Back to Journals » Journal of Pain Research » Volume 18
Critical Role of p38α MAPK Subclass in the Development of Pain Hypersensitivity After Hind Paw Incision
Authors Ishikawa D, Yamakita S , Oh-Hashi K, Amaya F
Received 28 August 2024
Accepted for publication 9 February 2025
Published 21 February 2025 Volume 2025:18 Pages 869—878
DOI https://doi.org/10.2147/JPR.S488494
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jinlei Li
Daiki Ishikawa,1 Shunsuke Yamakita,1 Kentaro Oh-Hashi,2– 4 Fumimasa Amaya1
1Department of Anesthesiology, Kyoto Prefectural University of Medicine, Kyoto, Japan; 2United Graduate School of Drug Discovery and Medical Information Sciences, Gifu University, Gifu, Japan; 3Center for One Medicine Innovative Translational Research (COMIT), Gifu University, Gifu, 501-1193, Japan; 4Department of Chemistry and Biomolecular Science, Faculty of Engineering, Gifu University, Gifu, 501-1193, Japan
Correspondence: Fumimasa Amaya, Department of Anesthesiology, Kyoto Prefectural University of Medicine, Kajii-Cho 465, Kamigyo-Ku, Kyoto, 602-0841, Japan, Tel +81-75-251-5633, Email [email protected]
Background: Deeper understanding of the mechanisms of postoperative pain is critical for developing more effective pain management strategies. The present animal study explored the function of four p38 mitogen-activated protein kinase (MAPK) subclasses (α, β, γ, and δ) in dorsal root ganglion (DRG) in the development of post-incisional pain hypersensitivity.
Methods: The amount of p38 MAPK subclass mRNA in the DRG of male Sprague-Dawley rats was quantified using real-time PCR. Localization of p38 MAPK expression was analyzed by immunohistochemistry using subclass-selective antibodies. The effects of a p38α MAPK inhibitor on plantar incision-induced pain hypersensitivity was assessed using behavioral tests to measure mechanical and thermal sensitivity. The impact of the inhibitor on phosphorylated p38 MAPK expression was also analyzed by immunohistochemistry.
Results: Four p38 MAPK subclass mRNA were identified in the DRG, with p38α, β, and γ MAPK showing significant expression. p38α and γ MAPK were identified in the DRG neurons, whereas p38β MAPK was distributed in satellite glial cells. Selective inhibition of p38α MAPK reduced both mechanical and thermal hypersensitivity following plantar incision. Treatment with the p38α MAPK inhibitor decreased the expression of phosphorylated p38 MAPK in the DRG.
Conclusion: These results demonstrated the distinct roles of p38 MAPK subclasses in the DRG, with p38α MAPK playing a dominant role in the development of pain hypersensitivity after tissue injury. Targeting p38α MAPK might be a promising therapeutic strategy for managing postoperative pain.
Keywords: p38 MAPK, tissue injury, hyperalgesia
Introduction
Despite recent advances in pain management, many surgical patients experience severe postoperative pain.1,2 Inadequate management of postoperative pain is associated with impaired rehabilitation, prolonged duration of hospital stay and adverse events.3 Their majorities are nociceptive, and most of the nociceptive pain can be treated by opioids. However, there is a challenge to use opioids for postoperative patients since their use carries the risk of adverse effects, tolerance, and addiction.4 Hence, continued research to examine the mechanisms of postoperative pain is essential for developing novel pain management strategies.
Tissue injury during surgery causes sensitization of primary afferents,5 causing functional changes in the primary afferent neurons in the dorsal root ganglion (DRG). Pro-inflammatory cytokines and neurotrophic factors are synthesized in damaged tissue, leading to an increase in neuronal excitability.6,7 Activation of intracellular signal transduction plays a crucial role in this increased excitability. Phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK) is one of the major signaling pathways in the primary afferent neurons.8 Phosphorylated p38 MAPK increases up-regulation of the sodium channel and neuropeptide expression and enhances neuronal excitability. p38 MAPK is involved in the pathophysiology of chronic pain after the nerve injury.9 In addition, p38 MAPK is phosphorylated in the DRG after the plantar incision, and its inhibition abolishes incision-induced pain hypersensitivity10,11 and pain chronicity.12 Despite the critical role of p38 MAPK was evident by preclinical evidences, development of p38 MAPK inhibitory drug has been unsuccessful due to unacceptable safety profiles.13 Subclass selective information is required to develop clinically feasible p38 inhibitor as analgesic drug.14
There are four known p38 MAPK subclasses (α, β, γ, and δ), which have similar amino acid sequences but distinctive activation mechanisms and downstream molecules.15 Their expression shows distinctive distribution in arthritis tissue.16 In the spinal cord, p38α and β MAPK have been identified in the neurons and glial cells of the dorsal horn, and their role in the development of pain hypersensitivity has been characterized.17,18 However, the expression and relative functions of the four p38 MAPK subclasses have not been examined in the DRG.
In the present study, we analyzed the expression of p38 MAPK subclasses in the rat DRG. We also investigated the effect of selective p38α MAPK inhibition on pain hypersensitivity and the phosphorylation of p38 MAPK after plantar incision.
Experimental Procedures
The experimental procedures were approved by the Kyoto Prefectural University of Medicine Animal Care Committee. All experiments were conducted in accordance with the guidelines of the National Institutes of Health, Science Council of Japan and the International Association for the Study of Pain.19
Male Sprague-Dawley rats (200–250 g, 6–8 weeks, Shimizu Laboratory Material), housed three rats per cage under a 12-hourly light/dark cycle, were the subjects of the experiments. The surgical procedures were performed under general anesthesia with 2% isoflurane. Animals have been randomly assigned to an experimental group. Sample size of each experimental group has been determine based on our previous studies. Male rats were used for these experiments since previous study showed lack of p38α and β MAPK signal involvement in the development of hyperalgesia in female rats.
Experiment 1: Expression and Localization of p38 MAPK Isoforms in the DRG
The expression levels of the four p38 MAPK isoforms were quantified by real-time PCR. For this, the left L5 DRGs were collected from untreated rats under deep anesthesia. RNA was extracted from the DRG using Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was synthesized from 1 µg of RNA using qPCR RT Master Mix (TOYOBO, Osaka, Japan). Quantitative RT-PCR was performed with a SYBR green master mixture kit, StepOnePlus (Thermo Fisher Scientific). The primer pairs used in this study are shown in Table 1. For thermal cycling, samples were first incubated at 95°C for 10 min. Each PCR cycle consisted of heating at 95°C for 15 seconds, followed by annealing and extension at 60°C for 1 minute. Samples were analyzed in duplicate. In order to standardize inter-individual variation of DRG tissue volume and RNA extraction efficacy, relative mRNA expression levels were calculated using the standard curve method and standardized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
 |
Table 1 Primers for Real-Time PCR Used in This Study |
Using isoform-specific antibodies, immunohistochemistry was performed to identify the distribution of p38 MAPK isoform expression. Rats were perfused transcardially with 0.9% NaCl and 10% neutralized formalin (Wako Pure Chemical Industries, Ltd., Osaka, Japan). The left L5 DRGs were collected from untreated rats and cryoprotected in 20% sucrose in a 0.1 M phosphate buffer (PB, pH 7.4) at 4°C overnight. The DRG was cut using a cryostat (10 µm, Leica Biosystems, Nussloch, Germany). Sections were incubated with Blocking One P (Nacalai Tesque Inc., Kyoto, Japan) at room temperature for 30 minutes prior to the primary antibody incubation.
To determine the characteristics of DRG cells that express p38α, β, or γ MAPK double-labeling immunohistochemistry with the neuronal marker neuronal nuclei (NeuN) or glial marker 3-phosphoglycerate dehydrogenase (3PGDH) was performed. Sections were incubated with p38α MAPK Rabbit Ab (1:200, Cell Signaling Technology) and anti-NeuN (1:100 Merck Millipore), p38β MAPK Rabbit Ab (1:100, Cell Signaling Technology) and anti-3PGDH (1:400 Merck Millipore) or p38γ MAPK antibody (1:200, Cell Signaling Technology) and anti-NeuN (1:100 Merck Millipore) in 0.1 M tris buffered saline (TBS) containing 0.1% Tween 20 and 1% blocking reagent at 4°C for three days. Sections were then incubated overnight at 4°C with Cy3 conjugated anti-rabbit (1:100 Merck Millipore) and FITC-conjugated anti-mouse (1:1000, Merck Millipore) secondary antibodies in 0.1 M TBS. Immunohistochemistry signals were visualized using a fluorescence microscope equipped with a digital camera system (Nikon, Tokyo, Japan).
Experiment 2: Effect of p38α MAPK Inhibitors on Plantar Incision-Induced Pain Hypersensitivity
Rats were randomly divided into the VX-702 group or vehicle group (N=5 for each group). In the VX-702 group, VX-702 (p38α MAPK inhibitor, 20 mg/kg dissolved in 10% dimethyl sulfoxide, Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) was administered intraperitoneally, and the vehicle group received the same amount of 10% dimethyl sulfoxide. All animals received plantar incision, and treated with VX-702 or vehicle prior to, day 1 and day 2 after the incision. Behavioral analysis was performed before drug administration on day 0 and 2 hours after drug administration on day 1 and 2; only behavioral analysis was performed on day 5. In order to explore effect of VX-702 in different dose, rats were randomly assigned into the VX-702 10 mg/kg, 20 mg/kg, 50 mg/kg and vehicle groups. Rats were treated with VX-702 or vehicle prior to and day 1 after the incision.
The behavioral analysis was performed by a single researcher (D.I.) who was blinded to the experimental conditions. Noxious mechanical sensitivity was assessed by measuring withdrawal thresholds against stimulation using von Frey filaments. For this, the animals were placed in a clear plastic chamber on a transparent elevated wire grid and allowed to move freely. After the animals were acclimated, withdrawal responses to mechanical stimuli were measured. A calibrated von Frey monofilament set (Muromachi Machinery, Tokyo, Japan) was used to measure the withdrawal responses to mechanical stimuli. The von Frey filament stimulus was applied from the incision point of the left paw. In this experiment, the lowest force that caused a clear withdrawal response at least twice in 10 stimuli was defined as the threshold. To avoid tissue damage, a cut-off stimulus value of 26 g was set before the experiment.
Noxious heat sensitivity was determined as the latency time for withdrawal against radiant heat (Ugo Basile, 37370, Italy). For this, rats were allowed to adapt in transparent plastic cages placed on a glass floor. The left hind paw was then exposed to a focused subfloor radiant heat source. To avoid burns, a cut-off latency of 20 seconds was set prior to the experiment. Each rat was tested three times at 5-minute intervals, and the mean of the three values was used as the withdrawal latency.
Experiment 3: Effect of the p38α MAPK Inhibitor on the Expression of Phosphorylated p38 MAPK After the Plantar Incision
Rats were randomly divided into naive, vehicle, and VX-702 groups (N=5 for each). In the VX-702 group, the p38α MAPK inhibitor, VX-702 (20 mg/kg dissolved in 10% dimethyl sulfoxide), was administered intraperitoneally, following which a plantar incision was made. Treatment dose of VX-702 was decided according to the previous studies.20 The vehicle group received the same amount of 10% dimethyl sulfoxide intraperitoneally followed by the plantar incision. Naive animals did not receive any treatment. On day 1, the left L5 DRG was collected and processed for immunohistochemistry to analyze phosphorylated p38 (p-p38) MAPK expression.
L5 DRG sections from rats of all three groups were incubated at 4°C for three days with p-p38 MAPK antibody (1:200, Cell Signaling Technology) in 0.1 M TBS containing 0.1% Tween 20 and 1% blocking reagent for 3 days. After washing with PBS, the sections were incubated overnight at 4°C with Cy3 conjugated anti-rabbit secondary antibody (1:100 Merck Millipore, Billerica, MA, USA) in 0.1 M TBS.
Plantar Incision Model
A plantar incision model was used in this study.21 A 1 cm long incision was made on the left plantar surface of the hindfoot 0.5 cm from the heel edge, and the skin, fascia, and plantaris muscle were penetrated with a No. 11 surgical blade. The skin was closed with 5–0 nylon sutures.
Assessment of Immunohistochemistry
The signal intensity of immunohistochemistry for p-p38 MAPK and the cell size of each DRG neuron were calculated using Image J software (National Institute of Mental Health, Bethesda, MD, USA) on a Windows computer system. The mean signal intensity and cross-sectional area of neurons with DAPI-positive nuclei were calculated. First, ROIs of all neurons present in the tissue were obtained by hand tracing. The ROIs were then applied to phospho-p38 MAPK stained images. This operation yielded the signal intensity and cross-sectional area of individual neurons present in the tissue. The relative signal intensity (RSI) of each neuron was calculated by subtracting the background signal intensity. Based on the scatterplot showing the relationship between RSI and cross-sectional area, we defined RSI = 50 as the borderline value to determine positive immunoreactivity in each neuron.
Statistical Analysis
Statistical analyses were performed using the Mann–Whitney test, Kruskal–Wallis test with Dunn’s multiple comparison test, or a two-way ANOVA with Bonferroni’s post-test on GraphPad Prism7 (GraphPad Software, La Jolla, CA, USA). Values of p < 0.05 were considered statistically significant. All data are presented as the mean ± SEM.
Results
Experiment 1: Expression and Localization of p38 MAPK Isoforms in the DRG
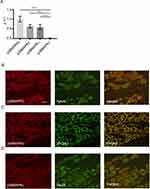
The results of real-time PCR analysis are shown in Figure 1A. We detected a considerable amount of p38α, β, and γ MAPK mRNA in the DRG. In contrast, the expression level of p38δ MAPK was significantly lower than that of the other three isoforms. Further analysis of the distribution of p38α, β, and γ MAPK expression by double-labeling immunohistochemistry showed that the expression of p38α MAPK was detected in NeuN-positive neurons (Figure 1B), whereas the expression of p38β MAPK was detected in 3PGDH-positive cells (Figure 1C). Expression of p38γ MAPK was also detected in NeuN-positive neurons (Figure 1D).
Experiment 2: Effect of the p38α MAPK Inhibitor on Plantar Incision-Induced Pain Hypersensitivity
Figure 2A demonstrates the results of mechanical hyperalgesia measured as the withdrawal threshold against von Frey stimulation. Behavioral analysis revealed that plantar incision induced a reduction of mechanical threshold against von Frey stimulation 1 day after the incision in the vehicle group. The mechanical hyperalgesia continued for at least 5 days after the treatment. Compared to the vehicle group, the mechanical threshold was significantly higher 1 and 2 days after the incision in the 20 mg/kg of VX-702 treated group. However, the mechanical threshold 5 days after the treatment was similar between the VX-702 and vehicle groups. Figure 2B demonstrates the results of thermal hyperalgesia evaluated as the latency time against radiant heat stimulation. Radiant heat latency was significantly reduced 1 day after the incision in the vehicle group, the reduction in heat latency returning to normal levels 5 days after the treatment. Heat latency was significantly longer in the 20 mg/kg of VX-702 treated group 1 day after the treatment, although no significant difference was observed between the VX-702 and vehicle groups 2 days and 5 days after the treatment.
Figure 2C shows the effect of different dose (10, 20 and 50 mg/kg) of VX-702 on mechanical sensitivity one day after the plantar incision one day after the incision. The 50 mg/kg and 20 mg/kg groups had significantly higher mechanical thresholds compared to the vehicle group. In contrast, the mechanical threshold of 10 mg/kg group was similar to the vehicle group. Figure 2D shows the effect of VX-702 on thermal sensitivity one day after the plantar incision with different dose (10, 20 and 50 mg/kg) one day after the incision. The heat latency was significantly longer in the 50 mg/kg group and the 20 mg/kg group compared to the vehicle group. In contrast, there was no significant difference between the 10 mg/kg and vehicle groups. Figure 2E demonstrates the timeline of the experimental procedure.
No symptoms of motor nerve blockade were observed in rats treated with 20 mg/kg of VX-702. Two hours after administration, there was no significant difference in the forward reflection time between the naive group and the 20 mg/kg group.
Experiment 3: Effect of the p38α MAPK Inhibitor on the Expression of Phosphorylated p38 MAPK After the Plantar Incision
In all three groups, naive, vehicle, and VX-702, p-p38 MAPK expression was mainly observed in the DRG neurons (Figure 3A). The signal intensity of p-p38 MAPK in neurons in each of the three groups is shown in Figure 3B. Neurons with intense p-p38 MAPK signals were observed in the vehicle group. The percentage of p-p38 MAPK positive neurons was significantly higher in the vehicle group than in the naive and VX-702 groups (Figure 3C).
Discussion
p38 MAPK in the sensory nervous system has been shown to be involved in the development of pain hypersensitivity in several animal models of abnormal pain. In the present study, different distributions of expression of four p38 MAPK isoforms were identified in the DRG. Additionally, selective inhibition of p38α MAPK successfully alleviated pain hypersensitivity and phosphorylation of p38 MAPK in the DRG after the plantar incision. These results demonstrated the distinctive characteristics of p38 MAPK subunits in the primary afferent neurons, and dominant function of p38α MAPK in the development of pain hypersensitivity after tissue injury.
Activation of p38 MAPK is considered as one of the main intracellular events leading to the sensitization of primary afferents in the various kinds of pain hypersensitivity. Ji et al demonstrated that activation of p38 MAPK in the DRG neurons after tissue inflammation leads to the development of inflammatory hyperalgesia.22 Injury to the spinal nerve has been shown to induce activation of p38 MAPK in injured23 and uninjured24 neighboring DRG neurons, leading to neuropathic pain. Activation of p38 MAPK is also associated with opioid induced hyperalgesia.25 In the DRG, p38 MAPK directly increases TRPV1,22,26 TRPA1,27 CGRP,28 and EPAC12 gene expression, and modulates tetrodotoxin-resistant sodium channel currents29 by phosphorylating Nav 1.8 voltage-gated sodium channels.30
Four isoforms of p38α, β, γ, and δ MAPK have been identified.31 Each isoform has distinct tissue distribution, activation mechanisms, and biological functions. p38α has a broad role in inflammation and the stress response, and its expression has been observed in various tissues.15 The biological functions and activation mechanisms of p38β are similar to those of p38α. p38β also shows broad expression. p38γ has been discovered in muscle tissue and is considered to regulate muscle function and differentiation.32 p38δ has been identified in the testis, pancreas, kidney and small intestine.33 These isoforms, despite their similarities, have distinct and sometimes overlapping roles in cellular signaling pathways, allowing the p38 MAPK family to regulate a wide range of biological processes. Among the four p38 MAPK isoforms, considerable amounts of p38α, β, and γ MAPK mRNA transcripts were observed in the DRG tissue. Histochemical analysis demonstrated that p38α MAPK was co-localized with NeuN, a selective marker of neuronal cells. On the other hand, p38β MAPK was co-localized with 3PGDH, a selective marker for satellite glial cells. The differential distribution between p38α and β MAPK observed in the present study was also previously reported in the dorsal horn of the spinal cord.17 p38γ MAPK was found to exist predominantly in the DRG neurons, and showed similar distribution to p38α MAPK. Similarities between p38α and γ MAPK expression have been reported in other organs as well.16
As we previously reported, plantar incision induces phosphorylation of p38 MAPK mainly in the neurons of the DRG.11 Based on our current histological observations, p38α or γ MAPK might be responsible for the induction of p38 MAPK phosphorylation in the DRG. Since p38α MAPK broadly regulates transcriptional activity and protein phosphorylation, we utilized a selective inhibitor of p38α MAPK to inhibit sensitization of sensory neurons by p38 MAPK. As we expected, VX-702, a selective inhibitor of p38α MAPK, successfully alleviated both mechanical and thermal pain hypersensitivity after the tissue injury. This is consistent with previous observations of the behavioral effect of FR167653, a non-selective p38 MAPK inhibitor, on pain hypersensitivity.10 VX-702 exhibits p38α MAPK inhibitory effect by direct inhibition of its phosphorylation.34 In our study, VX-702 successfully inhibited incision-induced phosphorylation of p38 MAPK, suggesting that phosphorylated p38 MAPK in the DRG are largely p38α isoform. This result also indicated systemic VX-702 treatment effectively suppress activation of p38 MAPK in the DRG. Our results collectively demonstrated the crucial role of p38α MAPK in p38 MAPK mediated sensitization of the primary afferents after tissue injury. Previous study demonstrated the crucial role of p38α MAPK in controlling nociceptive signals in the spinal cord.18 Together, the central role of p38α MAPK in the pathophysiology of nociceptive signaling within the spinal cord and DRG has now become evident.
Selective inhibition of p38 MAPK is a promising therapeutic target in several pathological situations.35–37 A previous clinical study showed that dilmapimod, another selective inhibitor of p38α MAPK, successfully alleviated neuropathic pain without serious side effects.38 Ongoing Phase 2 clinical trials to assess the therapeutic effect of p38α MAPK selective inhibitors also suggest superior tolerability of these drugs.39 Together, our observations implicate p38α MAPK as a promising therapeutic target for minimizing postoperative pain hypersensitivity.
Conclusion
The present study has several limitations to note. In this study, male rats were used for the analysis, and a role of p38α MAPK in female rats has not been evaluated. Previous study observed lack of p38 MAPK function in the development of hyperalgesia in female rats. p38α MAPK in the spinal cord has been reported to be involved in the development of hyperalgesia. The systemic administration of VX-702 suppresses p38α MAPK in the DRG, as evidenced by the decreased phosphorylation of p38 MAPK in that region. However, we cannot exclude the possibility that VX-702 also inhibited p38 MAPK in other brain lesion or peripheral tissues.
In conclusion, the present study demonstrated the distinct roles of p38 MAPK subclasses in the DRG, with p38α MAPK playing a dominant role in the development of pain hypersensitivity after tissue injury. Targeting p38α MAPK might be a promising therapeutic strategy for managing postoperative pain.
Abbreviations
MAPK, Mitogen-activated protein kinase; DRG, dorsal root ganglion; PB, phosphate buffer; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NeuN, neuronal nuclei; 3PGDH, 3-phosphoglycerate dehydrogenase; p-p38, phosphorylated p38.
Funding
SY was supported by Grants-in-Aid for Scientific Research from the Japan Society for Promotion of Science (22K16593). FA was supported by Grants-in-Aid for Scientific Research from the Japan Society for Promotion of Science (24K02540).
Disclosure
Dr Fumimasa Amaya reports personal fees from Maruishi Pharmaceutical Co.,Ltd., HISAMITSU PHARMACEUTICAL CO.,INC, DAIICHI SANKYO, INC., Nippon Zoki Pharmaceutical Co., Ltd., icu medical, Otsuka Pharmaceutical Co., Ltd., Shionogi & Co., Ltd., Kowa Pharmaceuticals, Inc., Sumitomo Dainippon Pharma Co., Ltd, AYUMI Pharmaceutical Corporation., Mundipharma, TSUMURA & CO., ONO PHARMACEUTICAL CO., LTD.; grants from Nobelpharma Co., Ltd., outside the submitted work. All the authors declare they have no other conflicts of interest in relation to this study.
References
1. Apfelbaum JL, Chen C, Mehta SS, aTJ G. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–540. doi:10.1213/01.ANE.0000068822.10113.9E
2. Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111(3):657–677. doi:10.1097/ALN.0b013e3181aae87a
3. Argoff CE. Recent management advances in acute postoperative pain. Pain Pract. 2014;14(5):477–487. doi:10.1111/papr.12108
4. Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia—when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet. 2019;393(10180):1558–1568. doi:10.1016/S0140-6736(19)30430-1
5. Amaya F, Izumi Y, Matsuda M, Sasaki M. Tissue injury and related mediators of pain exacerbation. Curr Neuropharmacol. 2013;11(6):592–597. doi:10.2174/1570159X11311060003
6. Spofford CM, Brennan TJ. Gene expression in skin, muscle, and dorsal root ganglion after plantar incision in the rat. Anesthesiology. 2012;117(1):161–172. doi:10.1097/ALN.0b013e31825a2a2b
7. Miura M, Sasaki M, Mizukoshi K, et al. Peripheral sensitization caused by insulin-like growth factor 1 contributes to pain hypersensitivity after tissue injury. Pain. 2011;152(4):888–895. doi:10.1016/j.pain.2011.01.004
8. Ji RR, Gereau R, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60(1):135–148. doi:10.1016/j.brainresrev.2008.12.011
9. Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci STKE. 2004;2004(252):reE14. doi:10.1126/stke.2522004re14
10. Mizukoshi K, Sasaki M, Izumi Y, Miura M, Watanabe M, Amaya F. Activation of p38 mitogen-activated protein kinase in the dorsal root ganglion contributes to pain hypersensitivity after plantar incision. Neuroscience. 2013;234:77–87. doi:10.1016/j.neuroscience.2013.01.001
11. Yamakita S, Matsuda M, Yamaguchi Y, Sawa T, Amaya F. Dexmedetomidine prolongs levobupivacaine analgesia via inhibition of inflammation and p38 MAPK phosphorylation in rat dorsal root ganglion. Neuroscience. 2017;361:58–68. doi:10.1016/j.neuroscience.2017.08.011
12. Matsuda M, Oh-Hashi K, Yokota I, Sawa T, Amaya F. Acquired exchange protein directly activated by cyclic adenosine monophosphate activity induced by p38 mitogen-activated protein kinase in primary afferent neurons contributes to sustaining postincisional nociception. Anesthesiology. 2017;126(1):150–162. doi:10.1097/ALN.0000000000001401
13. Chopra P, Kanoje V, Semwal A, Ray A. Therapeutic potential of inhaled p38 mitogen-activated protein kinase inhibitors for inflammatory pulmonary diseases. Expert Opin Investig Drugs. 2008;17(10):1411–1425. doi:10.1517/13543784.17.10.1411
14. Canovas B, Nebreda AR. Diversity and versatility of p38 kinase signalling in health and disease. Nat Rev mol Cell Biol. 2021;22(5):346–366. doi:10.1038/s41580-020-00322-w
15. Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773(8):1358–1375. doi:10.1016/j.bbamcr.2007.03.010
16. Korb A, Tohidast‐Akrad M, Cetin E, Axmann R, Smolen J, Schett G. Differential tissue expression and activation of p38 MAPK α, β, γ, and δ isoforms in rheumatoid arthritis. Arthritis Rheum. 2006;54(9):2745–2756. doi:10.1002/art.22080
17. Svensson CI, Fitzsimmons B, Azizi S, Powell HC, Hua XY, Yaksh TL. Spinal p38β isoform mediates tissue injury‐induced hyperalgesia and spinal sensitization. J Neurochem. 2005;92(6):1508–1520. doi:10.1111/j.1471-4159.2004.02996.x
18. Luo X, Fitzsimmons B, Mohan A, et al. Intrathecal administration of antisense oligonucleotide against p38α but not p38β MAP kinase isoform reduces neuropathic and postoperative pain and TLR4-induced pain in male mice. Brain Behav Immun. 2018;72:34–44. doi:10.1016/j.bbi.2017.11.007
19. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi:10.1016/0304-3959(83)90201-4
20. Ding C. Drug evaluation: VX-702, a MAP kinase inhibitor for rheumatoid arthritis and acute coronary syndrome. Curr Opin Invest Drugs. 2006;7(11):1020–1025.
21. Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64(3):493–502. doi:10.1016/0304-3959(95)01441-1
22. Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36(1):57–68. doi:10.1016/S0896-6273(02)00908-X
23. Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23(7):2517–2521. doi:10.1523/JNEUROSCI.23-07-02517.2003
24. Xu JT, Xin WJ, Wei XH, et al. p38 activation in uninjured primary afferent neurons and in spinal microglia contributes to the development of neuropathic pain induced by selective motor fiber injury. Exp Neurol. 2007;204(1):355–365. doi:10.1016/j.expneurol.2006.11.016
25. Horii Y, Matsuda M, Takemura H, Ishikawa D, Sawa T, Amaya F. Spinal and peripheral mechanisms individually lead to the development of remifentanil-induced hyperalgesia. Neuroscience. 2020;446:28–42. doi:10.1016/j.neuroscience.2020.08.014
26. Mizushima T, Obata K, Yamanaka H, et al. Activation of p38 MAPK in primary afferent neurons by noxious stimulation and its involvement in the development of thermal hyperalgesia. Pain. 2005;113(1–2):51–60. doi:10.1016/j.pain.2004.09.038
27. Yamamoto K, Chiba N, Chiba T, et al. Transient receptor potential ankyrin 1 that is induced in dorsal root ganglion neurons contributes to acute cold hypersensitivity after oxaliplatin administration. Mol Pain. 2015;11:11. doi:10.1186/s12990-015-0010-9
28. Wang Z, Chabot JG, Quirion R. On the possible role of ERK, p38 and CaMKII in the regulation of CGRP expression in morphine-tolerant rats. Mol Pain. 2011;7:68. doi:10.1186/1744-8069-7-68
29. Jin X, RWt G. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26(1):246–255. doi:10.1523/JNEUROSCI.3858-05.2006
30. Hudmon A, Choi JS, Tyrrell L, et al. Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci. 2008;28(12):3190–3201. doi:10.1523/JNEUROSCI.4403-07.2008
31. Qin JZ, Xin H, Qi XM, Chen G. Isoform-specific and cell/tissue-dependent effects of p38 MAPKs in regulating inflammation and inflammation-associated oncogenesis. Front Biosci. 2022;27(1):31. doi:10.31083/j.fbl2701031
32. Lechner C, Zahalka MA, Giot JF, Møller NP, Ullrich A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc Natl Acad Sci. 1996;93(9):4355–4359. doi:10.1073/pnas.93.9.4355
33. Goedert M. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J. 1997;16(12):3563–3571. doi:10.1093/emboj/16.12.3563
34. Han Y, Wang J, Zhang J, et al. VX-702 ameliorates the severity of sepsis-associated acute kidney injury by downregulating inflammatory factors in macrophages. J Inflamm Res. 2024;17:4037–4054. doi:10.2147/JIR.S464018
35. Damjanov N, Kauffman RS, Spencer‐Green GT. Efficacy, pharmacodynamics, and safety of VX‐702, a novel p38 MAPK inhibitor, in rheumatoid arthritis: results of two randomized, double‐blind, placebo‐controlled clinical studies. Arthritis Rheum. 2009;60(5):1232–1241. doi:10.1002/art.24485
36. Singh D, Smyth L, Borrill Z, Sweeney L, Tal-Singer R. A randomized, placebo-controlled study of the effects of the p38 MAPK inhibitor SB-681323 on blood biomarkers of inflammation in COPD patients. J Clin Pharmacol. 2010;50(1):94–100. doi:10.1177/0091270009347873
37. Christie JD, Vaslef S, Chang PK, et al. A randomized dose-escalation study of the safety and anti-inflammatory activity of the p38 mitogen-activated protein kinase inhibitor dilmapimod in severe trauma subjects at risk for acute respiratory distress syndrome. Crit Care Med. 2015;43(9):1859–1869. doi:10.1097/CCM.0000000000001132
38. Anand P, Shenoy R, Palmer JE, et al. Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury. Eur J Pain. 2011;15(10):1040–1048. doi:10.1016/j.ejpain.2011.04.005
39. Han J, Wu J, Silke J. An overview of mammalian p38 mitogen-activated protein kinases, central regulators of cell stress and receptor signaling. F1000Research. 2020;9:653. doi:10.12688/f1000research.22092.1
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.