Back to Journals » Infection and Drug Resistance » Volume 18
Coronavirus Disease 2019 Case Series in China: Sequelae and Effectiveness of Vaccination and Antiviral Drugs
Authors Ren M
Received 3 December 2024
Accepted for publication 14 February 2025
Published 25 February 2025 Volume 2025:18 Pages 1125—1133
DOI https://doi.org/10.2147/IDR.S499058
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Min Ren
Department of Emergency, Emergency and Critical Care Medical Center, Beijing Shijitan Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Min Ren, Department of Emergency, Emergency and Critical Care Medical Center, Beijing Shijitan Hospital, Capital Medical University, 10 Tieyi Road, Yangfangdian, Haidian District, Beijing, People’s Republic of China, Email [email protected]
Purpose of the Study: This study investigated post-coronavirus disease 2019 (COVID-19) sequelae among a sample of the Chinese population, and determined the statistical significance of correlations between age, sex, number of COVID-19 vaccinations, number of SARS-CoV-2 infections, development of pneumonia, use of specific drugs (namatevir/ritonavir, azvudine, molnupiravir), chronic underlying diseases, and post-COVID-19 sequelae.
Methods: Data from 869 patients, who visited the Emergency Department of Beijing Shijitan Hospital (Beijing, China) between December 7, 2022 to January 31, 2024, were collected. The criteria for admission: Age ≥ 14 years old, and diagnosed with ≥ 1 positive result on nucleic acid or antigen tests. Retrospectively analyzed the main manifestations of sequelae, statistical analysis of the relationship between age; sex; underlying diseases; number of COVID-19 vaccinations received; use of specific antiviral drugs for COVID-19 and prognosis, and statistical analyses, including logistic regression, were performed. Differences with P < 0.05 were considered to be statistically significant. Before entering the model, variables are screened using stepwise regression to ensure that the selected variables are the main ones related to the research question, thereby controlling for confounding factors. SPSS version 27 (IBM Corp. Armonk, NY, USA) was used for statistical analysis. In this study, the duration of sequelae ranged from 2 to 13 months.
Results and Conclusions: This study retrospectively analyzed 869 patients (415 male, 454 female), with an average age of 61.52 ± 23.09 years. There were 401 patients without underlying conditions and 468 patients with one or more underlying conditions. Regarding COVID-19 vaccination status, 320 patients were unvaccinated, while 576 patients received at least one dose of the COVID-19 vaccine. Additionally, 514 patients received antiviral medication, while 355 did not receive antiviral treatment. The primary manifestations of post-COVID-19 included shortness of breath, dizziness and weakness, chronic pneumonia, asthma, and reduced sense of smell. Individuals ≥ 70 years of age were more prone to COVID-19 pneumonia. Patients with underlying disease(s) exhibited statistically higher mortality rates after diagnosis of COVID-19. Vaccination against COVID-19 and the use of specific drugs had a statistically significant effect on mortality and the occurrence of post-COVID-19 sequelae. There was no statistically significant difference in COVID-19 infection rates between males and females, although males were more prone to COVID-19 pneumonia. Young women with COVID-19 often experienced no sequelae, and elderly males exhibited a high mortality rate.
Keywords: novel coronavirus, COVID-19 vaccine, post-COVID-19 sequelae, antiviral drugs
Introduction
Coronavirus disease 2019 (COVID-19) is spreading worldwide. According to data from the World Health Organization (WHO), as of November 8, 2023, the global cumulative number of confirmed COVID-19 cases exceeded 770 million, with > 6.97 million deaths. In China, > 99 million confirmed cases of COVID-19 and >120,000 deaths between January 3, 2020 and November 8, 2023, were reported to the WHO. Although the causative virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has undergone multiple mutations and transmissions, resulting in reduced virulence and decreased transmissibility, it continues to negatively affect human health and quality of life. The particular study period was chosen because it covers the 13 months following relaxation of dynamic COVID Zero policy, during which nearly everyone in China was infected at some point. At the beginning of China’s epidemic prevention policy, there were many severe cases, and the vast majority of individuals were infected with SARS-CoV-2 at one time or another. Since then, there have been multiple COVID-19 outbreaks, although the virus is currently at a low level of endemicity. In May 2023, the WHO recognized COVID-19 as an established and ongoing health issue, no longer constituting a public health emergency of international concern. The present study mainly examined the characteristics of SARS-CoV-2 infection, COVID-19 vaccines, and specific drugs, analyzing through statistical analysis of clinical data whether they have a statistically significant protective effect on individuals and the main post-COVID sequelae experienced among the Chinese population.
Methods
General information and data were collected from 869 patients (415 male, 454 female) who visited the Emergency Department of Beijing Shijitan Hospital (Beijing, China) between December 7, 2022 and January 31, 2024. Information were collected by reviewing patients’ medical records and searching the hospital information system (HIS). The criteria for admission: All participants were ≥ 14 years of age, and diagnostic standards for COVID-19 infection included individuals diagnosed with COVID-19 since December 7, 2022, with ≥ 1 positive result on nucleic acid or antigen tests. The key term in this study is “COVID-19 infection”, which is defined as a positive result for either nucleic acid or antigen testing. There is no specific group of patients that needs to be excluded. Information collected included the following: age; sex; underlying diseases; number of SARS-CoV-2 infections; development or absence COVID-19 pneumonia; use of specific antiviral drugs for COVID-19, such as namatevir/ritonavir, azvudine, molnupiravir; number of COVID-19 vaccinations received; and post-COVID-19 sequelae. In this study, the duration of sequelae ranged from 2 to 13 months. All relevant data were entered into a database using SPSS version 27 (IBM Corporation, Armonk, NY, USA), and statistical analyses, including logistic regression, were performed. The dependent variables in the regression model of this study include whether the patient develops pneumonia after COVID-19 infection, whether there are any sequelae, and whether the patient dies. The independent variables include age, sex, underlying conditions, vaccination status, and whether the patient took antiviral medication. Before variables are entered into the model, stepwise regression is used to screen them, ensuring that the selected variables are the main ones relevant to the research question. Differences with P < 0.05 were considered to be statistically significant.
Results
General information and data of 869 participants have no missing date, the general information of the patients is shown in Table 1. The clinical manifestations of acute COVID-19 infection are mainly fever, sore throat, nasal congestion, cough, fatigue, and body aches, with some patients also experiencing diarrhea and reduced sense of smell. Most of these symptoms gradually improve and disappear. However, some acute symptoms may persist or new symptoms may appear during the recovery phase, which are referred to as post-COVID sequelae. In this study, the duration of sequelae ranged from 2 to 13 months. The main post-COVID-19 sequelae in China and their percentages are summarized in Figure 1. Individuals > 70 years of age were more likely to develop COVID-19 pneumonia. Patients were divided into two groups, ≥ 70 and < 70 years of age, to analyze the rate of COVID-19 pneumonia. The results revealed a significant difference (Table 2). Patients with underlying diseases exhibited a significantly higher mortality rate after being diagnosed with COVID-19 (P < 0.01). Patients with underlying diseases exhibited a significantly higher rate of pneumonia (P < 0.01) (Table 3). Individuals who received higher number of COVID-19 vaccine doses were significantly less likely to experience sequelae (P < 0.01). There was a statistically significant difference in mortality among patients who received a higher number of COVID-19 vaccine doses versus those who did not (P<0.01) (Table 4). The difference in the incidence of sequelae between the group that took antiviral drugs and the group that did was statistically significant (P < 0.01). There was a statistical difference in mortality rate between the group that took antiviral drugs and the group that did not (P < 0.01) (Table 5). Males were significantly more likely than females to develop COVID-19 pneumonia (P < 0.01) (Table 6). The data demonstrated that young females infected with SARS-CoV-2 often did not experience sequelae, whereas older males exhibited a higher mortality rate from COVID-19 after infection (Figure 2). Finally, there was no statistical difference in COVID-19 rates between males and females (P > 0.05) (Table 7).
 |
Table 1 General Data |
 |
Table 2 The Statistical Regression Analysis Output on COVID-19 Infection in Relation to Age (P<0.01) |
 |
Table 3 The Statistical Regression Analysis Output on Death and COVID-19 Infection in Relation to Underlying Diseases (P<0.01) |
 |
Table 4 The Statistical Regression Analysis Output on Death and Post-COVID-19 Sequelae in Relation to COVID-19 Vaccination (P<0.01) |
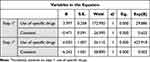 |
Table 5 The Statistic Regression Analysis Output on Death and Post-COVID-19 Sequelae in Relation to Antiviral Medication Usage. (P<0.01) |
 |
Table 6 The Statistical Regression Analysis Output on COVID-19 Infection in Relation to Gender (P<0.01) |
 |
Table 7 The Statistical Regression Analysis Output on Novel Coronavirus Infection in Relation to Gender (P>0.05) |
 |
Figure 1 Comparison of the frequencies of sequelae of COVID-19. |
Discussion
Results of the present study indicate that the primary manifestations of post-COVID conditions include breathlessness, fatigue, dizziness, chronic pneumonia, wheezing, loss of smell, chronic pneumonia, loss of taste, Parkinson’s disease, dementia, apathy, hair loss, myocardial damage, chronic rashes, lung nodules, decreased physical strength, tinnitus and deafness, decreased immunity, loss of appetite, increased blood sugar levels, sleepiness, increased blood pressure, graying of hair, reduced platelet count, renal insufficiency, weakened vagus nerve, excessive sweating, insomnia, headaches, hiccups, knee pain, bedridden state, were the most common. Results of the present study indicate that young adult females often do not experience post-COVID-19 conditions, although elderly males have a high mortality rate from COVID-19, and COVID-19 vaccines and specific drugs have a statistically significant protective effect on the population. The novel coronavirus (ie, SARS-CoV-2) is highly transmissible among humans and severely affects human health. All deaths (n = 161) in this study were caused by severe COVID-19 pneumonia in patients who were treated in the Emergency Department of Beijing Shijitan Hospital during the early stages of the pandemic policy, from December 7, 2022, to January 31, 2024. Most of these patients were > 70 years of age and had underlying disease(s). Various degrees of post-COVID-19 sequelae have affected contemporary life. Reducing the infection rate of SARS-CoV-2 and its sequelae has become a global issue.
This study included data from severely ill patients with pneumonia infected with SARS-CoV-2, who visited the Emergency Department of Beijing Shijitan Hospital at the beginning of China’s commencement of epidemic control policies, which resulted in a higher mortality rate. Subsequently, with several small-scale low-level outbreaks, both the incidence and mortality rates of severe pneumonia decreased significantly. Research indicates that the lungs are the primary target organs affected by coronaviruses.1 Extensive studies have shown that persistent breath-holding, shortness of breath, and other respiratory difficulties are among the most common symptoms in discharged patients.2 A study involving 110 non-critical patients revealed that lung function tests at discharge revealed varying degrees of impaired lung diffusion.3 Three months after discharge, approximately 55.7% of patients exhibited residual lung lesions (primarily ground-glass opacities [44.1%]), with 34.8% exhibiting impaired lung diffusion capacity, and 9.5% exhibiting other lung function abnormalities.4 These lung lesions and functional abnormalities remained prevalent during the six-month follow-up.5 In addition to respiratory symptoms, post-COVID sequelae often include reduced or loss of smell and taste. Research has shown that oral epithelial cells and taste buds highly express the angiotensin-converting enzyme 2 (ie, “ACE2”) receptor, leading to taste disturbances when the oral mucosa and taste buds are infected by SARS-CoV-2.6,7 Numerous studies have indicated that SARS-CoV-2 infection can cause olfactory and taste disorders, which are correlated.8,9 This aligns with the findings of the present study; however, the underlying mechanisms require further investigation. Fatigue, cough, and loss of smell are the most common lingering symptoms of COVID-19, although organ-specific damage has also been reported.10–12 A large cohort study including > 11 million samples was conducted. It has been confirmed that the risk to the cardiovascular system increases after SARS-CoV-2 infection, leading to various conditions such as arrhythmias, myocarditis, pericarditis, acute coronary artery disease, thromboembolic disorders, heart failure, and cardiac arrest.12 In addition to post-COVID conditions affecting the respiratory system, sense of smell and taste, and cardiovascular system, there can also be varying manifestations of sequelae in the kidneys, digestive system, hematological system, endocrine system, nervous system, immune system, skin, hair, and physical strength. These conditions significantly affect the health and quality of life of the general population. Medical professionals must consider protecting vulnerable populations, preventing infections, reducing infection rates, and decreasing the incidence of severe cases and mortality rates.
Results of the present study revealed that individuals > 70 years of age were more susceptible to COVID-19 pneumonia. Patients with underlying conditions were prone to developing COVID-19 pneumonia and had a higher mortality rate after infection, with all results showing statistically significant differences. In clinical practice, patients with COVID-19 pneumonia are mostly elderly, especially those with chronic underlying diseases. Other studies have reported that critically ill patients with COVID-19 are typically older, with an average age of 63 years, and have a higher risk for death due to hypertension, diabetes, and other underlying diseases, confirming that they are a high-risk group for novel coronavirus pneumonia.13
COVID-19 has become one of the most critical global public health emergencies in recent years, posing a severe threat to the health and lives of populations worldwide. Vaccination against the novel coronavirus (ie, COVID-19 vaccine) is the most cost-effective measure for protecting vulnerable populations, reducing the incidence of severe cases and mortality, and mitigating the harm caused by the virus. Countries are actively developing and administering COVID-19 vaccines, with recombinant proteins, viral vectors, inactivated vaccines, and mRNA vaccines dominating the market. As the transmissibility and immune evasion capabilities of variants increase, the effectiveness and durability of COVID-19 vaccines have become the focal points of current research, which indicates that a higher number of COVID-19 vaccinations is associated with lower morbidity and mortality rates from COVID-19, with the results demonstrating statistically significant differences. Research has shown that vaccination is one of the most effective measures for protecting vulnerable populations and reducing the severity and mortality of COVID-19. The ideal outcome of vaccination is prevention of infection; however, even when complete prevention is not possible, vaccination plays a significant role in reducing hospitalizations, and mitigating severe illness, and death.14 A recent study from Israel15 indicated that, among individuals who received 2 doses of the COVID-19 vaccine, the incidence of long-term COVID symptoms was significantly higher in the unvaccinated group 4 months post-infection than in the vaccinated group. The study also found that vaccination alleviated symptoms in infected individuals to the extent that the differences in “long COVID” symptoms between the vaccinated and non-infected groups were not statistically significant. Additionally, a study from the United States16 found that vaccination reduced the risk for persistent symptoms 28 days after infection. A study from the United Kingdom17 also suggested that the proportion of vaccinated patients developing long COVID symptoms 12 weeks post-infection was reduced by approximately 35% compared with non-vaccinated individuals.
This study indicates that the use of specific antiviral drugs significantly affects mortality and the occurrence of sequelae, there is a statistically significant difference. The National “Diagnosis and Treatment Protocol for Novel Coronavirus Infection (Trial Version 10)” specifies that nirmatrelvir tablets/ritonavir tablets and molnupiravir capsules are suitable for adult patients with mild to moderate symptoms within 5 days of onset who have high risk factors for progression to severe illness; azvudine tablets are used to treat adult patients with moderate COVID-19 infection.18 The National “Diagnosis and Treatment Protocol for Severe Cases of Novel Coronavirus Infection (Trial Version 4)” states that for patients with severe symptoms, especially those with a short duration of illness and a high viral load (Ct < 30), the aforementioned drugs can also be used.19 Currently, there are no drugs that can prevent COVID-19. These drugs do not prevent SARS-CoV-2 from invading healthy cells or directly kill the virus but work by blocking the virus’ polyprotein hydrolysis process after it invades human cells, thereby inhibiting viral replication and exerting an antiviral effect.
This study indicates there is no significant difference in infection rates between males and females; however, males are more likely than females to develop COVID-19 pneumonia, there is a significant difference. Young adult females with COVID-19 rarely experience sequelae, whereas older males exhibit higher mortality rates. Results of the present study are consistent with research related to the Wuhan epidemic, revealing no differences in the susceptibility and clinical symptoms of COVID-19 between males and females. However, males are more likely to experience severe inflammatory responses after developing COVID-19 pneumonia, and the absorption of lung lesions is slower in males than in females.20
This study has certain limitations due to time and sample size, and needs to be validated by large-scale research.
Conclusion
Sequelae of COVID-19 involve multiple systems addition to post-COVID conditions affecting the respiratory system, sense of smell and taste, and cardiovascular system, as well as varying manifestations of sequelae in the kidneys, digestive, hematological, endocrine, nervous, and immune systems, skin, hair, and physical strength. Young adult females often do not experience post-COVID-19 conditions, although elderly males have a high mortality rate from COVID-19, and COVID-19 vaccines and specific drugs have a statistically significant protective effect on the population, which should be promoted in clinical practice.
This information can provide guidance in clinical practice. Protective measures for elderly individuals with underlying diseases and the protective effects of COVID-19 vaccination and antiviral drugs merit promotion.
Data Sharing Statement
Data used in this study are available from the corresponding author upon reasonable request.
Ethical Statement
This study was approved by the Ethics Committee of Beijing Shijitan Hospital, Capital Medical University (Approval Number: IIT2024-147-002). Patient consent to review their medical records was not required by the IRB, and the reason for the exemption is that this study was a retrospective case analysis without any intervention. Patient data in this study database are strictly confidential and comply with the Declaration of Helsinki.
Disclosure
The author declares no conflicts of interest in this work.
References
1. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi:10.1016/S2213-2600(20)30076-X
2. Willi S, Lüthold R, Hunt A, et al. COVID-19 sequelae in adults aged less than 50 years: a systematic review. Travel Med Infect Dis. 2021;40:101995. doi:10.1016/j.tmaid.2021.101995
3. Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55:2001217. doi:10.1183/13993003.01217-2020
4. So M, Kabata H, Fukunaga K, et al. Radiological and functional lung sequelae of COVID-19: a systematic review and meta-analysis. BMC Pulm Med. 2021;21:97. doi:10.1186/s12890-021-01465-5
5. Mancini DM, Brunjes DL, Lala A, et al. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JAMA Heart Fail. 2021;9:927–937. doi:10.1001/jama.2021.1620
6. Sato T, Ueha R, Goto T, et al. Expression of ACE2 and TMPRSS2 in the upper and lower aerodigestive tracts of rats: implications for COVID 19 infections. Laryngoscope. 2021;131:E932–E939. doi:10.1002/lary.29004
7. Paderno A, Schreiber A, Grammatica A, et al. Smell and taste alterations in COVID-19: a cross-sectional analysis of different cohorts. Int Forum Allergy Rhinol. 2020;10:955–962. doi:10.1002/alr.22610
8. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi:10.1007/s00405-020-05965-1
9. Yan CH, Faraji F, Prajapati DP, et al. Association between chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10:806–813. doi:10.1002/alr.22579
10. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi:10.1016/j.eclinm.2021.101019
11. Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10:311–321. doi:10.1016/S2213-8587(22)00044-4
12. Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi:10.1038/s41591-022-01795-5
13. Chen L, Feng SY, Wang FX, Chen CM, Jiang X . Clinical diagnosis and treatment of critical patients with novel coronavirus pneumonia (12 cases). Chin J Clin Med. 2020:27(1): 32–35. doi:10.12025/j.issn.1008-6358.2020.20200236
14. Tenforde MW, Self WH, Adams K, et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326(20):2043–2054. doi:10.1001/jama.2021.19499
15. Kuodi P, Gorelik Y, Zayyad H, et al. Association between BNT162b2 vaccination and reported incidence of post-COVID-19 symptoms: cross-sectional study 2020-21, Israel. NPJ Vaccines. 2022;7:101. doi:10.1038/s41541-022-00478-x
16. Taquet M, Dercon Q, Harrison PJ. Six-month sequelae of postvaccination SARS-CoV-2 infection: a retrospective cohort study of 10,024 breakthrough infections. Brain Behav Immun. 2022;103:154–162. doi:10.1016/j.bbi.2022.03.016
17. Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22:43–55. doi:10.1016/S1473-3099(21)00460-6
18. Office of the National Health Commission Comprehensive Department of the National Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for novel coronavirus infection (Trial Version 10) [EB/OL]. 2023 [updated May 1, 2023].
19. Office of the National Health Commission Comprehensive Department of the National Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for severe cases of novel coronavirus infection (Trial Version 4) [EB/OL]. 2023 [updated May 1, 2023].
20. Xiong J, Zhu Y, Liu S, Zhang L, Ai W, Yan J, Deng J . [不同性别新型冠状病毒肺炎患者临床特征的比较] Comparison of clinical characteristics of patients with novel coronavirus pneumonia by sex. J Wuhan Univ Med Ed. 2021;42(1):15–18. Chinese. doi:10.14188/j.1671-8852.2020.0300
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


