Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
Corticosteroid Therapy for Diffuse Alveolar Hemorrhage with Respiratory Failure in Hematologic Malignancies: A Retrospective Cohort Study
Authors Ahn JH , Song KM, Huh JW, Lee JH, Hong SB, Lee JH , Lim CM, Lee KH, Koh Y
Received 1 February 2025
Accepted for publication 25 April 2025
Published 17 May 2025 Volume 2025:21 Pages 705—714
DOI https://doi.org/10.2147/TCRM.S520299
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Jee Hwan Ahn,1 Kyung Mee Song,1 Jin Won Huh,1 Jung-Hee Lee,2 Sang-Bum Hong,1 Je-Hwan Lee,2 Chae-Man Lim,1 Kyoo-Hyung Lee,2 Younsuck Koh1
1Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea; 2Department of Hematology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea
Correspondence: Younsuck Koh, Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-Ro 43-Gil, Songpa-Gu, Seoul, 05505, Republic of Korea, Email [email protected]
Background: Although DAH in hematopoietic stem cell transplantation recipients is commonly treated with systemic corticosteroids, the efficacy of steroid therapy on DAH with respiratory failure in hematologic malignancy patients has not been studied. We aimed to investigate the effectiveness of steroid therapy in hematologic malignancy patients who developed DAH with respiratory failure and required treatment in the intensive care unit (ICU).
Methods: Among DAH patients with leukemia, lymphoma, or multiple myeloma, those who were not admitted to the ICU were excluded. Included patients were classified into the steroid or control group according to steroid therapy. Patient data were retrospectively collected from electrical medical records. The primary outcome was ICU mortality.
Results: A total of 44 patients (steroid group, n = 32; control group, n = 12) were included. At the DAH diagnosis, the steroid group was less likely to have a history of previously treated solid malignancy (3% vs 25%; P = 0.025) compared to the control group. ICU mortality in the steroid group was not significantly different from that in the control group (66% vs 67%; P = 0.948). Only in the steroid group, the ratio of arterial oxygen partial pressure to fractional inspired oxygen was significantly improved after 2 days from the DAH diagnosis (151 ± 64 vs 120 ± 38 mm Hg; P = 0.039).
Conclusion: Hematologic malignancy patients who developed DAH with respiratory failure and were admitted to the ICU had high mortality, irrespective of steroid therapy.
Keywords: alveolar hemorrhage, hematologic malignancy, mortality, steroid
Background
Diffuse alveolar hemorrhage (DAH) is a rare clinical syndrome of various causes and in which hemorrhage occurs from alveolar capillaries into the alveolar space.1 DAH acutely presents with any combination of hemoptysis, anemia, respiratory failure, and diffuse infiltrates on chest radiograph. The causes of DAH are largely classified into immune causes, including vasculitis and anti-glomerular basement membrane antibody disease, and non-immune causes such as increased pulmonary capillary pressure, drugs, infections, and coagulopathy.2 The prognosis of DAH varies greatly depending on cause,2–4 and mortality from DAH following hematopoietic stem cell transplantation (HSCT) remains high, at 60–83%.5–8
While the treatment for DAH due to immune causes is immunosuppressive therapy based on systemic corticosteroids, the treatment for DAH due to non-immune includes correction of the underlying disease, antifibrinolytic agents, and supportive care such as oxygen therapy or mechanical ventilation. Correction of the underlying disease includes reducing left ventricular filling pressure with diuretics for left ventricular dysfunction or valvular heart disease, discontinuation of causative drugs, appropriate antimicrobial agents for infections, and transfusion for coagulopathy or thrombocytopenia.9 Empirically, tranexamic acid, aminocaproic acid, or activated recombinant factor VII have been used in the treatment of DAH although evidence on their efficacy is conflicting.9–12 For DAH in HSCT recipients, steroid therapy has been widely used because of a potential role against inflammation resulting from chemotherapy, graft-versus-host disease, and consequent cytokine release.13 Although some early case series and a retrospective study reported the effectiveness of steroid therapy,5,14,15 later retrospective studies showed that steroids did not improve survival in patients with DAH following HSCT,6,7 thus making the efficacy of steroid therapy uncertain. Steroid therapy can increase the risk of secondary infection due to immunosuppression; such infection may be life-threatening in critically ill patients on mechanical ventilation.16
DAH also occurs in patients with hematologic malignancy who have not undergone HSCT. To date, most data on steroid therapy are limited to DAH following HSCT. In patients with hematologic malignancies who have not undergone HSCT, inflammatory lung injuries related to neutrophil engraftment or graft-versus-host disease are absent. Therefore, the pathophysiology of DAH in this population may differ from that of HSCT-related DAH, and the anti-inflammatory effects of steroids may be less effective or even negligible. Nevertheless, steroid therapy has been empirically used to treat DAH in patients with hematologic malignancies—including at our center—despite the lack of solid evidence supporting its efficacy.17,18 This study aimed to evaluate the effectiveness of steroid therapy and to identify prognostic factors in hematologic malignancy patients who developed DAH with respiratory failure and required treatment in the intensive care unit (ICU).
Methods
Study Patients
This retrospective cohort study included all adult patients (aged ≥18 years) with leukemia, lymphoma, or multiple myeloma who developed DAH with acute respiratory failure and were admitted to the ICU of a tertiary referral teaching hospital from August 2005 to May 2018. DAH was diagnosed when the following criteria were met: acute onset hypoxemia with newly developed diffuse pulmonary infiltrates on a chest radiograph or computed tomography; and progressively bloodier aliquots on bronchoalveolar lavage (BAL).4,7 Patients who met the diagnostic criteria of DAH, but who were not admitted to the ICU, were excluded. Included patients were grouped into the steroid or control group according to steroid therapy for DAH. The protocols of this study were approved by the institutional review board of Asan Medical Center (IRB No. 2018–0981), which waived the need for informed consent because of the retrospective nature of the study. All data from study patients were anonymized prior to analysis, and the study was conducted in accordance with the Declaration of Helsinki.
Management of DAH
BAL was performed with fiberoptic bronchoscopy when DAH was suspected clinically. During BAL, lavage was done three times with saline, and the color of each aliquot was checked by the bronchoscopist. Differential cell counts, chemical analysis, cytological examinations, and microbiological tests for bacteria, mycobacteria, fungi (including Pneumocystis jirovecii), and viruses were routinely performed with BAL fluid.
In patients with respiratory distress or worsening hypoxemia despite oxygen therapy with a high-flow nasal cannula, mechanical ventilation was applied according to the lung-protective strategy. Platelet concentrates were transfused if thrombocytopenia (<50,000 cells/mm3) was present. Broad-spectrum antimicrobial agents were administered empirically in the absence of previously identified pathogens in respiratory specimens and were changed to definitive therapy after pathogens were cultured in BAL fluid. Steroid therapy and its dosage regimen were determined at the discretion of each patient’s attending physician: 1–2 mg/kg/day of intravenous methylprednisolone was started immediately after bronchoscopy with or without pulse therapy (500–1000 mg/day of methylprednisolone for 3 days). No immunosuppressants, other than steroid, were used for DAH.
Data Collection
Baseline and treatment data for study patients were obtained from hospital electronic medical records. The data collected included: age; sex; comorbidities; diagnosis and date of hematologic malignancy; date of last chemotherapy; HSCT and date; disease status of hematologic malignancy; diagnosis date of DAH; presence of overt hemoptysis; Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) scores on the diagnosis date of DAH; results of laboratory tests with BAL fluid; use of mechanical ventilation; ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2); blood glucose levels and total doses of insulin used during the first 4 days after the DAH diagnosis; and development of any new infection within 28 days after the DAH diagnosis. The diagnosis date of DAH was defined as the date of diagnostic bronchoscopy. The disease status of hematologic malignancy was classified as: “just diagnosed or during the first chemotherapy”; “controlled” (in complete remission); or “uncontrolled” (other than the previous two statuses).
The primary outcome was ICU mortality. Secondary outcomes were: hospital mortality; 60-day mortality from the DAH diagnosis; changes in PaO2/FiO2 ratio during the first 4 days after the DAH diagnosis; and new infections within 28 days from the DAH diagnosis. If a patient survived to discharge within 60 days after the DAH diagnosis, the survival status of the patient at day 60 was confirmed based on National Health Insurance Service data.
Statistical Analysis
Data are presented as either mean with standard deviation or median with interquartile range (IQR). Baseline and treatment variables were compared between the steroid and control groups using the Student’s t-test or Mann–Whitney U-test for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables. To analyze the effect of steroids on PaO2/FiO2 ratio over the first 4 days, repeated measures analysis of variance was used. Multivariable logistic regression analysis was done for steroid therapy and variables significantly associated with the primary outcome in univariable analysis. Cumulative survival rates for 60 days from the DAH diagnosis were compared by the Log rank test. A two-sided p value <0.05 was considered statistically significant. All analyses were performed using IBM SPSS Statistics version 21 (IBM Corp., Armonk, NY, USA).
Results
A total of 49 adult patients with hematologic malignancy developed DAH during the study period. After excluding 5 patients not admitted to the ICU, the remaining 44 patients were divided into the steroid (n = 32) and control (n = 12) groups based on steroid therapy (Figure 1). PaO2/FiO2 ratio on the day of the DAH diagnosis was <200 mm Hg in all included patients. Eight patients (18%) developed DAH before their first chemotherapy. In 12 patients (27%) who underwent HSCT, DAH occurred after a median of 56 days (IQR, 31–167 days) from HSCT. Half the study patients (n = 22) had at least one positive result on microbiological tests with BAL fluid: bacteria in 7 patients (Acinetobacter baumannii [n = 2], Enterobacter cloacae [n = 1], Legionella pneumophila [n = 1], non-tuberculous mycobacterium [n = 1], Staphylococcus aureus [n = 1], Stenotropomonas maltophilia [n = 1]); viruses in 11 patients (cytomegalovirus [n = 6], adenovirus [n = 2], parainfluenza virus type 3 [n = 2], herpes simplex virus 1 [n = 1], influenza virus type A [n = 1], respiratory syncytial virus [n = 1]); fungi in 4 patients (Pneumocystis jirovecii [n = 3], Candida albicans [n = 1]); and positive results on the galactomannan test in 5 patients.
 |
Figure 1 Flowchart of study inclusion. Abbreviation: DAH, diffuse alveolar hemorrhage. |
Table 1 shows demographic and clinical characteristics of the study patients at the DAH diagnosis. In the steroid group, one patient was previously treated for solid malignancy (non-small cell lung cancer), while 3 patients in the control group did (early gastric cancer, hepatocellular carcinoma, and bladder cancer; 3% vs 25%, P = 0.025). The steroid group tended to have a lower SOFA score (10.1 ± 3.6 vs 12.0 ± 2.4; P = 0.099) and be more likely to have a history of HSCT (34% vs 8%; P = 0.084), compared to the control group. Among the steroid group, 9 patients received pulse therapy followed by methylprednisolone at a mean dose of 1.7 ± 0.5 mg/kg/day, and the other 23 patients received methylprednisolone alone at a mean dose of 1.3 ± 0.5 mg/kg/day.
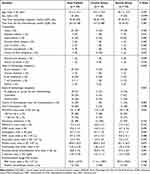 |
Table 1 Characteristics of Study Patients at the Diagnosis of Diffuse Alveolar Hemorrhage According to Steroid Therapy |
ICU mortality in the steroid group was 66% (21 of 32 patients), which was not significantly different from that in the control group (67%, 8 of 12 patients; P = 0.948). Secondary outcomes are presented in Table 2. Although there was no significant difference in PaO2/FiO2 ratio between the two groups over the first 4 days after the DAH diagnosis (P = 0.497 by repeated measures analysis of variance), PaO2/FiO2 ratio in the steroid group improved significantly after 2 days from the DAH diagnosis (P <0.05 with Bonferroni correction for each comparison; Figure 2). The proportions of patients who developed new infections within 28 days were comparable between the two groups (53% vs 50%, P >0.999). The steroid group had a tendency to receive insulin at a higher dose, which was not statistically significant (Table S1). As a result of multivariable logistic regression analysis for ICU mortality, PaO2/FiO2 ratio <100 mm Hg (adjusted OR, 12.62; 95% confidence interval [CI], 1.37–116.45) and SOFA score (adjusted OR, 1.33; 95% CI, 1.02–1.73) on the day of the DAH diagnosis were prognostic factors (Table 3). Kaplan-Meier survival curves according to steroid therapy, initial PaO2/FiO2 ratio, and history of HSCT are depicted in Figure 3.
 |
Table 2 Treatment Outcomes of Study Patients Depending on Steroid Therapy |
 |
Table 3 Results of Logistic Regression Analysis for Intensive Care Unit Mortality |
Discussion
In this retrospective study of DAH with respiratory failure in hematologic malignancy patients requiring ICU treatment, we found no significant difference in ICU mortality between patients who received steroid therapy and those who did not. Approximately two-thirds and three-quarters of all study patients died in the ICU and within 60 days from the DAH diagnosis, respectively. PaO2/FiO2 ratio <100 mm Hg and SOFA score, not steroid therapy, were prognostic factors for ICU mortality. Our results suggest that steroid therapy is not effective for DAH in patients with hematologic malignancy.
The present study is the first to evaluate the effect of steroid therapy on DAH with respiratory failure in hematologic malignancy patients. DAH cases with hematologic malignancy as the underlying disease have been reported intermittently as case reports or case series,17–21 or as a part of small retrospective studies about DAH.2–4 Although some of these DAH cases received steroid therapy, treatment outcomes according to steroid therapy were not reported. In 2020, Lee et al reported the effects of intrapulmonary thrombin administration in 15 ICU patients with DAH associated with hematologic malignancy.18 In that cohort, the mean SOFA score was 8 ± 1.9, the baseline PaO2/FiO2 ratio was 151 ± 63.9 mm Hg, and ICU mortality was 10 out of 15 patients (67%). The cohort’s severity and outcomes were comparable to ours. However, the efficacy of steroids could not be assessed because all patients in that study received steroid therapy. Steroids have been used without a clear basis in patients with hematologic malignancy who develop DAH, regardless of HSCT. However, in our study, a low PaO2/FiO2 ratio and a high SOFA score at the DAH diagnosis were independent predictors of ICU mortality. These findings suggest that the severity of accompanying respiratory and other organ failure—rather than the use of steroid therapy—may be the primary determinant of outcomes in DAH patients with hematologic malignancy admitted to the ICU.
Regarding the potential pathogenesis of DAH in HSCT recipients, the following factors have been suggested: lung tissue injury from pretransplant chemotherapy, radiation, unidentified infection, and dimethyl sulfoxide; vasculopathy of small arteries and thrombotic microangiopathy; prolonged thrombocytopenia; and inflammation and consequent cytokine release due to neutrophil engraftment and graft-versus-host disease.13,22 To control these inflammatory responses, and based on anecdotal benefits, DAH in HSCT recipients is treated with steroids. However, previous studies showed conflicting results. In the first retrospective study evaluating the effectiveness of steroids, DAH patients treated with >30 mg/day of methylprednisolone had significantly lower in-hospital mortality than those who did not (67% vs 91%).5 Conversely, three later retrospective studies reported that steroid therapy had limited effectiveness as a DAH treatment6 and was not associated with 60-day mortality (74% with steroids vs 75% without steroids; P = 0.28)7 or hospital mortality (61% with steroids vs 48% without steroids; P = 0.22).23 DAH in patients with hematologic malignancy who have not undergone HSCT has some underlying conditions similar to DAH in HSCT recipients, including chemotherapy, infection, and coagulopathy. In our study, patients who did not receive HSCT had high 60-day mortality, which was comparable to the mortality in patients who received HSCT (both 75%). This finding suggests that the prognosis of DAH in patients with hematologic malignancy is poor, irrespective of HSCT.
In our study, although PaO2/FiO2 ratio over time did not differ significantly between the two groups, PaO2/FiO2 ratio in the steroid group was significantly improved after two days from the DAH diagnosis; this was unlike the findings in the control group. The numerical trend for steroid therapy to improve oxygenation was not reported in previous studies in HSCT recipients with DAH. Physicians may have administered steroids to DAH patients with hematologic malignancy because short-term improvements in oxygenation are often noticed with steroid therapy. However, in our cohort, all five patients who were initially extubated but eventually died in the ICU belonged to the steroid group. Of these, three died from newly developed infections a median of 10 days (range, 8–13 days) after extubation, and one patient died 11 days after extubation due to recurrent DAH. Although the sample size is too small to assess the association between steroid therapy and post-extubation ICU mortality, these findings raise concerns that short-term improvement in oxygenation in the steroid group may not translate into meaningful clinical benefit. Further investigations are needed about the efficacy of steroids on oxygenation and its clinical significance.
One of several limitations of the current study is that diagnostic criteria for DAH did not include the presence of hemosiderin-laden macrophages in BAL fluid or alveolar hemorrhage in lung-biopsy specimens; our center does not routinely perform iron staining on BAL fluid. Potentially, therefore, some DAH cases may not have been included in the study. However, because our study subjects were DAH patients admitted to the ICU due to respiratory failure, most of the patients who developed clinically significant DAH and underwent BAL were likely included. Secondly, patients’ attending physicians determined steroid therapy regimens without a standardized protocol. Resultant variation in the effect size of steroid therapy may have influenced the study results. However, both the steroid doses administered and steroid pulse therapy were not associated with ICU mortality in our logistic regression analysis. Lastly, the single-center, retrospective study design with a small sample size limits both the statistical power to detect differences in the primary outcome between the two groups and the generalizability of the findings. A previous study by Metcalf et al demonstrated a benefit of steroid therapy in DAH following BMT, reporting 30-day estimated mortality rates of 43% in the steroid group and 84% in the control group.5 Based on these assumptions, the calculated power of our study was 71% with a two-sided alpha error of 0.05, which is considered suboptimal. However, we believe the lack of statistically significant differences in our study is not due to insufficient power, but rather reflects the absence of a clinically meaningful benefit of steroids in this population. This interpretation is supported by the fact that outcomes for our study patients were comparable to those in previous studies of DAH in HSCT recipients. Moreover, DAH in patients with hematologic malignancies is rare, making a prospective observational or interventional study nearly infeasible.
Conclusions
Hematologic malignancy patients who developed DAH with respiratory failure and were admitted to the ICU had high ICU and 60-day mortalities irrespective of steroid therapy, thus suggesting that steroids may not improve the prognosis of DAH in these patients. Decisions about steroid therapy should be carefully made because there is no clear evidence that steroids are beneficial in DAH patients with hematologic malignancy. To validate the efficacy of steroid therapy or to identify alternative effective treatments in these patients, multicenter prospective studies would be valuable.
Abbreviations
DAH, diffuse alveolar hemorrhage; HSCT, hematopoietic stem cell transplantation; ICU, intensive care unit; BAL, bronchoalveolar lavage; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; PaO2/FiO2, ratio of arterial oxygen partial pressure to fractional inspired oxygen; IQR, interquartile range; CI, confidence interval.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The protocol of this study was approved by the institutional review board of Asan Medical Center (IRB No. 2018-0981), which waived the need for informed consent because of the retrospective nature of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Lara AR, Schwarz MI. Diffuse alveolar hemorrhage. Chest. 2010;137(5):1164–1171. doi:10.1378/chest.08-2084
2. de Prost N, Parrot A, Picard C, et al. Diffuse alveolar haemorrhage: factors associated with in-hospital and long-term mortality. Europ Resp J. 2010;35(6):1303–1311. doi:10.1183/09031936.00075309
3. Jin SM, Yim JJ, Yoo CG, et al. Aetiologies and outcomes of diffuse alveolar haemorrhage presenting as acute respiratory failure of uncertain cause. Respirology. 2009;14(2):290–294. doi:10.1111/j.1440-1843.2008.01444.x
4. Rabe C, Appenrodt B, Hoff C, et al. Severe respiratory failure due to diffuse alveolar hemorrhage: clinical characteristics and outcome of intensive care. J Crit Care. 2010;25(2):230–235. doi:10.1016/j.jcrc.2009.04.009
5. Metcalf JP, Rennard SI, Reed EC, et al. Corticosteroids as adjunctive therapy for diffuse alveolar hemorrhage associated with bone marrow transplantation. Am J Med. 1994;96(4):327–334. doi:10.1016/0002-9343(94)90062-0
6. Lewis ID, DeFor T, Weisdorf DJ. Increasing incidence of diffuse alveolar hemorrhage following allogeneic bone marrow transplantation: cryptic etiology and uncertain therapy. Bone Marrow Transplant. 2000;26(5):539–543. doi:10.1038/sj.bmt.1702546
7. Majhail NS, Parks K, Defor TE, Weisdorf DJ. Diffuse alveolar hemorrhage and infection-associated alveolar hemorrhage following hematopoietic stem cell transplantation: related and high-risk clinical syndromes. Biol Blood Marrow Transplant. 2006;12(10):1038–1046. doi:10.1016/j.bbmt.2006.06.002
8. Rathi NK, Tanner AR, Dinh A, et al. Low-, medium- and high-dose steroids with or without aminocaproic acid in adult hematopoietic SCT patients with diffuse alveolar hemorrhage. Bone Marrow Transplant. 2015;50(3):420–426. doi:10.1038/bmt.2014.287
9. Park JA. Treatment of Diffuse Alveolar Hemorrhage: controlling Inflammation and Obtaining Rapid and Effective Hemostasis. Int J Mol Sci. 2021;22(2):793. doi:10.3390/ijms22020793
10. Wanko SO, Broadwater G, Folz RJ, Chao NJ. Diffuse alveolar hemorrhage: retrospective review of clinical outcome in allogeneic transplant recipients treated with aminocaproic acid. Biol Blood Marrow Transplant. 2006;12(9):949–953. doi:10.1016/j.bbmt.2006.05.012
11. Gupta S, Jain A, Warneke CL, et al. Outcome of alveolar hemorrhage in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2007;40(1):71–78. doi:10.1038/sj.bmt.1705695
12. Elinoff JM, Bagci U, Moriyama B, et al. Recombinant human factor VIIa for alveolar hemorrhage following allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(7):969–978. doi:10.1016/j.bbmt.2014.03.015
13. Afessa B, Tefferi A, Litzow MR, Krowka MJ, Wylam ME, Peters SG. Diffuse Alveolar Hemorrhage in Hematopoietic Stem Cell Transplant Recipients. Am J Respir Crit Care Med. 2002;166(5):641–645. doi:10.1164/rccm.200112-141CC
14. Chao NJ, Duncan SR, Long GD, Horning SJ, Blume KG. Corticosteroid therapy for diffuse alveolar hemorrhage in autologous bone marrow transplant recipients. Ann Intern Med. 1991;114(2):145–146. doi:10.7326/0003-4819-114-2-145
15. Raptis A, Mavroudis D, Suffredini A, et al. High-dose corticosteroid therapy for diffuse alveolar hemorrhage in allogeneic bone marrow stem cell transplant recipients. Bone Marrow Transplant. 1999;24(8):879–883. doi:10.1038/sj.bmt.1701995
16. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954–963. doi:10.1093/clinids/11.6.954
17. Spira D, Wirths S, Skowronski F, et al. Diffuse alveolar hemorrhage in patients with hematological malignancies: HRCT patterns of pulmonary involvement and disease course. Clin Imag. 2013;37(4):680–686. doi:10.1016/j.clinimag.2012.11.005
18. Lee J, Rhee CK, Kim SC, et al. Use of intrapulmonary administration of thrombin in hematological malignancy patients with alveolar haemorrhage: a case series. Medicin. 2020;99(20):e20284. doi:10.1097/MD.0000000000020284
19. Russi E, Odermatt B, Joller-Jemelka HI, Spycher MA. Alveolar haemorrhage as a presenting feature of myeloma. Europ Resp J. 1993;6(2):267–270. doi:10.1183/09031936.93.06020267
20. Azoulay E, Fieux F, Moreau D, et al. Acute monocytic leukemia presenting as acute respiratory failure. Am J Respir Crit Care Med. 2003;167(10):1329–1333. doi:10.1164/rccm.200206-554OC
21. Nanjappa S, Jeong DK, Muddaraju M, Jeong K, Hill ED, Greene JN. Diffuse Alveolar Hemorrhage in Acute Myeloid Leukemia. Cancer Control. 2016;23(3):272–277. doi:10.1177/107327481602300310
22. Lynch Y, Vande Vusse LK. Diffuse Alveolar Hemorrhage in Hematopoietic Cell Transplantation. J Intensive Care Med. 2024;39(11):1055–1070. doi:10.1177/08850666231207331
23. Zhang Z, Wang C, Peters SG, et al. Epidemiology, Risk Factors, and Outcomes of Diffuse Alveolar Hemorrhage After Hematopoietic Stem Cell Transplantation. Chest. 2021;159(6):2325–2333. doi:10.1016/j.chest.2021.01.008
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



