People’s Republic of China, Email [email protected]
Abstract: Functional dyspepsia (FD) is a common functional gastrointestinal disorder characterized by epigastric pain and symptoms related to meal consumption. According to recent diagnostic criteria, FD can be classified as epigastric pain syndrome (EPS) or postprandial discomfort syndrome (PDS), based on the predominant symptoms. The pathophysiology of FD can be influenced by various factors, including microinflammation, gastrointestinal infections, abnormal gastroduodenal motility, visceral hypersensitivity, disturbances in the brain-gut axis, and psychological factors. As a result, its management requires a comprehensive approach that includes both pharmacological and non-pharmacological interventions such as psychological therapies and adjunctive treatments. However, despite the availability of various treatment options, no contemporary pharmacological agents have been approved specifically for FD. In this context, Traditional Chinese Medicine (TCM) has emerged as a promising alternative that offers notable efficacy and safety in managing FD. Various TCM modalities, including herbal prescriptions, acupuncture, moxibustion, and music therapy have demonstrated therapeutic potential. In this article, we discuss the pathophysiology of FD from a modern medical perspective and describe integrative treatment strategies based on TCM, highlighting the potential benefits of combining the traditional and contemporary approaches.
Keywords: FD, EPS, PDS, duodenum, TCM
Introduction
Functional gastrointestinal disorders (FGIDs) are a prevalent syndrome characterized by gastrointestinal (GI) symptoms of indeterminate etiology, often manifesting as alterations in bowel sensitivity, peristalsis, or both.1 Functional dyspepsia (FD) and irritable bowel syndrome (IBS) are among the most widely recognized conditions within this category. FD is defined as a clinical syndrome marked by chronic symptoms localized to the duodenum,2 including epigastric pain, burning sensations in the epigastric region, and postprandial fullness or early satiety persisting for a minimum duration of six months.3 According to the most recent Rome IV diagnostic criteria, FD is classified into two subtypes based on predominant symptoms: epigastric pain syndrome (EPS) and postprandial discomfort syndrome (PDS).4 The economic burden of FD is substantial, affecting patients, healthcare systems, and society.4 Nevertheless, the current treatment modalities for FD within the framework of modern medicine remain inadequate.5 In contrast, Traditional Chinese Medicine (TCM) has demonstrated certain levels of efficacy and safety 6 in the management of FD, warranting further investigation.
Herein, we designed this review to comprehensively discuss the pathophysiology of FD through the lens of modern medical research while also exploring the therapeutic potential of TCM in managing FD. By integrating insights from both modern and traditional approaches, this review describes potentially effective treatment strategies and addresses the gaps in the current understanding of FD management.
Epidemiology and Clinical Characteristics and of FD
FD is a prevalent and often challenging condition to manage given its complex clinical presentation and the necessity to rule out organic causes. In this section, we provide an understanding of the epidemiology and clinical characteristics of FD for enhancing diagnosis and effective management, emphasizing the importance of thorough clinical assessment and appropriate diagnostic procedures.
FD can affect individuals of any age and is known for its substantial impact on work productivity and health-related quality of life (HRQOL). In the United States, annual healthcare costs associated with FD are estimated to be as high as $18 billion, reflecting the economic burden of the condition.7,8 Epidemiological studies conducted in Nordic countries and Italy have reported a high prevalence of FD and its frequent coexistence with other functional gastrointestinal disorders (FGIDs).9–12 Additionally, approximately 40–50% of FD patients report symptoms consistent with gastroesophageal reflux disease (GERD).13,14 EPS is commonly associated with non-erosive reflux disease, whereas PDS tends to overlap with functional heartburn.15,16 Meta-analyses indicate that individuals with FD are approximately eight times more likely to have irritable bowel syndrome (IBS) compared to the general population, underscoring the frequent overlap between these conditions.17,18
FD is primarily characterized by symptoms such as epigastric pain, burning sensation, and feeling of fullness, often accompanied by early satiety during meals. According to diagnostic criteria, these symptoms must have been present for at least three months and persist for a minimum duration of six months to establish a diagnosis of FD.4 One notable clinical feature associated with FD is the potential for weight loss, which may occur in some patients.19 The Rome IV criteria, currently recognized as the most comprehensive standard for diagnosing FD, categorize the disorder into two subtypes based on the predominant symptoms: epigastric pain syndrome (EPS) and postprandial distress syndrome (PDS).20 In EPS, symptoms such as epigastric pain and burning sensations are not necessarily associated with food intake and may occur even during fasting, often relieved by eating.21,22 In contrast, PDS is predominantly characterized by postprandial fullness and early satiety following meals. Although PDS is more commonly observed than EPS, it is not uncommon for patients to experience symptoms of both subtypes simultaneously.9,23 Additionally, a significant proportion of patients with FD may exhibit symptoms that overlap with IBS.20,24
The diagnosis of FD necessitates the exclusion of organic gastrointestinal diseases, as the presence of symptoms alone is insufficient for a definitive diagnosis.25,26 According to the Rome IV criteria, FD should only be diagnosed after comprehensive evaluation to rule out potential organic causes.4,27
Due to the nonspecific nature of FD symptoms, it can be challenging to identify the underlying cause based solely on the clinical presentation. A thorough medical history and physical examination are essential, with particular attention to recent changes in medication that can affect gastrointestinal function.28–30 Initial laboratory investigations should include a complete blood count (CBC) and testing for Helicobacter pylori (H. pylori), especially in regions with a high prevalence of H. pylori infection.31 Testing methods may involve immunoglobulin G-based serological assays, urease breath tests, or stool antigen tests. However, false-negative results can occur if proton pump inhibitors (PPIs) are used alongside urease breath tests or stool antigen tests.32
Erosive gastritis is sometimes observed in patients with FD, but its prevalence is relatively low, and routine screening for this condition offers limited diagnostic value.33 Diagnostic procedures typically include upper endoscopy and biopsy, which may reveal findings such as erosive esophagitis (observed in 20% of cases) or peptic ulcer disease (noted in 6%).24 Endoscopic evaluation is particularly recommended for older patients, those presenting with atypical symptoms, or those who do not respond to initial treatment.31,34 In cases where symptoms persist despite treatment or when clinical indicators suggest impaired gastric motility, gastric emptying studies may be warranted, as a considerable proportion of FD patients may exhibit delayed gastric emptying.35
Pathophysiological Mechanisms
FD is a multifactorial disorder characterized by a combination of gastrointestinal and central nervous system dysfunctions (Figure 1). Despite significant research efforts, the underlying mechanisms remain poorly understood, primarily because of the heterogeneity of symptoms and the complex interplay between various physiological systems. This section explores the current understanding of the pathophysiological mechanisms of FD, focusing on factors such as gastrointestinal motility disturbances, visceral hypersensitivity, immune responses, alterations in the gut-brain axis, and the role of the gastrointestinal microbiota.
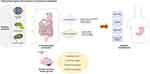 |
Figure 1 Pathophysiological mechanisms of FD. |
The current literature suggests that FD symptoms arise from a complex interplay between the gastroduodenal region and central nervous system. The inherent ambiguity surrounding FD may impede a comprehensive understanding of its pathophysiology as precise definitions of the condition are challenging to establish. Although many individuals experience symptoms of indigestion, these symptoms do not constitute a distinct disease in isolation. The characteristics of peptic ulcer disease illustrate this point; although dyspeptic symptoms may persist over time, they do not define a disease entity. The term “chronic” in this context does not imply continuous symptoms but rather indicates that individuals with peptic ulcers experience indigestion more frequently than their healthy counterparts. For instance, according to the Rome IV criteria, “chronic” is defined as experiencing feelings of fullness or early satiety two or more times per week or upper abdominal pain at least once per week for a duration of three months or more.36 Furthermore, the onset of FD symptoms is often precipitated by factors such as fatigue and stress, leading to the hypothesis that an individual’s atypical response to stress is a fundamental aspect of FD.37 However, the determinants of this stress response vary. There are numerous etiological factors contributing to FD, with many being directly or indirectly associated with its pathophysiology, thereby reinforcing the notion of FD as a multifactorial disorder. Alterations in gastrointestinal motility and sensitivity are regarded as the primary mechanisms underlying FD.
The symptoms of PDS are believed to stem from gastrointestinal dysfunction,38,39 which subsequently results in gastric overload.40,41 Additionally, a partial impairment of gastric accommodation may exacerbate these symptoms.39,42 Delayed gastric emptying is primarily linked to manifestations such as nausea, vomiting, and a sensation of fullness following meals.43 Nevertheless, a clear relationship between symptom alleviation and acceleration of gastric emptying has not been established.44,45 A systematic review indicated that enhancing gastric emptying for 20 minutes led to a significant improvement in symptoms.45
Symptoms associated with EPS are believed to arise from mechanical hypersensitivity of the stomach.46,47 Research has also examined the influence of chemical hypersensitivity, particularly regarding the heightened sensitivity of the duodenum and reduced clearance of both exogenous and endogenous acids.48,49 The presence of exogenous acids in the duodenum, which exceed physiological levels, impedes gastric accommodation during meals.50 Figure 2 provides an overview of the mechanisms leading to FD and the symptoms of different FD subtypes. According to the Rome IV criteria, FD is categorized as a disorder involving gut-brain interactions.51 Research indicates that stress may disrupt the balance of intestinal microbiota, subsequently influencing the functionality and behavior of the central nervous system, a phenomenon referred to as the “brain-gut axis”.52 However, there is a paucity of research substantiating this concept.53 A longitudinal study revealed that individuals with depression who do not exhibit gastrointestinal symptoms at baseline may develop FD, whereas patients with FD who do not present with psychological complications at baseline may experience anxiety and depression.54
 |
Figure 2 Subtypes of FD and the potential treatment approaches. |
The etiology of barrier defects and immune activation observed in FD remains unclear. Although duodenal acid perfusion studies do not accurately replicate the physiological changes that occur in the distal duodenum, they indicate that exposure to duodenal acid and modifications in gastric sensorimotor function may be contributing factors.55–57 Despite normal gastric acid secretion in individuals with FD, duodenal acid perfusion has been shown to induce gastric relaxation, increase duodenal mucosal hyperpermeability, and activate mast cells 58 in experiments involving FD patients.
The role of immune activation in the morbidity mechanism of FD is apparent in a post-infectious environment.59 A particular study found that antibodies against cytolethal distending toxin B, which is produced by gram-negative bacteria associated with acute gastroenteritis, were more prevalent among individuals exhibiting both FD and IBS than among healthy control subjects.60 This finding indicates that FD following infection may be inadequately researched and comprehended.
Compared to the rectum, the bacterial flora in the duodenum exhibits a lower density but greater diversity.61 The duodenum is predominantly inhabited by gram-positive aerobes, whereas the colon is primarily populated by obligate anaerobes.62 A preliminary investigation involving nine patients diagnosed with FD indicated an increase in the overall number of streptococcal and contrast cells in duodenal mucosal-associated microbiota (MAM) relative to control subjects, which correlated with the severity of meal-related symptoms and overall quality of life.63 However, this study was limited by its small sample size and absence of data regarding pharmacological treatments. Additionally, there is a lack of information concerning overlap with IBS. Notably, IBS overlap has been linked to dysbacteriosis in both fecal and duodenal samples.64,65 Consequently, further research and repeated studies are essential to elucidate that duodenal autocrine and paracrine mechanisms play a significant role in mucosal defense against acid and in the process of luminal digestion.66 Hormones produced by the gut or pancreas are crucial for brain signaling,67 which subsequently activates the duodenal-gastric feedback mechanism through either chemical or mechanical stimuli, thereby influencing gastric emptying. Chamber experiments have demonstrated that the permeability of the duodenal mucosa is elevated, whereas the resistance between epithelial cells is diminished in patients with FD.68 Furthermore, the expression of specific intercellular adhesion proteins, such as tight junctions, cohesin junctions, and descasein, has been found to be significantly reduced. These alterations may be linked to the increased permeability and low-grade mucosal inflammation in the duodenum. A study involving Belgian patients with FD corroborated an earlier report regarding the presence of duodenal eosinophils in Swedish adults.69 Nonetheless, a limitation of in vitro studies is that they primarily assess the integrity of the epithelial layer, neglecting the roles of superficial mucus and innervation of the enteric nervous system, both of which also contribute to mucosal barrier function. Thus, there remains a significant gap in research regarding these factors, particularly the role of the gut microbiota in the pathophysiology of FD.
Two recent systematic reviews 70,71 have highlighted the limitations associated with FD and systemic inflammatory responses. The presence of duodenal mast cells in the Leuven cohort may be attributed to the overlap between FD and IBS, as duodenal mast cells have been identified as a characteristic of IBS in another investigation.72,73 Furthermore, eosinophil mast cell signaling may be linked to stress-induced hyperpermeability.74 Two studies 75,76 indicated that an elevated eosinophil count in the duodenal mucosa is correlated with postprandial symptoms. The association between duodenal eosinophilia and meal-related symptoms can be elucidated by alterations in duodenal-gastric reflexes and a dysregulated gastric response to food intake.75
Although various hypotheses have been proposed, including motility disorders, hypersensitivity, and immune activation, no single mechanism can fully explain this condition. Thus, further research is necessary to elucidate the interplay between these factors and develop targeted therapeutic approaches that address the diverse symptoms experienced by patients with FD.
Treatment
The management of FD involves a multifaceted approach that encompasses both pharmacological and nonpharmacological strategies (Table 1). Given the complex and multifactorial nature of FD, treatment is often tailored to target specific symptoms and their underlying mechanisms. This section outlines the current therapeutic approaches for FD, focusing on pharmacological interventions, including acid suppression, antibiotic therapy, prokinetics, anti-inflammatory agents, antidepressants, and probiotics, as well as non-pharmacological treatments, such as cognitive behavioral therapy (CBT).
 |
Table 1 Mechanisms of Action of Pharmacological Therapies for FD |
Helicobacter Pylori Identification and Treatment
All prominent social guidelines regarding the management of FD advocate the implementation of noninvasive testing for H. pylori infection. In instances where the test yields a positive result,77 subsequent eradication therapy for H. pylori is recommended. Among patients with FD who test positive for H. pylori, the symptoms that exhibit the most significant improvement following eradication therapy include epigastric pain and burning sensations associated with EPS, whereas symptoms such as postprandial fullness and early satiety show a more modest response.78 Nevertheless, it is important to note that only a limited proportion of H. pylori-positive FD patients will experience symptomatic relief following eradication therapy. When compared to trials involving PPIs, the methodologies for H. pylori testing and treatment demonstrate comparable efficacy in alleviating symptoms, with the potential for greater cost-effectiveness.79 The Kyoto Consensus posits that if patients with FD and H. pylori infection exhibit a positive response to eradication therapy and this response is sustained, the appropriate diagnosis is H. pylori-associated dyspepsia. Conversely, most patients whose symptoms persist despite successful eradication therapy are classified as having FD unless alternative diagnoses are established.80
Acid Suppression Therapy
PPIs including omeprazole, esomeprazole, and pantoprazole are frequently prescribed for the management of FD. Acid suppression therapy, particularly through the use of PPIs, is recommended as an initial intervention to alleviate FD symptoms. Evidence indicates that PPIs 77 demonstrate greater efficacy than placebo, irrespective of the treatment regimen or duration of administration. Although recent apprehensions regarding the adverse effects associated with prolonged PPI use have been largely addressed, the recommendation remains to utilize the lowest effective dose as a preferred strategy.81,82 According to the Rome IV consensus, PPIs were deemed ineffective for the PDS subgroup,36 a conclusion supported by findings from a previous meta-analysis.83
Antibiotic Therapy
Rifaximin, a selectively absorbed antibiotic with limited systemic absorption, has demonstrated efficacy in the management of IBS.84 A study investigating the effects of rifaximin on FD revealed that the antibiotic significantly enhanced the remission rates of dyspeptic symptoms, including belching, postprandial fullness, and bloating.85
Anti-Inflammatory Therapy
A recent cross-sectional study suggested that anti-inflammatory effects may contribute to a reduction in duodenal eosinophilia;86 however, this finding requires validation in a prospective study involving a subgroup defined by the Rome IV criteria. The 2006 Cochrane review 87 referenced an earlier investigation in which a histamine-2 receptor antagonist (H2RA) demonstrated the ability to inhibit gastric acid secretion, in contrast to a placebo-controlled study involving PPIs. Nevertheless, owing to the absence of high-quality comparative trials and the superior anti-inflammatory properties of PPIs, the guidelines established by the American College of Gastroenterology and the Canadian Association of Gastroenterology advocate for the use of PPIs as the first-line treatment rather than H2RAs.77 Additionally, a recent retrospective case-control study conducted in Australia indicated a potential benefit from the combined blockade of histamine-1 and histamine-2 receptors, revealing a trend towards a higher baseline duodenal eosinophil count in responders than in non-responders.88
Prokinetic Drugs
Given the correlation between gastric physiological abnormalities and FD, the administration of agents that promote gastric secretion may be particularly advantageous for patients, particularly those exhibiting the PDS variant characterized by significant post-meal fullness and satiety symptoms.89–91 A meta-analysis has demonstrated that cisapride, which functions as both a 5-HT4 receptor agonist and 5-HT3 antagonist, can lead to a comprehensive improvement in FD symptoms.92 However, cisapride is currently not available in the market because of its adverse effects on cardiac function.93
Domperidone is classified as an antiemetic that functions as a D2 receptor antagonist. Its market presence has increased, notwithstanding the absence of substantial evidence demonstrating its efficacy in FD. Although domperidone exhibits lower thermosensitivity in its binding to the D2 receptor located in the substantia nigra, existing data indicate a potential risk for QT interval prolongation and the occurrence of ventricular arrhythmias.94,95
Tegaserod is a recognized agonist of the 5-HT4 receptor and has been utilized in the management of irritable bowel syndrome with constipation (IBS-C). Research indicates that tegaserod notably enhances gastric emptying.96 In a Phase II clinical trial, tegaserod demonstrated greater efficacy than placebo in alleviating symptoms associated with PDS; however, this finding was not corroborated in a subsequent Phase III trial.97,98
Buspirone, a 5-HT1A receptor agonist, is indicated for the treatment of generalized anxiety disorder (GAD) and is recognized as an adjunctive therapy for major depressive disorder. In a study conducted by Tack et al,42 which involved a small 4-week crossover FD trial, buspirone significantly alleviated overall dyspeptic symptoms, as well as specific symptoms, including postprandial fullness, early satiety, and bloating. However, the study indicated that buspirone did not affect gastric sensitivity or objective measures of gastric emptying in solid foods; instead, it was found to enhance both gastric emptying and holding capacity for the same quantity of food.
Antidepressants
Antidepressant medications serve as effective neuromodulators for patients experiencing chronic refractory digestive symptoms.99,100 These pharmacological agents are particularly beneficial for individuals presenting with coexisting psychiatric disorders and non-gastrointestinal pain, as well as for those exhibiting overlapping FGIDs symptoms. The therapeutic efficacy of antidepressants does not appear to be directly linked to any specific impact on comorbid psychological factors.101 Rather, it is posited that these medications function by disrupting or modulating the interpretation of peripheral signals originating from the gastrointestinal tract.102 Consequently, it is anticipated that antidepressants will enhance the overall symptoms of FD with a minimal influence on gastroduodenal physiology. Before initiating antidepressant therapy for FD, it is essential to comprehend patient perceptions and expectations to maximize adherence to treatment. Tricyclic antidepressants (TCAs) such as amitriptyline and nortriptyline are recognized as the most effective pharmacological options. As a first-line treatment, TCAs exert multiple effects on neurotransmitter systems, including the inhibition of the reuptake of biogenic amines such as serotonin and norepinephrine, in addition to exhibiting postsynaptic antimuscarinic and antihistaminic properties.103 It is important to note that the dosages of TCAs prescribed for this indication are typically much lower than those conventionally employed in the treatment of depression, often commencing at 10–25 mg taken at bedtime. Adverse effects present a significant challenge for the administration of these medications, commonly resulting in sedation, dry mouth, urinary retention, sexual dysfunction, and dizziness. Patients experiencing these side effects may find secondary amine TCAs, which possess a reduced anticholinergic profile, to be more tolerable.101
Selective Serotonin Norepinephrine Reuptake Inhibitors (SNRIs), including venlafaxine, desvenlafaxine, and duloxetine, represent viable alternatives for addressing the central aspects of IBS.104 Notably, venlafaxine has demonstrated peripheral effects on gastric function, particularly in enhancing the tolerance to increased postprandial gastric volume. However, findings from several multicenter clinical trials indicate that asymptomatic patients receiving venlafaxine did not exhibit significant differences in outcomes compared to those receiving a placebo, a discrepancy that may be partially attributed to the specific endpoints utilized in the studies.105
Probiotics
Probiotics have been shown to enhance health-related quality of life in patients with FD by inhibiting Hp, although their effectiveness has also been established in patients with FD who are not infected with H. pylori.106 Current literature has highlighted the benefits of specific probiotic strains, suggesting variations in both their therapeutic effects and underlying mechanisms. For instance, a study investigating the daily consumption of yogurt containing 109 colony-forming units of probiotics for 12 weeks revealed promising results.107 In H. pylori-negative FD patients, this regimen led to notable alleviation of gastric symptoms, particularly those associated with PD). However, the characteristic symptoms of EPS showed no significant improvement. These findings underscore the strain-specific differences in targeting FD symptoms. Another study identified a higher frequency of bile acids in gastric juice samples of FD patients than in control subjects,108 suggesting a potential pathogenic role. While yogurt consumption did not reduce bile acid levels, the administration of probiotics was associated with the restoration of gut-like bacteria, indicating their role in microbiome modulation and highlighting the distinct mechanisms by which probiotics may benefit FD patients, including microbial balance and gut-bacterial interactions. Moreover, small intestinal bacterial overgrowth (SIBO) has emerged as a potential biomarker and therapeutic target for FD. In this regard, probiotics have been shown to play a role in addressing SIBO-related symptoms, although standardized treatment protocols have yet to be established. To further refine clinical applications, additional research on probiotic strains, their observed benefits, and mechanisms of action is essential to elucidate strain-specific efficacy and optimize therapeutic strategies for FD management.
Cognitive Behavioral Therapy
Psychological and behavioral interventions, particularly cognitive behavioral therapy (CBT), may prove beneficial in the management of FD, particularly among patients who associate the severity of their condition with life stressors or who present with concurrent psychiatric disorders.109–111 Although the overall quality of studies and the level of evidence supporting these therapeutic approaches for FD remain limited, a recent review by the American College of Gastroenterology and the Canadian Association of Gastroenterology identified 12 trials, all of which demonstrated significant symptom improvement in comparison with control groups.77 Generally, patients with functional dyspepsia exhibit enhancement in overall functioning following psychotherapy, alongside improvements in HRQOL and psychological complications.112,113 Furthermore, recent investigations have indicated that self-guided CBT 114 and Internet-based CBT 115,116 can also lead to substantial symptom alleviation in individuals with IBS.
Collectively, managing FD remains a clinical challenge owing to the variability in symptoms and the lack of FDA-approved treatments specifically for FD. Pharmacological therapies, though often utilized off-label, can alleviate symptoms to varying degrees, whereas non-pharmacological interventions, particularly CBT, offer additional symptom management. Continued research on targeted therapies and the integration of both traditional and novel approaches will be crucial for improving patient outcomes.
Cognition of FD in TCM
TCM offers a unique perspective for the diagnosis and treatment of FD, emphasizing the importance of syndrome differentiation and holistic management. Unlike the biomedical approach, TCM conceptualizes FD as a consequence of imbalances among various physiological and psychological factors, including emotional disturbances, dietary irregularities, and exogenous pathogenic influences. This section describes the TCM understanding of FD, including its classification into distinct syndromes and the underlying pathophysiological concepts.
Expert consensus on TCM diagnosis and treatment of functional dyspepsia (2023) 117 is the consensus on diagnosis and treatment, based on the naming characteristics of TCM diseases, on the basis of summarizing the academic viewpoints of predecessors and contemporary physicians.118 To better correspond to FD diagnosis and subtype division, experts unanimously defined EPS as “stomach pain” in TCM. PDS is attributed to the treatment of “stuffiness and fullness” “in TCM.119
TCM considers FD to be the result of many factors, such as emotional disorders, excessive fatigue, congenital endowment deficiency, improper diet, and feeling exogenous pathogens.117 Exogenous pathogenic factors such as cold, heat, and dampness attack the stomach, resulting in obstruction of qi movement in the epigastrium, which leads to food stagnation and damage to the spleen and stomach. Anxiety and anger lead to failure of the liver to disperse, invasion of the stomach, disharmony of the stomach, and even qi stagnation and blood stasis. Weakness of the spleen and stomach, failure of transportation and transformation, obstruction of qi movement, or weakness of Yang qi in the middle energizer can lead to spleen deficiency, qi stagnation, and disharmony of the stomach, resulting in this disease.120 FD is located in the stomach and is closely associated with the liver and spleen.
According to consensus,117 FD is divided into five syndromes: spleen deficiency, qi stagnation syndrome, liver-stomach disharmony syndrome, spleen-stomach damp-heat syndrome, spleen-stomach weakness syndrome, and cold-heat intermingled syndrome.
The TCM approach to FD highlights the significance of individualized diagnosis and treatment, taking into account diverse etiological factors and clinical presentations. By categorizing FD into specific syndromes, TCM practitioners can tailor therapeutic strategies to address the root causes of symptoms, offering a comprehensive and integrative framework for FD management. Further studies are warranted to validate the efficacy of the TCM approaches in modern clinical practice.
Treatment of FD with TCM
TCM has gained considerable attention for its potential in managing functional dyspepsia (FD), particularly in patients experiencing symptoms related to gastrointestinal dysfunction and sleep disturbances. Various TCM formulations have been investigated for their therapeutic effects, demonstrating their ability to alleviate symptoms, regulate gastrointestinal hormones, and improve the quality of life. This section discusses the evidence supporting the use of TCM in FD treatment, including findings from recent clinical trials and studies. A summary of these results is shown in Table 2.
 |
Table 2 TCM Treatments for FD and Their Mechanisms of Action |
Yuan Ruixing et al 121 conducted a randomized controlled trial (RCT) to evaluate the efficacy of Shugan Jieyu Xiaopi Fang after one month of treatment in 96 patients with FD associated with liver-stomach disharmony and insomnia, randomized into either an observation group (received Shugan Jieyu Xiaopi Fang) or a control group (received standard treatment). Clinical efficacy was evaluated using TCM syndrome scores, Nepean Dyspepsia Symptom Index (NDSI), Nepean Dyspepsia Life Quality Index (NDLQI), Pittsburgh Sleep Quality Index (PSQI), gastrointestinal hormones (motilin, gastrin, ghrelin), neurotransmitters (5-HT, norepinephrine, γ-aminobutyric acid), inflammatory cytokines (interleukin-6, tumor necrosis factor-alpha), and adverse reactions. They reported that Shugan Jieyu Xiaopi Fang significantly alleviated symptoms, improved sleep quality, modulated gastrointestinal hormone and neurotransmitter levels, reduced inflammation, and had a favorable safety profile. Mingming et al 122 evaluated the combined efficacy.
In addition, Fan Mingming et al 122 evaluated the efficacy of Chaizhu Liwei Yin combined with domperidone in 80 elderly patients with FD related to spleen deficiency and qi stagnation accompanied by sleep disorders. The patients were randomly assigned to receive either combined treatment or domperidone alone for four weeks, and their clinical outcomes, including TCM syndrome scores, NDI, and PSQI, were assessed before and after treatment. They found that combined therapy significantly improved gastrointestinal symptoms, sleep quality, and overall quality of life compared to domperidone alone.
Wang et al 123 conducted a multicenter, randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of Simotang oral liquid in treating PDS, a subtype of FD. The trial included 240 participants from 12 centers randomly assigned to either the experimental group receiving Simotang oral liquid or the control group receiving placebo, each for four weeks. The findings indicated that Simotang oral liquid effectively and safely alleviated the PDS symptoms.
Further evidence of the benefits of TCM in FD treatment was provided by Liu Fangfang et al,124 who investigated the efficacy of Zhizhu Kuanzhong capsules combined with mosapride in patients with FD characterized by spleen deficiency and qi stagnation. A total of 102 patients were randomized to receive either combination therapy or mosapride alone for one month. This study evaluated clinical efficacy, gastrointestinal hormone levels, gastric emptying rates, and adverse reactions. Combination therapy significantly regulated gastrointestinal hormone levels, enhanced gastric emptying, and demonstrated favorable safety, indicating its potential as a therapeutic approach for FD.
Collectively, the application of TCM in treating FD showed promising outcomes, especially when integrated with conventional therapies. Clinical studies have highlighted the efficacy of TCM formulations in symptom relief, modulation of gastrointestinal function, and enhancement of patient well-being. Although these findings support the role of TCM as a complementary treatment, further research is needed to standardize protocols and assess long-term safety and effectiveness.
Acupuncture Treatment of FD
Acupuncture has been increasingly studied as a potential therapeutic approach for FD, especially for its effects on gastrointestinal motility, brain-gut peptides, inflammatory responses, and the associated psychological symptoms. Several studies have investigated different acupuncture protocols, highlighting their potential to alleviate FD symptoms through various mechanisms. Herein, we describe the methods of acupuncture and moxibustion treatment for FD and provide a summary in Table 3.
 |
Table 3 Acupuncture Treatments for FD and Their Mechanisms of Action |
Zhou Jingying et al 125 conducted an animal study to evaluate the effects of electroacupuncture at the “Neiguan” and “Zhongwan” acupoints, individually and in combination, on gastric motility and brain-gut peptides in FD rats. The study involved 56 specific pathogen-free (SPF) male Sprague-Dawley rats, which were randomly assigned to a normal group or a model group, with FD induced using a 0.1% sucrose-iodoacetamide solution and the modified small platform method. After establishing the model, the rats were divided into four subgroups: the model, Neiguan, Zhongwan, and Neiguan-Zhongwan groups. Electroacupuncture was applied bilaterally at the acupoints for 30 minutes daily for seven days. The results indicated that the combined stimulation of “Neiguan-Zhongwan” significantly enhanced gastric motility, alleviated dyspeptic symptoms, and modulated brain-gut peptides, showing superior efficacy compared to individual acupoint stimulation.
Xiaojuan et al 126 explored the combination of Yimu Futu acupuncture therapy and abdominal massage in patients with FD related to liver depression and spleen deficiency. In this randomized study involving 120 FD patients, the observation group received acupuncture in addition to standard treatment and abdominal massage, whereas the control group received standard treatment alone. The therapeutic outcomes included TCM symptom scores, gastrointestinal hormone levels, and intestinal flora composition. The combined approach significantly improved clinical symptoms, regulated gastrointestinal hormones, and restored intestinal microbiota balance compared to the control group.
Kang et al 127 investigated the effect of electroacupuncture on intestinal mucosal permeability in FD rats, focusing on the TLR4/ERK signaling pathway. Fifty male Sprague-Dawley rats were randomly assigned to the control, model, electroacupuncture (EA), and TAK242 (TLR4 inhibitor) groups. Electroacupuncture at “Zusanli” (ST 36) and “Taichong” (ST 40) was administered daily for 14 days. Overall, the results indicate that the intervention reduced the gastric residual rate, improved intestinal propulsion, decreased inflammatory markers, and restored mucosal barrier integrity, likely through the downregulation of TLR4-ERK pathway proteins.
Cheng et al 128 examined the effects of acupuncture on gastrointestinal motility and serotonin (5-HT) system in rats with FD and depression-like symptoms. The rats were divided into the normal, model, acupuncture, and fluoxetine groups. The acupuncture group received treatment at “Baihui”, “Zhongwan”, and bilateral “Zusanli” for seven days. Their findings demonstrated that acupuncture improved gastrointestinal motility, alleviated depression-like behaviors, and modulated brain-gut peptide levels, particularly by regulating the 5-HT system.
Taken together, these studies indicate that acupuncture may offer therapeutic benefits in FD by enhancing gastric motility, regulating brain-gut peptides, improving intestinal mucosal integrity, and addressing comorbid psychological symptoms. The ability of acupuncture to modulate the brain-gut axis, particularly through specific acupoint combinations, suggests a potential complementary role in FD management. Further research is warranted to confirm these findings and establish standardized protocols.
Comprehensive Treatment of FD with TCM
Comprehensive treatment approaches combining TCM with acupuncture have shown promise in managing FD, particularly in cases characterized by spleen deficiency, qi stagnation, and liver-stomach disharmony. Recent RCTs have explored the synergistic effects of herbal formulations and acupuncture (Figure 3 and Table 4), highlighting their potential to improve gastrointestinal motility, regulate gastrointestinal hormone levels, and alleviate symptoms associated with FD.
 |
Table 4 Comprehensive TCM Approaches for FD and Their Mechanisms of Action |
 |
Figure 3 TCM mechanism for Intervention in FD. |
In this regard, Han Jing et al 129 conducted an RCT to evaluate the efficacy of Tiaoqi Hewei Decoction combined with acupuncture targeting the Zhongwan, bilateral Zusanli, Neiguan, and Tianshu acupoints in 98 FD patients with spleen deficiency and qi stagnation, who were randomly assigned to receive either Mosapride Citrate Tablets with acupuncture (control group) or Tiaoqi Hewei Decoction with acupuncture in addition to Mosapride Citrate Tablets (observation group) for four weeks. They found that the combined treatment significantly improved gastric emptying, regulated gastrointestinal hormones, and alleviated symptoms compared with the control group, demonstrating its potential in enhancing the overall therapeutic efficacy for FD. In addition, Fan Mingming et al 130 assessed the clinical efficacy and safety of combining Chaizhu Liwei Yin with acupuncture targeting the bilateral Pishu, Shangwan, Zhongwan, Tianshu, Taichong, and Ganshu points in patients with FD. In this RCT involving 90 participants, the treatment group received combined therapy, while the control group was treated with domperidone alone for four weeks. Their results showed that the combined approach significantly improved TCM syndrome scores, enhanced gastrointestinal motility, and alleviated symptoms such as epigastric pain, fullness, burning sensations, anorexia, nausea, vomiting, and fatigue, demonstrating superior efficacy compared with domperidone alone. Moreover, Zhao Wenlin et al 131 investigated the combination of Yuejubaohe Pill and acupoint catgut embedding in 123 FD patients with liver-stomach disharmony randomly assigned to receive either standard treatment with acupoint catgut embedding (control group) or Yuejubaohe Pill in addition to the control treatment (study group). They reported that the combined therapy significantly enhanced serum gastrointestinal hormone levels, improved pepsinogen levels, and facilitated gastric motility, resulting in better clinical outcomes compared with the control group. Finally, Ji Weipeng et al 132 explored the efficacy of Jieyu Fuwei Powder combined with acupoint catgut embedding in FD patients characterized by liver-stomach disharmony. The RCT involved 62 patients randomly divided into a treatment group receiving Jieyu Fuwei Powder and acupoint catgut embedding and a control group receiving oral mosapride citrate. They reported that combination therapy significantly reduced serum levels of calcitonin gene-related peptide (CGRP) and Substance P (SP), while increasing motilin (MTL) and gastrin (GAS) levels, suggesting that the combined treatment was more effective in alleviating FD symptoms compared to the control group, likely due to the modulation of brain-gut peptide levels.
Collectively, comprehensive TCM treatment combining herbal formulations with acupuncture or catgut embedding has the potential to alleviate FD symptoms, enhance gastrointestinal motility, and regulate hormone levels. These findings support the therapeutic benefits of integrating TCM and acupuncture in FD management, although further studies are warranted to optimize treatment protocols and assess their long-term efficacy.
Advantages and Mechanistic Differences of TCM in Improving FD Symptoms
TCM offers several advantages in the management of FD compared to conventional drug treatments, such as its holistic approach to TCM, which aims to address the root causes of FD rather than merely alleviate symptoms. TCM therapies, including herbal formulations and acupuncture, have demonstrated potential in regulating gastrointestinal function, modulating the gut-brain axis, and enhancing overall well-being without adverse effects commonly associated with pharmacological treatments.
Unlike conventional drug therapies, which often target specific physiological pathways (eg, acid suppression or motility enhancement), TCM approaches consider the balance of the entire body’s systems. For instance, herbal formulations are tailored to individual patient presentations, addressing not only gastrointestinal symptoms, but also associated psychological and systemic factors. Acupuncture, another TCM modality, has been shown to influences brain-gut peptides and modulates neuroimmune responses, thereby contributing to symptomatic relief through multifaceted mechanisms.
In contrast, Western medicine typically focuses on symptomatic management through pharmacological interventions such as PPIs, prokinetic agents, or antibiotics. These treatments primarily aim to alleviate symptoms, such as epigastric pain, bloating, or delayed gastric emptying. However, they may not address the underlying factors, such as emotional stress or systemic imbalances, which are considered integral to TCM theory. Thus, a combination of both Western and TCM approaches might lead to improved treatment outcomes, as shown in Figure 4. Collectively, TCM offers a more individualized and comprehensive therapeutic approach, potentially improving FD symptoms through mechanisms distinct from those of western medicine. By targeting the body’s holistic state rather than isolated symptoms, TCM may contribute to both short-term relief and long-term regulation of gastrointestinal function, whereas Western medicine may provide instant relief to patients by soothing their acute symptoms. Further research is warranted to elucidate these mechanisms and to optimize the integration of TCM with conventional treatments for FD.
 |
Figure 4 Pathways of integrative TCM and modern medical treatment for FD. |
Conclusion
FD has historically been regarded as the most prevalent functional gastrointestinal disorder; however, there is growing recognition that it may not be solely a functional condition.133–136 However, the pathophysiological mechanisms underlying FD remain unclear. Symptoms related to meals in patients with PDS and those in overlapping symptom groups have been linked to low-grade inflammation, which is attributed to increased permeability of the duodenal mucosa and alterations in submucosal neuronal structures.137–139 This correlation indicated the potential of these factors to serve as diagnostic or prognostic indicators. Nonetheless, it remains uncertain whether these changes are causative or are a consequence of an unidentified etiology. Additional research is warranted to explore the relationship between gastric dysfunction resulting from duodenal inflammation and GERD as well as the potential for overlapping IBS contingent upon the extent of intestinal inflammation.
In terms of therapeutic approaches, existing treatments demonstrate limited effectiveness, primarily addressing symptoms and sensorimotor functions of the stomach, rather than the underlying pathologies associated with the duodenum. The integration of TCM with contemporary medical practices offers a holistic treatment option. Nonetheless, it is important to acknowledge that the underlying mechanisms of TCM remain poorly understood. Future research should focus on elucidating these mechanisms. Despite this, the combined application of traditional Chinese and Western medical treatments for FD remains a viable therapeutic strategy (Figure 3).
In considering future perspectives, it is essential that the categorization of FD subtypes incorporate various underlying pathophysiological mechanisms. This approach could facilitate targeted therapeutic interventions, moving beyond symptom-based treatment paradigms. However, significant progress remains to be made in investigating the pathophysiology and treatment modalities for FD.
Abbreviations
FGIDs, Functional gastrointestinal disorders; GI, Gastrointestinal; FD, Functional dyspepsia; IBS, Irritable bowel syndrome; EPS, Epigastric pain syndrome; PDS, Postprandial discomfort syndrome; TCM, Traditional Chinese Medicine; CBC, Complete blood count; H. pylori, Helicobacter pylori; PPIs, Proton pump inhibitors; HRQOL, Health-related quality of life; MAM, Mucosal-associated microbiota; FDA, Food and Drug Administration; H2RA, Histamine-2 receptor antagonist; GAD, Generalized anxiety disorder; TCAs, Tricyclic antidepressants; SNRIs, Serotonin Norepinephrine Reuptake Inhibitors; CBT, Cognitive behavioral therapy; RCT, Randomized controlled trial; NDSI, Nepean Dyspepsia Symptom Index; NDLQI, Nepean Dyspepsia Life Quality Index; PSQI, Pittsburgh Sleep Quality Index; NDI, Nepean Dyspepsia Index; SPF, Specific pathogen-free; SD, Sprague-Dawley; H&E, Hematoxylin and eosin; ELISA, Enzyme-linked immunosorbent assay; CCK, Cholecystokinin; EA, Electroacupuncture; DAO, Diamine oxidase; LPS, Lipopolysaccharide; D-LA, D-lactic acid; MBP, Myelin basic protein; SS, Somatostatin; MTL, Motilin; TPH1, Tryptophan hydroxylase 1; CGRP, Calcitonin-gene-related peptide; SP, Substance P; GAS, Gastrin.
Funding
Natural Science Foundation of Heilongjiang Province (LH2023H079).
Disclosure
The authors declare that they have no affiliation with, or involvement in, any organization or entity with any financial interest in the subject matter or materials discussed in this paper.
References
1. Christopher JB, Douglas AD, Nicholas JT, et al. Functional gastrointestinal disorders: advances in understanding and management. Lancet. 2020; 396(10263):1664–74.
2. Gregory SS, Prakash CG. Functional dyspepsia: diagnostic and therapeutic approaches. Drugs. 2020; 80(13):1319–36.
3. S Guilhermebecker, M Luizedmundo, F Carlosferrnandodem, et al. Influence of organic and functional dyspepsia on work productivity: the HEROES-DIP study. Value Health. 2011; 14:S126–9.
4. Stanghellini V, Talley NJ, CHAN F, et al. Rome IV - gastroduodenal disorders. Gastroenterology. 2016; 150:1380–1392 doi:10.1053/j.gastro.2016.02.011
5. Nicholas JT. Functional dyspepsia: advances in diagnosis and therapy. Gut Liver. 2017; 11(3):349.
6. Jiaqi Z, Ting C, Yongtian W, et al. Insights and future prospects of TCM in the treatment of functional dyspepsia. Phytomedicine. 2024; 127:155481.
7. Lacy BE, Weiser KT, Kennedy AT, et al. Functional dyspepsia: the economic impact to patients. Aliment Pharmacol Ther. 2013; 38(2). 170–177 doi:10.1111/apt.12355
8. Brook RA, Kleinman NL, Choung RS, et al. Functional dyspepsia impacts absenteeism and direct and indirect costs. Clin Gastroenterol Hepatol. 2010; 8(6): 498–503. doi:10.1016/j.cgh.2010.03.003
9. Zagari RM, Law GR, Fuccio L, et al. Epidemiology of functional dyspepsia and subgroups in the Italian general population: an endoscopic study. Gastroenterology. 2010; 138(4): 1302–1311. doi:10.1053/j.gastro.2009.12.057
10. P Aro, Talley NJ, Ronkainen J, et al. Anxiety is associated with uninvestigated and functional dyspepsia (Rome III Criteria) in a Swedish population-based study. Gastroenterol -Baltimore Philadelphia. 2009;137(1):94–100.
11. Bernersen B, Johnsen R, Straume B. Non-ulcer dyspepsia and peptic ulcer: the distribution in a population and their relation to risk factors. Gut. 1996; 38(6): 822–825. doi:10.1136/gut.38.6.822
12. Kaji M, Fujiwara Y, Shiba M, et al. Prevalence of overlaps between GERD, FD and IBS and impact on health-related quality of life. J Gastroenterol Hepatol. 2010; 25(6): 1151–1156. doi:10.1111/j.1440-1746.2010.06249.x
13. Ohara S, Kawano T, T Kouzuk, Kouzu T. Survey on the prevalence of GERD and FD based on the Montreal definition and the Rome III criteria among patients presenting with epigastric symptoms in Japan. J Gastroenterol. 2011; 46: 603–611. doi:10.1007/s00535-011-0382-1
14. Vakil N, Halling K, Ohlsson L, et al. Symptom overlap between postprandial distress and epigastric pain syndromes of the Rome III dyspepsia classification. Am J Gastroenterol. 2013; 108(5): 767–774. doi:10.1038/ajg.2013.89
15. Noh YW, Jung HK, Kim SE, et al. Overlap of erosive and non-erosive reflux diseases with functional gastrointestinal disorders according to Rome III criteria. J Neurogastroenterol Motil. 2010; 16(2): 148–156. doi:10.5056/jnm.2010.16.2.148
16. Savarino E, Pohl D, Zentilin P, et al. Functional heartburn has more in common with functional dyspepsia than with non-erosive reflux disease. Gut. 2009; 58(9): 1185. doi:10.1136/gut.2008.175810
17. Ford AC, Marwaha A, Lim A, et al. Systematic review and meta-analysis of the prevalence of irritable bowel syndrome in individuals with dyspepsia. Clin Gastroenterol Hepatol. 2010; 8(5): 401–409. doi:10.1016/j.cgh.2009.07.020
18. Choi J, Kim N, Yoon H, et al. Overlap between irritable bowel syndrome and functional dyspepsia including subtype analyses. J Gastroenterol Hepatol. 2017; 32:1553–1561. doi:10.1111/jgh.13756
19. Tack J, Jones MP, Karamanolis G, et al. Symptom pattern and pathophysiological correlates of weight loss in tertiary-referred functional dyspepsia. Neurogastroenterol Motil. 2010; 22(1): 29–e5. doi:10.1111/j.1365-2982.2008.01240.x
20. Olafur SP, William EW, Miranda ALVT, et al. Rome IV diagnostic questionnaires and tables for investigators and clinicians. Gastroenterology. 2016;2016:S0016–5085.
21. Suzuki H, Kusunoki H, Kamiya T, et al. Effect of lansoprazole on the epigastric symptoms of functional dyspepsia (ELF study): a multicentre, prospective, randomized, double-blind, placebo-controlled clinical trial. United Eur Gastroenterol J. 2013; 1(6): 445–452. doi:10.1177/2050640613510904
22. Yu-Jen F, Jyh-Ming L, Chieh-Chang C, et al. Distinct aetiopathogenesis in subgroups of functional dyspepsia according to the Rome III criteria. Gut. 2014; 64(10):1517–1528.
23. Tack J, Talley NJ. Functional dyspepsia--symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol. 2013; 10(3): 134–141. doi:10.1038/nrgastro.2013.14
24. Ford AC, Marwaha A, Sood R, Moayyedi P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut. 2015;64(7):1049–57.
25. Fang YJ, Liou JM, Chen CC, et al. Distinct aetiopathogenesis in subgroups of functional dyspepsia according to the Rome III criteria. Gut. 2015; 64(10): 1517–1528. doi:10.1136/gutjnl-2014-308114
26. Seon-Young Park, Acosta, Andrés A, et al. Gastric motor dysfunction in patients with functional gastroduodenal symptoms. Am J Gastroenterol. 2017; 112: 1689–1699. doi:10.1038/ajg.2017.264
27. Sayuk GS, Elwing JE, Lustman PJ, et al. High somatic symptom burdens and functional gastrointestinal disorders. Clin Gastroenterol Hepatol. 2007; 5(5): 556–562. doi:10.1016/j.cgh.2006.11.024
28. Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol. 2006; 12(17): 2660–2666. doi:10.3748/wjg.v12.i17.2661
29. Bytzer P, Hallas J. Drug-induced symptoms of functional dyspepsia and nausea. A symmetry analysis of one million prescriptions. Aliment Pharmacol Ther. 2000; 14(11): 1479–1484. doi:10.1046/j.1365-2036.2000.00862.x
30. Hallas J, Bytzer P. Screening for drug related dyspepsia: an analysis of prescription symmetry. Eur J Gastroenterol Hepatol. 1998;10:27–32.
31. Talley N. Practice parameters committee of the American college of gastroenterology guidelines for the management of dyspepsia. Am J Gastroenterol. 2005; 100:2324–37.
32. Monteiro L, Oleastro M, Lehours P, et al. Diagnosis of Helicobacter pylori infection. Helicobacter. 2010; 14(s1): 8–14. doi:10.1111/j.1523-5378.2009.00707.x
33. Petrarca L. Dyspepsia and celiac disease: prevalence, diagnostic tools and therapy. World J Methodol. 2014; 4 (3): 8. doi:10.5662/wjm.v4.i3.189
34. Moayyedi P, Lacy BE, Andrews CN, et al. ACG and CAG clinical guideline: management of dyspepsia (vol 112, pg 988, 2017). Am J Gastroenterol. 2017; 2017(9): 112.
35. Petrarca L, Nenna R, Mastrogiorgio G, et al. Dyspepsia and celiac disease: prevalence, diagnostic tools and therapy. World J Methodol. 2014; 4: 189
36. Stanghellini V, Chan FK, Hasler WL, et al. Gastroduodenal disorders. Gastroenterology. 2016; 150(6): 1380–1392.
37. Miwa H. Why dyspepsia can occur without organic disease: pathogenesis and management of functional dyspepsia. J Gastroenterol. 2012; 47: 862–871. doi:10.1007/s00535-012-0625-9
38. Tack J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–52.
39. Tack J, Demedts I, Meulemans A, et al. Role of nitric oxide in the gastric accommodation reflex and in meal induced satiety in humans. Gut. 2002; 51(2): 219–224. doi:10.1136/gut.51.2.219
40. Piessevaux H, Tack J, Walrand S, et al. Intragastric distribution of a standardized meal in health and functional dyspepsia: correlation with specific symptoms. Neurogastroenterol Motil. 2003; 15(5): 447–455. doi:10.1046/j.1365-2982.2003.00431.x
41. Caldarella MP, Azpiroz F, Malagelada JR. Antro-fundic dysfunctions in functional dyspepsia. Gastroenterology. 2003; 124(5): 1220–1229. doi:10.1016/S0016-5085(03)00287-7
42. Tack J, Janssen P, Masaoka T, et al. Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2012; 10(11): 1239–1245. doi:10.1016/j.cgh.2012.06.036
43. Sarnelli G, Caenepeel P, Geypens B, et al. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol. 2003; 98(4): 783–788. doi:10.1111/j.1572-0241.2003.07389.x
44. Janssen P, Harris MS, Jones M, et al. The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am J Gastroenterol. 2013; 108(9): 1382–1391. doi:10.1038/ajg.2013.118
45. Vijayvargiya P, Camilleri M, Chedid V, et al. Effects of promotility agents on gastric emptying and symptoms: a systematic review and meta-analysis. Gastroenterology. 2019; 156:1650–1660. doi:10.1053/j.gastro.2019.01.249
46. Tack J, Caenepeel P, Fischler B, et al. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001; 121(3): 526–535. doi:10.1053/gast.2001.27180
47. Vandenberghe J, R Vos, Persoons P, et al. Dyspeptic patients with visceral hypersensitivity: sensitization of pain specific or multimodal pathways? Gut. 2005; 54(7): 914–919. doi:10.1136/gut.2004.052605
48. Oshima T, Tomita T, Okugawa T, et al. Generation of dyspeptic symptoms by direct acid and water infusion into the stomachs of functional dyspepsia patients and healthy subjects. Aliment Pharmacol Ther. 2012; 35(1): 175–182. doi:10.1111/j.1365-2036.2011.04918.x
49. Samsom M, Verhagen Mamt, Henegouwen GPV, et al. Abnormal clearance of exogenous acid and increased acid sensitivity of the proximal duodenum in dyspeptic patients. Gastroenterology. 1999; 116(3): 515–520. doi:10.1016/S0016-5085(99)70171-X
50. Lee K-J, Vos R, Janssens J, Tack J. Influence of duodenal acidification on the sensorimotor function of the proximal stomach in humans. AJP Gastrointestinal Liver Physiol. 2004; 286(2): 278G–84. doi:10.1152/ajpgi.00086.2003
51. Vanuytsel T, Van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014; 63(8): 1293. doi:10.1136/gutjnl-2013-305690
52. Palma GD, Blennerhassett P, J Lu, et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat Commun. 2015; 6: 7735. doi:10.1038/ncomms8735
53. Lee I-S, Wang H, Chae Y, Preissl H, Enck P. Functional neuroimaging studies in functional dyspepsia patients: a systematic review. Neurogastroenterol Motil. 2016; 28(6): 793–805. doi:10.1111/nmo.12793
54. Koloski NA, Jones M, Kalantar J, et al. The brain--gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012; 61(9): 1284. doi:10.1136/gutjnl-2011-300474
55. L Kwangjae, K Jinhong, C Sungwon. Dyspeptic symptoms associated with hypersensitivity to gastric distension induced by duodenal acidification. J Gastroenterol Hepatol. 2006; 21(3):515–20.
56. Magnus S, Rita V, Jozef J, et al. Acid infusion enhances duodenal mechanosensitivity in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2003; 285(2):G309–15.
57. Vanuytsel T, Karamanolis G, Oudenhove LV, Vos R, Tack J. Influence of ondansetron on gastric sensorimotor responses to short duodenal acid infusion in healthy volunteers. Neurogastroenterol Motil. 2010; 23(3):226–e115.
58. Vanheel H, Farré R, Beeckmans D. Duodenal acid perfusion increases duodenal permeability and activates the duodenogastric reflex, independently from mast cell activation. United Eur Gastroenterol J. 2017; 5(Supplement): 1.
59. Kindt S, Tertychnyy A, De Hertogh G, et al. Intestinal immune activation in presumed post‐infectious functional dyspepsia. Neurogastroenterol Motil. 2009; 21(8): 832–e56. doi:10.1111/j.1365-2982.2009.01299.x
60. Sheehan D, Moran C, Shanahan F. The microbiota in inflammatory bowel disease. J Gastroenterol. 2015; 50: 495–507. doi:10.1007/s00535-015-1064-1
61. Li G, Yang M, Zhou K, et al. Diversity of duodenal and rectal microbiota in biopsy tissues and luminal contents in healthy volunteers. J Microbiol Biotechnol. 2015; 25(7): 1136–1145. doi:10.4014/jmb.1412.12047
62. Simrén M, Barbara G, J Flinth, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013; 62(1): 159–176. doi:10.1136/gutjnl-2012-302167
63. Zhong L, Shanahan ER, A RAJ, et al. Dyspepsia and the microbiome: time to focus on the small intestine. Gut. 2017; 66(6): 1168–1169. doi:10.1136/gutjnl-2016-312574
64. Kerckhoffs AP, Samsom M, Van Der Rest ME, et al. Lower bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World J Gastroenterol. 2009; 15(23): 2887. doi:10.3748/wjg.15.2887
65. Giamarellos-Bourboulis E, Tang J, Pyleris E, et al. Molecular assessment of differences in the duodenal microbiome in subjects with irritable bowel syndrome. Scand J Gastroenterol. 2015; 50(9): 1076–1087. doi:10.3109/00365521.2015.1027261
66. Rnnestad I, Akiba Y, Kaji I, et al. Duodenal luminal nutrient sensing. Curr Opin Pharmacol. 2014; 19: 67–75. doi:10.1016/j.coph.2014.07.010
67. Mori H, Verbeure W, Schol J, et al. Gastrointestinal hormones and regulation of gastric emptying. Current Opinion Endocrinol Diab Obes. 2022; 2: 29.
68. Hanne V, Maria V, V Tim, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut. 2013; 63(2):262–71.
69. Nicholas JT, Marjorie MW, Pertti A, et al. Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case-control study. Clin Gastroenterol Hepatol, 2007, 5(10):1175–1183
70. Du L, Chen B, Kim JJ, Chen X, Dai N. Micro-inflammation in functional dyspepsia: a systematic review and meta-analysis. Neurogastroenterol Motil, 2018, 30(4).
71. Grace B, Georgia C, Andrea M, et al. Evidence for local and systemic immune activation in functional dyspepsia and the irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2019; 114(3):429–36.
72. Xiaohong W, Xiaopei L, Wenqing G, et al. Quantitative evaluation of duodenal eosinophils and mast cells in adult patients with functional dyspepsia. Ann Diagn Pathol. 2015; 19(2):50–6.
73. Hai-Peng Y, Xiao-Pei L, Wen-Rong Y, et al. Inducible nitric oxide synthase in the duodenal mucosa is associated with mast cell degranulation in patients with functional dyspepsia. Ann Clin Lab Sci. 2015; 45(5):522–7.
74. V Tim, Sander VW, Hanne V, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2013; 63(8):1293–9.
75. Marjorie MW, Kavita RA, Lisa SES, et al. Duodenal eosinophilia and early satiety in functional dyspepsia: confirmation of a positive association in an Australian cohort. J Gastroenterol Hepatol. 2013; 29(3):474–9.
76. Wauters L, Nightingale S, Talley NJ, Sulaiman B, Walker MM. Functional dyspepsia is associated with duodenal eosinophilia in an Australian paediatric cohort. Aliment Pharmacol Ther. 2017; 45(10):1358–64.
77. Moayyedi PM, Lacy BE, Andrews CN, et al. ACG and CAG clinical guideline: management of dyspepsia. Official J Ame College Gastroenterol. 2017; 112(7): 988–1013. doi:10.1038/ajg.2017.154
78. L Lan, Yu J, Chen Y-L, et al. Symptom-based tendencies of Helicobacter pylori eradication in patients with functional dyspepsia. World J Gastroenterol. 2011; 17(27): 3242.
79. O’morain C. Role of Helicobacter pylori in functional dyspepsia. World J Gastroenterol. 2006; 12(17): 2677. doi:10.3748/wjg.v12.i17.2677
80. Sugano K, Tack J, J Kuiperse, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015; 64(9): 1353–1367. doi:10.1136/gutjnl-2015-309252
81. Vaezi MF, Yang Y-X, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology. 2017; 153(1): 35–48. doi:10.1053/j.gastro.2017.04.047
82. Moayyedi P, Leontiadis GI. The risks of PPI therapy. Nat Rev Gastroenterol Hepatol. 2012; 9(3): 132–139. doi:10.1038/nrgastro.2011.272
83. Pinto‐sanchez MI, Yuan Y, Bercik P, et al. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst Rev. 2017; 2017(3):1.
84. Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011; 364(1): 22–32. doi:10.1056/NEJMoa1004409
85. Tan VP, Liu KS-H, Lam F, et al. Randomized clinical trial: rifaximin versus placebo for the treatment of functional dyspepsia. Aliment Pharmacol Ther. 2017; 45(6): 767–776. doi:10.1111/apt.13945
86. Potter MD, Wood NK, Walker MM, et al. Proton pump inhibitors and suppression of duodenal eosinophilia in functional dyspepsia. Gut. 2019; 68(7): 1339–1340. doi:10.1136/gutjnl-2018-316878
87. Moayyedi P, Shelly S, Deeks JJ, et al. Pharmacological interventions for non‐ulcer dyspepsia. Cochrane Database Syst Rev. 2006; 2006(4):1.
88. Potter MD, Goodsall TM, Walker MM, et al. Dual histamine blockade for the treatment of adult functional dyspepsia: a single centre experience. Gut. 2019;2019:1.
89. K Vandenhoute, Carbone F, Tack J. Postprandial distress syndrome: stratification and management. Expert rev gastroenterol hepatol. 2019; 13(1): 37–46. doi:10.1080/17474124.2019.1543586
90. Carbone F, Tack J. Gastroduodenal mechanisms underlying functional gastric disorders. Dig Dis. 2014; 32(3): 222–229. doi:10.1159/000357854
91. Tack J, Camilleri M. New developments in the treatment of gastroparesis and functional dyspepsia. Curr Opin Pharmacol. 2018; 43: 111–117. doi:10.1016/j.coph.2018.08.015
92. Pittayanon R, YUAN Y, Bollegala NP, et al. Prokinetics for functional dyspepsia: a systematic review and meta-analysis of randomized control trials. Official J Ame College Gastroenterol. 2019; 114(2): 233–243. doi:10.1038/s41395-018-0258-6
93. Tack J, Camilleri M, Chang L, et al. Systematic review: cardiovascular safety profile of 5‐HT 4 agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther. 2012; 35(7): 745–767. doi:10.1111/j.1365-2036.2012.05011.x
94. Leelakanok N, Holcombe A, Schweizer ML. Domperidone and risk of ventricular arrhythmia and cardiac death: a systematic review and meta-analysis. Clin Drug Invest. 2016; 36: 97–107. doi:10.1007/s40261-015-0360-0
95. Morris AD, Chen J, Lau E, et al. Domperidone-associated QT interval prolongation in non-oncologic pediatric patients: a review of the literature. Canadian J Hospital Pharm. 2016; 69(3): 224. doi:10.4212/cjhp.v69i3.1560
96. Degen L, Matzinger D, Merz M, et al. Tegaserod, a 5‐HT4 receptor partial agonist, accelerates gastric emptying and gastrointestinal transit in healthy male subjects. Aliment Pharmacol Ther. 2001; 15(11): 1745–1751. doi:10.1046/j.1365-2036.2001.01103.x
97. Chey WD, Howden CW, Tack J, et al. Long-term tegaserod treatment for dysmotility-like functional dyspepsia: results of two identical 1-year cohort studies. Dig Dis Sci. 2010; 55: 684–697. 3 doi:10.1007/s10620-009-1049-0
98. Vakil N, Laine L, Talley NJ, et al. Tegaserod treatment for dysmotility-like functional dyspepsia: results of two randomized, controlled trials. Off J Ame College Gastroenterol. 2008; 103(8): 1906–1919.
99. Clouse RE, Prakash C, Anderson RJ, et al. Antidepressants for functional gastrointestinal symptoms and syndromes: a meta-analysis. Gastroenterology. 2001; 5(120): A642. doi:10.1016/S0016-5085(01)83189-9
100. Lu Y, Chen M, Huang Z, et al. Antidepressants in the treatment of functional dyspepsia: a systematic review and meta-analysis. PLoS One. 2016; 11(6): e0157798. doi:10.1371/journal.pone.0157798
101. Clouse R. Antidepressants for irritable bowel syndrome. Gut. 2003; 52(4): 598–599. doi:10.1136/gut.52.4.598
102. Morgan V, Pickens D, Gautam S, et al. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005; 54(5): 601–607. doi:10.1136/gut.2004.047423
103. Gillman P. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007; 151(6): 737–748. doi:10.1038/sj.bjp.0707253
104. Grover M, Camilleri M. Effects on gastrointestinal functions and symptoms of serotonergic psychoactive agents used in functional gastrointestinal diseases. J Gastroenterol. 2013; 48: 177–181. doi:10.1007/s00535-012-0726-5
105. Van Kerkhoven LA, Laheij RJ, Aparicio N, et al. Effect of the antidepressant venlafaxine in functional dyspepsia: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2008; 6(7): 746–752. doi:10.1016/j.cgh.2008.02.051
106. Igarashi M, Nakae H, Matsuoka T, et al. Alteration in the gastric microbiota and its restoration by probiotics in patients with functional dyspepsia. BMJ Open Gastroenterol. 2017; 4(1): e000144. doi:10.1136/bmjgast-2017-000144
107. Ohtsu T, Takagi A, Uemura N, et al. The ameliorating effect of Lactobacillus gasseri OLL2716 on functional dyspepsia in Helicobacter pylori-uninfected individuals: a randomized controlled study. Digestion. 2017; 96(2): 92–102. doi:10.1159/000479000
108. Tziatzios G, Giamarellos-Bourboulis EJ, Papanikolaou IS, et al. Is small intestinal bacterial overgrowth involved in the pathogenesis of functional dyspepsia? Med Hypotheses. 2017; 106: 26–32. doi:10.1016/j.mehy.2017.07.005
109. Calvert EL, Houghton LA, Cooper P, et al. Long-term improvement in functional dyspepsia using hypnotherapy. Gastroenterology. 2002; 123(6): 1778–1785. doi:10.1053/gast.2002.37071
110. Cheng C, Yang F-C, S Jun, et al. Flexible coping psychotherapy for functional dyspeptic patients: a randomized, controlled trial. Psychosomatic Med. 2007; 69(1): 81–88. doi:10.1097/01.psy.0000249734.99065.6f
111. Orive M, Barrio I, Orive V, et al. A randomized controlled trial of a 10 week group psychotherapeutic treatment added to standard medical treatment in patients with functional dyspepsia. J Psychosomatic Res. 2015; 78(6): 563–568. doi:10.1016/j.jpsychores.2015.03.003
112. Haug TT, Wilhelmsen I, Svebak S, et al. Psychotherapy in functional dyspepsia. J Psychosomatic Res. 1994; 38(7): 735–744. doi:10.1016/0022-3999(94)90026-4
113. Faramarzi M, Azadfallah P, Book HE, et al. The effect of psychotherapy in improving physical and psychiatric symptoms in patients with functional dyspepsia. Iranian J Psych. 2015; 10(1): 43.
114. Lackner JM, Jaccard J, Krasner SS, et al. Self-administered cognitive behavior therapy for moderate to severe irritable bowel syndrome: clinical efficacy, tolerability, feasibility. Clin Gastroenterol Hepatol. 2008; 6(8): 899–906. doi:10.1016/j.cgh.2008.03.004
115. Andersson E, LJÓTSSON B, Smit F, et al. Cost-effectiveness of internet-based cognitive behavior therapy for irritable bowel syndrome: results from a randomized controlled trial. BMC Public Health. 2011; 11: 1–7. doi:10.1186/1471-2458-11-215
116. Lindfors P, ljótsson B, Bjornsson E, et al. Patient satisfaction after gut‐directed hypnotherapy in irritable bowel syndrome. Neurogastroenterol Motil. 2013; 25(2): 169–e86. doi:10.1111/nmo.12022
117. Luqing Zhao, Zhaohong S, Shengsheng Z. Expert consensus on diagnosis and treatment of functional dyspepsia in TCM (2023). Chin J TCM. 2024;39(3):1372–1378.
118. Luqing Zhao, Shengsheng Z. Experience and thinking of TCM treatment of functional dyspepsia. Beijing J TCM. 2011; 30 (01): 32–34.
119. Shanghai University of TCM, Institute of Chinese Medical History and Literature, China Academy of TCM, Fujian University of TCM, et al. Terminology of Clinical Diagnosis and Treatment of TCM Part 1: Disease [Z]. State Administration of Market Supervision and Administration; National Standardization Management Committee. 2023:244
120. Zhang Shengsheng Zhouq. Expert consensus on TCM diagnosis and treatment of epigastric pain (2017). J TCM. 2017; 58 (13): 1166–1170.
121. Ruixing Y, Dingpeng H, Peishan W, et al. Observation on the effect of Shugan Jieyu Xiaopi Fang in the treatment of functional dyspepsia complicated with insomnia of liver-stomach disharmony type. Chin J TCM. 2024;1(1):1–13.
122. Mingming F, Yichuan Z, Yongmei L, et al. Clinical study of Chaishuli Weiyin combined with domperidone in the treatment of senile FD of spleen deficiency and qi stagnation with sleep disorders. J Med Res. 2024; 53 (08): 137–140.
123. Fengyun W, Fang L, Yinglian X, et al. Multicenter clinical study of Simotang oral liquid in the treatment of postprandial dyspepsia syndrome of functional dyspepsia. Chin J TCM. 2024;4(1):1–9.
124. Fangfang L, Jiehong W, Yongpan X, et al. Effect of Zhizhu Kuanzhong Capsule combined with mosapride on gastrointestinal hormones and gastric emptying in patients with functional dyspepsia of spleen deficiency and qi stagnation type. Clin Med Res Pract. 2024; 9 (9): 125–128.
125. Jingying Z, Hongzhi Y, Qian L, et al. Effect of electroacupuncture at “Neiguan-Zhongwan” on gastric motility and related brain-gut peptides in rats with functional dyspepsia Chin J TCM. 2024; 39 (9): 4653–4658.
126. Xiaojuan F, Hao Y, Kejun L, et al. Effect of Yimu Futu acupuncture combined with abdominal massage on gastrointestinal hormones and intestinal flora in patients with functional dyspepsia of liver depression and spleen deficiency type. Adv Mod Biomed. 2024; 24 (15): 2878–2881.
127. Zhaoxia K, Dongmei C. Effect of electroacupuncture on intestinal mucosal permeability in rats with functional dyspepsia based on TLR4/ERK pathway. J TCM. 2024; 30 (5): 29–35.
128. Cheng Z, Guoqi Z, Jingji W, et al. Study on the mechanism of acupuncture improving functional dyspepsia with depression-like behavior in rats. Acupuncture Res. 2025;50:1–8.
129. Han J, Xu X, Sun YX. Effect of Tiaoqi Hewei Decoction combined with acupuncture on gastrointestinal hormones in patients with functional dyspepsia due to spleen deficiency and qi stagnation. Shaanxi TCM. 2024; 45 (5): 622–626.
130. Mingming F, Yongmei L, Yichuan Z, et al. Clinical observation on senile functional dyspepsia with spleen deficiency and qi stagnation treated by acupuncture combined with Chaishuli Weiyin. J Liaoning Univ TCM. 2024; 26 (04): 16–20.
131. Wenlin Z, Hongwei H, Yipin F, et al. Effect of Yueju Baohe Pill combined with acupoint catgut embedding on gastric motility, serum gastrointestinal hormones and pepsinogen in patients with functional dyspepsia of liver-stomach disharmony type. Adv Mod Biomed. 2024; 24 (06): 1109–1113.
132. Weipeng J, Yan Y, Kun L, et al. Clinical efficacy of Jieyu Fuwei Powder combined with acupoint catgut embedding in the treatment of functional dyspepsia of liver-stomach disharmony type and its effect on brain-gut peptides. J Nanjing Univ TCM. 2023; 39 (08): 783–787.
133. Vanheel H, Vicario M, Vanuytsel T, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut. 2014; 63(2): 262–271. doi:10.1136/gutjnl-2012-303857
134. Liebregts T, Adam B, Bredack C, et al. Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Official J Ame College Gastroenterol. 2011; 106(6): 1089–1098. doi:10.1038/ajg.2010.512
135. Wauters L, Nightingale S, Talley NJ, et al. Functional dyspepsia is associated with duodenal eosinophilia in an Australian paediatric cohort. Aliment Pharmacol Ther. 2017; 45(10): 1358–1364. doi:10.1111/apt.14045
136. Kindt S, Van Oudenhove L, Broekaert D, et al. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2009; 21(4): 389–398. doi:10.1111/j.1365-2982.2008.01220.x
137. Cirillo C, Bessissow T, Desmet A-S, et al. Evidence for neuronal and structural changes in submucous ganglia of patients with functional dyspepsia. Official J Ame College Gastroenterol. 2015; 110(8): 1205–1215. doi:10.1038/ajg.2015.158
138. Talley NJ, Walker MM, Aro P, et al. Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case-control study. Clin Gastroenterol Hepatol. 2007; 5(10): 1175–1183. doi:10.1016/j.cgh.2007.05.015
139. Walker MM, Aggarwal KR, Shim LS, et al. Duodenal eosinophilia and early satiety in functional dyspepsia: confirmation of a positive association in an A ustralian cohort. J Gastroenterol Hepatol. 2014; 29(3): 474–479. doi:10.1111/jgh.12419
140. McBride D, Hardoon S, Walters K, Gilmour S, Raine R. Explaining variation in referral from primary to secondary care: cohort study. BMJ. 2010;341:c6267.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

