Back to Journals » Cancer Management and Research » Volume 17
Correlation of PET/CT Maximal Standardized Uptake Values with Clinicopathologic Parameters of Aggressive Non-Hodgkin’s Lymphoma
Received 7 March 2025
Accepted for publication 11 May 2025
Published 22 May 2025 Volume 2025:17 Pages 975—984
DOI https://doi.org/10.2147/CMAR.S522628
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Yingci Li,1 Dongbo Wu,2 Mohan Tian1
1Department of PET/CT-MR Center, Harbin Medical University Cancer Hospital, Harbin, Heilongjiang, 150081, People’s Republic of China; 2Department of Radiology, Harbin Medical University Cancer Hospital, Harbin, Heilongjiang, 150081, People’s Republic of China
Correspondence: Mohan Tian, Department of PET/CT-MR Center, Harbin Medical University Cancer Hospital, No. 150, Haping Road, Nangang District, Harbin City, Heilongjiang, 150081, People’s Republic of China, Email [email protected]
Objective: To investigate the correlation between the maximum standardized uptake value (SUVmax) of positron emission tomography (PET)/computed tomography (CT) scanning imaging with the clinicopathologic parameters of aggressive non-Hodgkin’s lymphoma (NHL) and its diagnostic value.
Methods: A total of 128 patients with NHL were retrospectively selected. PET/CT examination was performed to calculate SUVmax, and clinicopathological data were collected. Pearson correlation test was used to analyze the correlation between them, and the relevant indicators were detected according to the pathological results. ROC curve was used to analyze the diagnostic value.
Results: The level of PET/CT SUVmax was positively correlated with the levels of β 2-microglobulin (β 2-MG), lactate dehydrogenase (LDH), Ki-67, tumor size and bone marrow infiltration (P< 0.05). The PET/CT SUVmax and serum β 2-MG, LDH and Ki-67 levels in the aggressive group were significantly higher than those in the indolent group (P< 0.05). The AUC of the combined detection of PET/CT SUVmax, β 2-MG, LDH and Ki-67 in the diagnosis of aggressive NHL was 0.920, and the combined detection had a high diagnostic value.
Conclusion: PET/CT SUVmax was correlated with a variety of clinicopathological parameters. Combined detection of PET/CT SUVmax has high diagnostic value in aggressive NHL.
Keywords: PET/CT maximum standardized uptake values, β 2-MG, LDH, Ki-67, aggressive non-Hodgkin’s lymphoma
Introduction
Non-Hodgkin’s lymphoma (NHL) is the most common hematological malignancy in clinical practice, with numerous biological and clinical heterogeneity subtypes.1 According to the World Health Organization (WHO) and International Agency for Research on Cancer (ICC) classification, it contains many biological and clinical heterogeneous subtypes, mainly divided into B-cell lymphoma and T-cell lymphoma.2,3 Although NHL usually manifests as aggressive spreading disease or rapidly growing mass, with the characteristics of multicentric origin and long-distance spread, not all patients have a poor prognosis.4 Taking diffuse large B-cell lymphoma (DLBCL) treated with CHOP-R (cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab) as an example, about 60% of patients can be cured by first-line treatment. However, there are still some patients who have resistance to radiotherapy, chemotherapy and immunotherapy, which affects their lives and health.5 Hematopoietic stem cell transplantation is one of the standard treatments for patients with relapsed or refractory disease after chemotherapy. However, due to potential comorbidities and chemotherapy-resistant diseases, about 40% of patients may not be suitable for hematopoietic stem cell transplantation, and about 50% of patients who receive this treatment may relapse.6 Therefore, early and accurate diagnosis and appropriate treatment are very important to improve the prognosis of patients with NHL.
Positron emission tomography (PET) / Computed tomography (CT) scanning imaging combines both PET and CT techniques. Imaging that combines metabolic abnormalities and tumor morphological changes plays an important role in the diagnosis, staging, efficacy monitoring, and outcome prediction of various cancers.7 Standardized uptake value Maximum (SUVmax) is the most widely analyzed semi quantitative parameter in PET/CT. Research has found that SUV can reflect changes in glucose metabolism and proliferation activity of tumor cells.8 However, due to differences in individual patient situations, different variables may have an impact on the standardization of measurements.9
In this study, 128 patients with NHL treated in our hospital were selected as study subjects. We investigated the correlation between PET/ CT SUVmax with the clinicopathologic parameters of NHL and the diagnostic value of PET/CT SUVmax, aiming to provide reference for accurately assessing the invasiveness of NHL and evaluating the prognosis.
Materials and Methods
General Materials
A total of 128 patients with NHL treated in our hospital from June 2021 to May 2024 were retrospectively selected as the research objects. In order to reduce research bias, only patients with DLBCL and follicular lymphoma (FL) were included in this study. No patients with FL transformed to DLBCL were screened. The inclusion process was shown in Figure 1. Inclusion criteria: (1) All patients met the guidelines for the diagnosis and treatment of lymphoma,10 and had been diagnosed through pathological testing. (2) All patients had complete clinical data. (3) All patients underwent PET/CT SUVmax examination. Exclusion criteria: (1) Patients with Hodgkin’s lymphoma; (2) Patients with hepatitis B, hepatitis C, cirrhosis or liver injury; (3) Patients who had received previous relevant treatment for NHL in the past. This study was ratified by the Ethics Committee of our hospital.
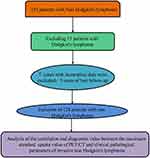 |
Figure 1 Process diagram for patient inclusion. |
PET/CT Detection
The PET/CT examination was conducted using the Discovery 710 Clarity scanner (GE Healthcare, USA). 18F-FDG was synthesized by PETtrace cyclotron and Siemens Explora FDG4 chemical synthesis module, with a radiochemical purity of not less than 95%. The patients should fast for at least 6 hours before the examination. If blood glucose was lower than 6.1 mmol/L (non-diabetes patients) or 11.1 mmol/L (diabetes patients), the injection dose should be set to 3.7 MBq/kg. After 1 hour of intravenous injection, CT (parameter settings were listed as follows: voltage 120 kV, current intensity 40 mA, layer thickness 5 mm, interval 5 mm) and PET (parameter settings were listed as follows: 3D mode, 2 minutes/bed, layer thickness 4 mm. The brain scanning ranged from the top of the skull to the plane of the mandible, and the body scanning ranged from the base of the skull to the proximal femur) scanning were performed. Data analysis was conducted by two or more experienced diagnostic physicians who were capable of independently reading images. Visual and semi quantitative analysis methods were used to analyze and interpret images. A circular Region of interest (ROI) was drawn on PET-CT slices registered in the axial, coronal, or sagittal planes. SUVmax was calculated using the following standard formula and could be adjusted according to body weight: SUVmax =average ROI activity (MBq/mL) / [injection dose (MBq)/body weight (kg)].
Collection of Clinical Pathological Parameters
Clinical and pathological parameters of patients were collected, including the age (≤ 55 years, >55 years), gender, tumor size (≤ 2 cm, >2 cm), bone marrow infiltration, clinical staging (Stage I + Stage II, Stage III + Stage IV), International Prognostic Index (IPI) (≤ 3 points, >3 points), B symptoms, globulin (≤ 19 g/L, >19 g/L), albumin (≤ 40 g/L, >40 g/L), ferritin (≤ 390 ng/mL, >390 ng/mL), β2-microglobulin (β2-MG) (≤ 2.6 mg/L,>2.6 mg/L), lactate dehydrogenase (LDH) (≤ 245 U/L, >245 U/L), cell proliferation nuclear antigen-67 (the Ki-67) (≤ 30%, >30%), etc.
Detection Method
The levels of β2-MG and LDH were detected by enzyme-linked immunosorbent assay (ELISA), and the level of Ki-67 was detected by immunohistochemistry. The specific operation was carried out according to the relevant kit instructions.
Malignant Diagnosis
Given the aggressive course of mantle-cell lymphoma, the different treatment options for T-cell NHL and the highly variable disease course, only 74 cases of DLBCL representing aggressive NHL, and 54 cases of follicular NHL, were included in this study to reduce study bias. According to the results of pathological examination, the patients were divided into aggressive group (DLBCL cases) and indolent group (follicular NHL cases).
Correlation Analysis
The correlation between PET/CT SUVmax and clinical pathological parameters was analyzed through Pearson correlation test. The clinical pathological parameters taken for analysis were the indicators with significant differences in Table 1.
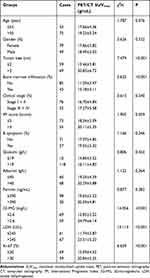 |
Table 1 The Relationship Between PET/CT SUVmax and Clinical Data ( |
Diagnostic Value Analysis
Establish ROC curves were established to analyze the diagnostic value of PET/CT SUVmax, β2-MG, LDH and Ki-67 alone and in combination for invasive NHL.
Statistical Analysis
Statistical analysis was conducted using SPSS 23.0. The measurement data that conformed to the normal distribution were presented as mean ± standard deviation, and independent sample t-test was used for inter group comparison. The correlation between PET/CT SUVmax and clinical pathological parameters was analyzed using Pearson correlation test. The diagnostic value of combined detection by PET/CT SUVmax, β2-MG, LDH, and Ki-67 for invasive NHL was analyzed using ROC curve analysis. The statistical test level was set at P<0.05.
Results
The Relationship between PET/CT SUVmax and Clinical Data
PET/CT SUVmax levels were not associated with gender, age, clinical stage, albumin, ferritin, globulin, IPI score, and B symptoms (P>0.05). PET/CT SUVmax levels increased significantly in aggressive NHL patients with the increase of β2-MG (t=7.479), LDH (t=5.625), Ki-67 (t=14.056), tumor size (t=14.113) and bone marrow infiltration (t=6.659) (P<0.05, Table 1).
The Correlation between PET/CT SUVmax and Clinical Pathological Parameters
Pearson correlation analysis revealed that PET/CT SUVmax levels were significantly positively correlated with tumor size (r=0.416), bone marrow infiltration (r=0.308), and the levels of β2-MG (r=0.314), LDH (r=0.552), and Ki-67 (r=0.468) (P<0.05, Table 2 and Figure 2).
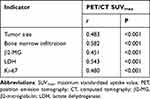 |
Table 2 The Correlation Between PET/CT SUVmax and Clinical Pathological Parameters |
The Linear-Regression Analysis
Linear regression analysis was performed with tumor size, bone marrow infiltration (yes = 1, no = 0), β2-MG, LDH and Ki-67 levels as independent variables and PET/CTSUVmax as dependent variable. The results showed that the regression equation between tumor size and PET/CTSUVmax was Y=2.15X1+5.2 (X1 was tumor size, Y was the predicted value of PET/CTSUVmax), and the regression coefficient of 2.15 was statistically significant (P<0.05). The regression equation between bone marrow infiltration and PET/CTSUVmax was Y= 3.5 X2+10.5 (X2 was bone marrow infiltration), and the regression coefficient 3.5 was statistically significant (P<0.05). The regression equation between β2-MG and PET/CTSUVmax was Y=1.8X3+8.3 (X3 was the level of β2-MG), and the regression coefficient 1.8 was statistically significant (P<0.05). The regression equation between LDH and PET/CTSUVmax was Y=2.5X4+12.2 (X4 was LDH level), and the regression coefficient 2.5 was statistically significant (P<0.05). The regression equation between Ki-67 and PET/CTSUVmax was Y=2.3X5+9.5 (X5 was the level of Ki-67), and the regression coefficient 2.3 was statistically significant (P<0.05).
Analysis of PET/CT SUVmax, β2-MG, LDH, and Ki-67 Levels in Patients with Different Degrees of Malignancy
Compared with the inert group, the invasive group had much higher levels of PET/CT SUV max (t=15.334) and serum β2-MG (t=8.352), LDH (t=13.278), and Ki-67 (t=22.559) (P<0.05) (Table 3).
 |
Table 3 Analysis of PET/CT SUVmax, β2-MG, LDH and Ki-67 Levels in Patients with Different Degrees of Malignancy ( |
ROC Curve Analysis
ROC curve analysis confirmed that the AUCs of PET/CT SUV max, β2-MG, LDH, and Ki-67 for the diagnosis of invasive NHL were 0.786, 0.805, 0.851 and 0.830, respectively. The combined detection of the four indicators had an AUC of 0.920, specificity of 82.43%, sensitivity of 96.30%, and Jordan index of 0.787. The combined detection had higher diagnostic value for invasive NHL (Table 4 and Figure 3).
 |
Table 4 Diagnostic Value of PET/CT SUVmax, β2-MG, LDH and Ki-67 for Invasive NHL |
 |
Figure 3 ROC curve analysis of PET/CT SUVmax, β2-MG, LDH, and Ki-67 in the diagnosis of invasive NHL. |
Discussion
NHL is a malignant tumor originating from the lymphatic system. Radiochemotherapy and immunotherapy are typical treatment methods. However, approximately 20% to 30% of patients may develop resistance to the aforementioned treatments, affecting their life and health.1 NHL typically appears in an invasive or inert form, with invasive patients having a lower 5-year survival rate and a poorer prognosis.11 Therefore, early diagnosis and selection of appropriate treatment options are of great significance for improving the prognosis of NHL patients.
At present, PET/CT imaging has become the mainstream clinical imaging tool for staging, treatment evaluation, and follow-up evaluation of lymphoma patients. PET/CT imaging not only provides high-resolution anatomical information, but also information about the metabolic activity of lesions.12 PET/CT SUVmax is the ratio of the radioactive activity of the imaging agent taken up by the local lesion to the total injected dose of radioactive activity. In recent years, there has been some controversy over the factors related to PET/CT SUVmax in lymphoma. Patients with FL in different stages had different SUVmax, and high SUVmax may be associated with more advanced diseases.13 There are also studies suggesting that high SUVmax are often associated with poorer prognosis. For example, patients with high SUVmax of inert lymphoma are commonly accompanied by shorter progression free survival (PFS) and overall survival (OS), leading to shorter and higher mortality rates.14 A study has found that PET/CT SUVmax is significantly correlated with the prognosis of NHL patients. The higher the SUVmax is, the greater the possibility of invasion is, and the poorer the prognosis of the patient is.15 In this study, it was found that PET/CT SUVmax levels were significantly positively correlated with tumor size and bone marrow infiltration. Previous studies have found that high levels of circulating tumor cells and large volume lesions may be independent risk factors for B-cell malignancy.16 Larger tumor volume accompanied by more neovascularization and abundant blood supply can promote the development of tumor malignancy and enhanced metabolism of lesion tissue, leading to an increase in PET/CT SUVmax levels.17 Bone marrow is the most common site of extranodal involvement in lymphoma patients. Thus, the bone marrow status is a key step in evaluating the initial examination of lymphoma patients and can provide key information for treatment decision-making. PET/CT detection confirmed that patients with bone marrow infiltration had significantly lower survival time than those without bone marrow infiltration. Therefore, PET/CT can be used to evaluate the prognosis of patients with bone marrow infiltration.13 According to reports, the sensitivity and specificity of PET/CT in diagnosing bone marrow infiltration in invasive NHL are much higher than those in inert NHL.18 In the study of follicular lymphoma, it was found that PET/CT SUVmax levels were markedly higher in patients with bone marrow involvement than in unaffected patients,19 which was similar to the results of this study. In summary, understanding the relationship between PET/CT SUVmax with tumor size and bone marrow infiltration is beneficial for improving lesion detection rate.
In addition, our present study found that PET/CT SUVmax was closely related to levels of β 2-MG, LDH, and Ki-67. The combined detection could effectively evaluate the invasiveness of patients with invasive NHL. A study has found that higher levels of β2-MG may indeed be related to MHC-I. MHC-I deficiency is more common in cancer cells, which may help cancer cells evade immune responses and progress to metastasis. It can be seen that β2-MG levels may be one of the indicators reflecting tumor cell proliferation activity.20 LDH has become an important prognostic biomarker for tumor diseases, and serum LDH levels are significantly negatively correlated with the survival rate of lymphoma patients. The diagnostic value of PET/CT SUVmax combined with LDH in NHL invasiveness is higher than that of LDH alone, with an AUC of 0.831, sensitivity and specificity of 63.10% and 80.00%, respectively.21 In this study, the combined detection of multiple indicators including PET/CT SUVmax, β2-MG, LDH, and Ki-67 showed a diagnostic AUC of 0.920, specificity of 82.43%, and sensitivity of 96.30%, which was higher than the combined diagnosis of PET/CT SUVmax and LDH, further demonstrating the accuracy of the combination of various indicators in this study. Ki-67 is a nuclear protein related to the cell cycle, expressed in all the stages except the resting phase of cell division. Ki-67 reflects the rate of tumor cell proliferation, which is closely related to the ability of tumor cell infiltration, metastasis, and implantation.22 Previous studies have found that hepatocellular carcinoma patients with Ki-67 positive lesions have stronger FDG uptake ability.23 The stronger the ability of tumor tissue to uptake FDG is, the larger the SUV is, and the stronger the diagnostic ability for NHL invasiveness is. Combined detection using ET/CT SUVmax, β2-MG, LDH, and Ki-67 levels is beneficial for clarifying the patient’s condition and conducting timely intervention and treatment.
Conclusion
In general, this study confirmed that PET/CT SUVmax was significantly correlated with tumor size, bone marrow infiltration, β2-MG, LDH, Ki-67 and other clinicopathological parameters. The combined detection of these indicators had high value in the diagnosis of aggressive NHL, which provided a strong basis for clinical diagnosis. In this study, patients with DLBCL and FL were selected as the study subjects, which reduced the research bias caused by the diversity of histological types and made the results more reliable. However, NHL also includes other histological types. Follow-up studies can further expand the sample coverage to explore the relationship between PET/CT SUVmax and clinicopathological parameters in different histological types and its diagnostic value.
However, this study has some limitations due to its small sample size and single-center study. Further studies with larger sample size and multicenter studies are needed to further verify the results of this study, so as to evaluate aggressive NHL more accurately and provide more reliable guidance for the treatment and prognosis of patients.
Abbreviations
SUV max, maximum standardized uptake value; PET, positron emission tomography; CT, computed tomography; NHL, non-Hodgkin’s lymphoma; β2-MG, β2-microglobulin; LDH, lactate dehydrogenase; IPI, International Prognostic Index; ELISA, Enzyme linked immunosorbent assay; IHC, Immunohistochemistry; MALT, mucosa associated lymphoid tissue; SLL, small lymphoid lymphoma; MCL, mantle cell lymphoma; DLBCL, diffuse large B-cell lymphoma; BL, Burkitt lymphoma; EATL, enteropathy associated T-cell lymphoma; ALCL, anaplastic large cell lymphoma; PTCL, peripheral T-cell lymphoma.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 helsinki declaration and its later amendments or comparable ethical standards.
The study was approved by the Ethics Committee of The Harbin Medical University Cancer Hospital.
Patient Consent for Publication
Informed consent was obtained from participants for the participation in the study and all methods were carried out in accordance with relevant guidelines and regulations.
Funding
No funding was received for this paper.
Disclosure
The authors declare that they have no competing interests.
References
1. Thandra KC, Barsouk A, Saginala K, et al. Epidemiology of non-Hodgkin’s lymphoma. Med Sci. 2021;9(1):5.
2. Falini B, Martino G, Lazzi S. A comparison of the International Consensus and 5th World Health Organization classifications of mature B-cell lymphomas. Leukemia. 2023;37(1):18–34. doi:10.1038/s41375-022-01764-1
3. Abro B, Maurer MJ, Habermann TM, et al. Real-world impact of differences in the WHO and ICC classifications of non-Hodgkin lymphoma: a LEO cohort study analysis. Blood. 2024;144(19):2063–2066. doi:10.1182/blood.2024025681
4. Newcomb RA, Johnson PC, Yang D, et al. Coping and perception of prognosis in patients with indolent non-Hodgkin’s lymphoma. Oncologist. 2024;29(5):441–449. doi:10.1093/oncolo/oyad295
5. Dai L, Chen H, Tan Q, et al. Identification of novel prognostic autoantibodies in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone via a high-throughput antigen microarray. Cancer. 2024;130(8):1257–1269. doi:10.1002/cncr.35158
6. Zoellner AK, Unterhalt M, Stilgenbauer S; European Mantle Cell Lymphoma Network, et al. Long-term survival of patients with mantle cell lymphoma after autologous haematopoietic stem-cell transplantation in first remission: a post-hoc analysis of an open-label, multicentre, randomised, Phase 3 trial. Lancet Haematol. 2021;8(9):e648–e657. doi:10.1016/S2352-3026(21)00195-2
7. Kiamanesh Z, Ayati N, Sadeghi R, et al. The value of FDG PET/CT imaging in outcome prediction and response assessment of lymphoma patients treated with immunotherapy: a meta-analysis and systematic review. Eur J Nucl Med Mol Imaging. 2022;49(13):4661–4676. doi:10.1007/s00259-022-05918-2
8. Pepe P, Pepe L, Tamburo M, et al. 68Ga-PSMA PET/CT and prostate cancer diagnosis: which SUVmax value? In Vivo. 2023;37(3):1318–1322. doi:10.21873/invivo.13211
9. Yin J, Wang H, Zhu G, et al. Prognostic value of whole-body dynamic 18F-FDG PET/CT Patlak in diffuse large B-cell lymphoma. Heliyon. 2023;9(9):e19749. doi:10.1016/j.heliyon.2023.e19749
10. McCarten KM, Nadel HR, Shulkin BL, et al. Imaging for diagnosis, staging and response assessment of Hodgkin lymphoma and non-Hodgkin lymphoma. Pediatr Radiol. 2019;49(11):1545–1564. doi:10.1007/s00247-019-04529-8
11. Alkrekshi A, Kassem A, Park C, Tse W. Risk of non-Hodgkin’s lymphoma in HCV patients in the United States between 2013 and 2020: a population-based study. Clin Lymphoma Myeloma Leuk. 2021;21(11):e832–e838. doi:10.1016/j.clml.2021.06.014
12. Geng H, Lian K, Zhang W. Prognostic value of 18F-FDG PET/CT tumor metabolic parameters and Ki-67 in pre-treatment diffuse large B-cell lymphoma. Quant Imaging Med Surg. 2024;14(1):325–334. doi:10.21037/qims-23-702
13. Jitani AK, Dutta S, Mandal PK, et al. Utility of 18F-fluorodeoxyglucose PET-CT scan in detecting bone marrow involvement in lymphoma. Indian J Med Res. 2021;154(5):691–698. doi:10.4103/ijmr.IJMR_1420_19
14. Qian L, Yan M, Zhang W, et al. Prognostic value of interim 18F-FDG PET/CT in T-cell lymphomas. Leuk Lymphoma. 2020;61(4):927–933. doi:10.1080/10428194.2019.1697815
15. Xia X, Wang Y, Yuan J, et al. Baseline SUVmax of 18F-FDG PET-CT indicates prognosis of extranodal natural killer/T-cell lymphoma. Medicine. 2020;99(37):e22143. doi:10.1097/MD.0000000000022143
16. Ohata S, Takenaka K, Sugiyama D, et al. Bone marrow infiltration is a distinctive risk factor for rituximab infusion-related reactions in CD20-positive B-cell non-Hodgkin lymphoma. Adv Hematol. 2022;2022(1):3688727. doi:10.1155/2022/3688727
17. Kallergi M, Georgakopoulos A, Lyra V, et al. Tumor size measurements for predicting Hodgkin’s and non-Hodgkin’s lymphoma response to treatment. Metabolites. 2022;12(4):285. doi:10.3390/metabo12040285
18. Chen S, Wang S, He K, et al. PET/CT predicts bone marrow involvement in paediatric non-Hodgkin lymphoma and may preclude the need for bone marrow biopsy in selected patients. Eur Radiol. 2018;28(7):2942–2950. doi:10.1007/s00330-018-5306-5
19. Annunziata S, Cuccaro A, Tisi MC, Hohaus S, Rufini V. FDG-PET/CT at the end of immuno-chemotherapy in follicular lymphoma: the prognostic role of the ratio between target lesion and liver SUVmax (rPET). Ann Nucl Med. 2018;32(5):372–377. doi:10.1007/s12149-018-1243-2
20. Li H, Shao G, Zhang Y, et al. Nomograms based on SUVmax of 18F-FDG PET/CT and clinical parameters for predicting progression-free and overall survival in patients with newly diagnosed extranodal natural killer/T-cell lymphoma. Cancer Imaging. 2021;21(1):9. doi:10.1186/s40644-020-00379-y
21. Qi J, Gu C, Wang W, et al. Elevated lactate dehydrogenase levels display a poor prognostic factor for non-Hodgkin’s lymphoma in intensive care unit: an analysis of the MIMIC-III database combined with external validation. Front Oncol. 2021;11(1):753712. doi:10.3389/fonc.2021.753712
22. Menon SS, Guruvayoorappan C, Sakthivel KM, Rasmi RR. Ki-67 protein as a tumour proliferation marker. Clin Chim Acta. 2019;491:39–45. doi:10.1016/j.cca.2019.01.011
23. Yin Y, Liu J, Sun R, et al. Exploring the efficacy of 18F-FDG PET/CT in hepatocellular carcinoma diagnosis: role of Ki-67 index and tumor differentiation. Abdom Radiol. 2023;48(11):3408–3419. doi:10.1007/s00261-023-04027-4
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.