Back to Journals » Drug Design, Development and Therapy » Volume 19
Crop Wild Relatives (CWRs) in the United Arab Emirates: Resources for Climate Resilience and Their Potential Medicinal Applications
Authors Manoharan R, Nair CS, Nishanth D, Subramanian R, Xie X, Ren M, Jaleel A
Received 4 October 2024
Accepted for publication 5 February 2025
Published 3 March 2025 Volume 2025:19 Pages 1515—1525
DOI https://doi.org/10.2147/DDDT.S497800
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Muzammal Hussain
Ramya Manoharan,1 Chythra Somanathan Nair,1 Drishya Nishanth,1 Radhakrishnan Subramanian,1 Xiulan Xie,2 Maozhi Ren,2 Abdul Jaleel1
1Department of Integrative Agriculture, College of Agriculture and Veterinary Medicine, United Arab Emirates University, Al Ain, United Arab Emirates; 2Laboratory of Space Biology, Institute of Urban Agriculture, Chinese Academy of Agricultural Sciences, Chengdu, People’s Republic of China
Correspondence: Abdul Jaleel, Department of Integrative Agriculture, College of Agriculture and Veterinary Medicine, United Arab Emirates University, Al Ain, United Arab Emirates, Email [email protected]
Abstract: Global climate change threatens the production, growth, and sustainability of plants. Crop wild relatives (CWRs) offer a practical and sustainable solution to these climatic issues by boosting genetic diversity and crop resilience. Even though CWRs are wild relatives of domesticated plants, they are nevertheless mostly neglected. This review focuses on the possible application of CWRs, which are found in the United Arab Emirates (UAE) and are known for their abiotic stress tolerance and potential medicinal properties. In olden days, traditionally, CWRs has been used as medicine for various ailments as they are rich in phytochemical compounds. However, the medicinal potential of these wild plant species is decreasing at an alarming rate due to climate change stress factors. The medicinal potential of these native crop wild plant species must be investigated because they could be a useful asset in the healthcare sector. Research on pangenomics studies of certain CWRs is also highlighted in the review, which reveals genetic variability caused due to climate change stress factors and how these genetic variability changes affect the production of secondary metabolites that have potent medicinal value. This provides insights into developing personalized medicine, in which particular CWRs plant species can be chosen or modified to generate medicinal compounds. Despite their superior medicinal properties, many CWRs in the UAE are still not well understood. Finding the desired genes coding for the biosynthesis of specific phytochemicals or secondary metabolites may help us better understand how these substances are synthesized and how to increase their production for a range of treatments.
Keywords: crop wild relatives, CWRs, genetic resource, phytochemicals, pangenomics, medicinal applications
Introduction
Crop wild relatives (CWRs) encompass wild crop species that share genetic connections with economically significant crops that are essential for food, forage, medicinal, ornamental, forestry, oil, and fiber production.1 These untamed relatives play a pivotal role in enhancing agricultural production and sustaining eco-friendly agro-ecosystems. They serve as a vital reservoir of genes conferring resistance to biotic and abiotic factors such as pests, diseases, drought, and extreme temperatures. Significantly, the integration of genes from wild relatives is a common practice in the creation of modern varieties for many major crops. Crop wild relatives also enhance the nutritional profile in domesticated plants.2 In one of the studies, researchers have identified CWRs of wheat, fruits and condiments with potential medicinal properties.3 Comparative studies in Egypt have highlighted variations in phytochemical properties between cultivated plants and their wild relatives, with certain wild species demonstrating greater nutrient density and antioxidant activity.4 Crop wild relative species also provide essential resources such as animal feed and building materials. As climate change and ecosystem instability intensify, CWRs will likely become vital in ensuring future food security.5
On a global scale, utilization of CWRs in breeding programs has demonstrated significant economic benefits for the agriculture sector. In 2020, annual economic contribution of CWRs to the global economy was roughly estimated to be more than $150 billion.6,7 Studies suggest that, since 1945, incorporation of CWRs in crop breeding improved the crop yields by around 30%, that is an estimated US $100 billion worldwide.8,9 For example, in tomato plants, integrating genes from a wild crop species resulted in a 2.4% rise in its solids content, contributing to an annual value of $250 million. Similarly, genes of three wild peanut varieties showed enhanced resistance to root-knot nematodes, resulting in annual global savings of approximately $100 million.10 These research studies of using wild crop species genes in breeding programs highlight its significant economic benefits. Despite the invaluable potential of CWRs to enhance some crop varieties in breeding programs, their conservation, both in their natural habitats (in situ) and outside their natural habitats (ex situ), has been neglected for many years, placing them at risk of extinction.
Studies have been conducted to estimate the loss of CWR diversity and its conservation strategies. In the United States, conservation data for 600 CWRs indicate that 7% are critically endangered in their natural habitats, 50% are endangered, 28% are vulnerable, 11% are near-threatened, and 3% are least concern.11 Another study revealed a significant underrepresentation of their diversity in gene banks. In total, 29% lack germplasm accessions, and for 23% there are only fewer accessions available.12 A conservation study in Italyfound that 23 CWRs out of 29 species lack a gene pool. Additionally, insufficient data is available regarding their ex-situ and in-situ conservation. Out of the identified species, 16 were deemed high priority for ex-situ conservation, while 22 were prioritized for in-situ conservation efforts.13 In the Arabian Peninsula, excessive grazing by goats and camels poses a significant threat to plants, including crop wild relatives. As CWRs grow in a wild environment without any protective measures, they are susceptible to depletion. This issue is especially pronounced in interior deserts, where the breakdown of traditional pastoral practices often leads livestock to venture into the desert areas.14,15 The Arabian Peninsula faces additional challenges from the global warming phenomenon, both physically and biologically. Extreme temperature, rainfall, frequent floods and drought are some of the stress factors that cause sand dunes formation, encroachment of sand and dust storms and intensify the loss of plant diversity.16,17 These factors could lead to the extinction of CWR species that holds significant agronomic and medicinal value. Therefore, it is essential to conserve, study and utilize the crop wild relative species for the future. This review paper highlights the CWRs found in the United Arab Emirates that possess important medicinal properties.
CWRs in the United Arab Emirates (UAE)
The climatic condition of UAE experiences very less rainfall and during the summer season, the daytime temperatures can exceed even 50 degrees. This extreme climatic condition is reflected in the soil’s fertility that are very poor, but rich in other minerals like gypsum and lime. Due to extreme heat, the organic matter content in the soil is very low (less than 1%), limiting the improvement of physical properties required for healthy plant growth. Additionally, the high calcium carbonate levels in the soil create high buffering capacity and challenges its fertility causing unavailability of phosphorus and certain micronutrients. CWRs plant species in this UAE region might possess extreme adaptations to these climatic conditions which are essential in increasing the agricultural productivity. These wild plant species possess a global significance due to their ability to withstand challenges like extreme heat, drought and salinity. This makes them crucial for utilization of CWRs in a sustainable way.14 Crop wild relatives contain numerous abiotic stress tolerance genes that conferred resistance to many climate change stress factors such as heat, salinity and cold,18 serving as a reservoir of alleles with substantial agronomic significance. CWRs plant species exhibit greater genetic and phenotypic diversity compared to domesticated species. CWRs provides breeders an extensive gene pool that proves to be a valuable resource. Furthermore, this resource is expected to expand the genetic foundation of cultivated varieties by incorporating economically crucial genes, essential for addressing the demands posed by food crises and climate change.19 In this comprehensive review, we have compiled information on the CWRs found in the United Arab Emirates (shown in Figure 1 and Table 1)20 and their potential utilization for medicinal applications and plant abiotic stress tolerance. A total of 87 crop wild relative species belonging to various families like Leguminosae, Gramineae, and Cucurbitaceae have been reported in the UAE.
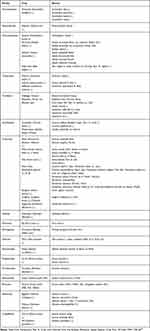 |
Table 1 Crop Wild Relatives (CWRs) and Species in the United Arab Emirates |
 |
Figure 1 Number of CWRs species reported in the United Arab Emirates (UAE). |
Multiple studies have demonstrated CWRs as an important source of tolerance to abiotic stress and withstanding global climatic changes. Within the parameter of shifting climate strategies, drought has emerged as a key factor hampering crop output. Despite having various tolerance genes or quantitative trait loci (QTLs) singled out among the tomato’s wild relatives, their performance has not reached the required targets.21 It has also been observed that, in tomato breeding programs for climate change resilience, the utilization of CWRs significantly increases the chance of high adaptive trait diversity.22 The CWR plant species “Solanum pennellii” utilizes water more efficiently in drought soil under a unique and distinct dryland adaptation mechanism. On the other hand, salt tolerance in coastal areas was observed in both “Solanum cheesmanii” and “Solanum peruvianum” due to an adaptation mechanism that was developed by the intricate root system.23 As for the salt stress tomatoes, some CWRs such as S. pennellii and S. pimpinellifolium show a level of tolerance but the rest suffer as a result of diminished and in some cases drastic reductions to their productivity. With the recent advancements in technology, especially gene editing and sequencing,24 it was discovered that S. pimpinellifolium also possess F1 resistant traits, genes that exhibit tolerance to salinity and other abiotic stress on the root system. In tomatoes, the germination process has shown several QTLs, with some solely linked to S. pimpinellifolium and S. pennellii ability to resist salinity.25,26 Moreover, at vegetative and reproductive growth stages, numerous QTLs from S. pimpinellifolium have been documented specific to the fruit number, fruit weight, and fruit yield.27,28 It also analyzed the major associated genes with their QTLs and those genes could potentially be transferred into cultivated tomato genotypes suitable for salt stress tolerance breeding.29 Cold stress results in a low water and nutrient absorption in plants, causing the cells to be starving for nutrients. On the other hand, heat stress causes radical changes in the physiology and metabolism of the plants.30 It has been suggested that plants employ tolerance mechanisms against chilling conditions by regulating gene expression of oxygen-free radicals scavenging protective proteins against the cold stress.31 A major enzyme catalase has a scavenging role of ROS, which is important as it detoxifies hydrogen peroxide in the cell cytoplasm.32
A third of the world’s principal agricultural production is made up of legume plants, which are also important sources of food for both humans and animals.33 The most well-known legume fodder, Medicago sativa, or alfalfa, is grown on 32 million hectares of land worldwide in more than 80 nations.34 Given the focus on characteristics linked to high production, the extended domestication of alfalfa plants may have resulted in a decreased tolerance for severe abiotic and biotic stressors. The secret to accelerating Medicago sativa breeding is to use genetic variations associated with agronomic traits found in wild cousins that are closely linked to cultivated Medicago.35 Thus, genomic information derived from wild species provides important information for improving traits linked to stress tolerance in bean feeds. Access to genetic resources rich in alleles adapted to harsh conditions is essential for creating alfalfa varieties with increased resistance to environmental difficulties. Wild species in the genus Medicago, such as Medicago falcata, Medicago ruthenica,36 Medicago polymorpha,37 and Medicago truncatula (a model plant for legumes),38 are closely related to M. sativa.39 M. ruthenica most likely evolved strong defenses against harsh environmental factors such as saline soil, dryness, and subfreezing temperatures. Among the species of Medicago, M. ruthenica is a species that is relatively rare and highly adapted to severe conditions. M. ruthenica’s potential usefulness in these settings is assessed favorably. The exact functions of abiotic stress-related genes, such as those from M. ruthenica’s AP2/ERF family, MYB/MYB-related family, bZIP, bHLH and WRKY, in granting stress resistance are still unclear, though.40,41 These genes are essential for a number of regulatory processes that react to different abiotic stressors.
Many studies have shown that factors responsible for transcription, like bZIP, AP2/ERF, and WRKY, play crucial roles in transmitting the ABA signal and orchestrating plant’s stress responses through their interactions.42 Being close relative with alfalfa, M. ruthenica shares similarities with alfalfa in terms of genome size, life cycle and pollination system, and is additionally a perennial species. Notably, it is a wild species with several accessions that grows extensively in dry and/or semi-arid areas and exhibits a high level of drought stress resistance. This trait has made it a significant source of parental material for climate-resilient alfalfa breeding, which in turn improves alfalfa’s resistance to unfavorable environments.43,44 Studies revealed that, among the legume species tested, seedlings of M. ruthenica exhibited the highest drought resistance. The ability of M. ruthenica to withstand drought was further demonstrated when it was found that, in contrast to other legume forages, M. ruthenica seedlings were mostly unaffected by the same treatment. By using molecular breeding techniques, it is possible to improve agronomic features linked to high yield and exceptional resistance to environmental stress by comprehending the genome of M. ruthenica and discovering its resistance genes.36,43
In comparison to their wild equivalent, Vigna, cultivated variants of the Vigna species usually known as cowpeas have shown heightened vulnerability to drought. Notably, V. hainiana and V. stipulacea are renowned for their ability to withstand high temperatures, whilst V. kirkii and V. heterophylla show heightened degrees of drought resistance.45 Furthermore, because of their gene expression linked to proline and ABA biosynthesis, V. trilobata, V. exilis, and V. riukiensis have been found to be drought-resistant.46 Mechanisms like antioxidant capacity, the presence of tiny leaves that improve heat dissipation, and leaf hairiness are thought to be responsible for the wild Vigna species’ resistance to heat and drought.47 Vigna riukiuensis is unique in that it can endure high temperatures because of its vast root system and small leaves.48–50 Quantitative trait loci (QTLs) associated with salt resistance were identified in V. marina ssp. oblonga, a type known for its resilience to salinity. These findings present an opportunity for integrating salt tolerance traits into cultivated cowpea varieties.51 Given the significance of wild Vigna relatives in the enhancement of cultivated Vigna,52,53 the ongoing efforts in crossbreeding are more crucial. More research should be done on the varied genetic composition of wild cowpea germplasm,54 since it is an important source of abiotic stress resistance mechanisms.55 Numerous studies have been conducted on CWRs for agronomic purposes, but research on their medicinal properties remains limited.
Medicinal Properties of CWRs in the UAE
CWRs are valued not only for their agricultural significance, but also possess invaluable medicinal properties. Naturally many CWRs contain phytochemicals, with medicinal properties, including antioxidants, antimicrobials, anti-inflammatory and anti-cancer activities.20 Exploring plant extracts from CWRs could unveil their pharmaceutical promise. In some regions, local communities have traditional knowledge about CWRs and their medicinal properties. They were being used for treating skin disorders, wounds, burns, bruises, cold, cough, swollen joints, fertility, childbirth, post and prenatal care.56 However, in the UAE, research into CWRs and their medicinal attributes remains limited. With their genetic diversity, CWRs contains novel compounds for drug discovery. Researchers can isolate compounds from CWRs in the UAE to unlock potential for developing new drugs aimed at combating diseases. Previous research studies have shown the medicinal potential of some species of CWRs found in the UAE as represented in Table 2. Pangenomics to study the medicinal property of CWRs.
 |
Table 2 Medicinal Properties of CWRs in the United Arab Emirates (UAE) |
Pangenomic studies are highly valuable to study the CWRs or wild relatives of domesticated plants with medicinal properties. Pangenomic studies help to understand genetic variations and how different plant varieties produce various medicinal compounds. Pangenome represents the entire set of genes of any given plant species. All plants species share the core gene component, whereas the dispensable or variable gene component is only present in some species as represented in Figure 2. Pangenomic studies have been conducted on CWRs to enhance agronomic traits in agriculture.6 However, such studies focusing on the medicinal applications of CWRs remain limited. Pangenomic research offers valuable opportunities to uncover genetic diversity that has been lost over time. By exploring CWRs, it may be possible to identify and recover medicinal properties diminished due to climate change and associated stress factors. These studies can uncover how plants adapt to varying environmental conditions, which may impact the production of secondary metabolites, the compounds typically responsible for their medicinal properties. Cannabis sativa is a globally significant drug producing plants. The pangenomic study of cannabis plant has been constructed with 181 new and 12 old genomes. High levels of genetic and structural variation with a single cannabis species has been discovered. There are acyl-lipid thioesterase (ALT) gene variants linked to fatty acid chain length variation and the synthesis of the uncommon cannabinoids cannabidiol varin (CBDV) and tetrahydrocannabinol varin (THCV). Cannabis contains over 100 chemical compounds known as ‘cannabinoids’ with therapeutic effects that can be used for medical conditions such as cancer, inflammatory rheumatoid arthritis, epilepsy, headache, migraine, chronic pain and dermatitis.79 Another study focused on plants of the Panax genus, known for producing ginsenosides – secondary metabolites with significant medicinal value. The content and composition of ginsenosides were analyzed across 20 different Panax species. The study reported diverse biological activities associated with the structural and genetic variations of these ginsenosides, across the species highlighting their potential to optimize ginsenosides production for use in treating cancer, diabetes, cardiovascular diseases, and neurological disorders.80 These pangenomics studies are increasingly important as they offer a comprehensive view of the genetic makeup of medicinal plants, enabling more effective use of these plants in traditional and modern medicine.
 |
Figure 2 Medicinal properties of CWRs studied pangenomically. |
Conclusion
CWRs are plant species that shares genetic traits with domesticated crops. CWRs are essential for phytoremediation, soil rehabilitation, sustainable agriculture, and a host of other industrial and medical uses. Although CWRs are widely distributed in the United Arab Emirates, little is known about their amazing therapeutic qualities, which include antibacterial, anticancer, antioxidant, and anti-inflammatory effects. Because of their high genetic variety, CWRs are important resources for enhancing the production of therapeutic compounds. Limited research on CWRs medicinal plants has revealed genetic and structural variations that lead to the increased secondary metabolites production, which are often key medicinal compounds. CWRs contain special phytochemicals and metabolites that have invaluable medicinal properties which are not found in domesticated plants. Investigating these phytochemical compounds produced by CWRs may result in novel therapies. Conserving and investigating CWRs is essential given the escalating threat posed by climate change. Pangenomic studies can assist in finding the genetic changes in CWRs which is due to climate change stress factors. This provides an insight on how CWRs adapt to climate change, and determines how they affect the synthesis of phytochemicals with potential medicinal properties. CWRs plant species containing high levels of medicinal compounds can be chosen by examining the particular genetic variations, leading to more potent treatments. Pangenomic studies on CWRs is becoming more significant as they offer a thorough insight about their genetic composition. This understanding enables more efficient utilization of these plant species (CWRs) in both traditional and modern medicine while promoting the sustainable use of natural resources in the United Arab Emirates.
Funding
Authors received no specific funding for this work. APC was covered by Research and Sponsored Projects Office of United Arab Emirates University.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Heywood V, Casas A, Ford-Lloyd B, Kell S, Maxted N. Conservation and sustainable use of crop wild relatives. Agric Ecosyst Environ. 2007;121(3):245–255. doi:10.1016/j.agee.2006.12.014
2. Khoury CK, Greene SL, Krishnan S, Miller AJ, Moreau T. A road map for conservation, use, and public engagement around North America’s crop wild relatives and wild utilized plants. Crop Sci. 2019;59(6):2302–2307. doi:10.2135/cropsci2019.05.0309
3. Kumar A, Choudhary A, Landol S, Singh N, Singh N. Evaluation of crop wild relatives from Lahaul Valley, Himachal Pradesh, India. J Curr Opin Crop Sci. 2021;2(3):318–339. doi:10.62773/jcocs.v2i3.79
4. Eissa NM, Ibrahim AA, Soliman MI. Comparative studies among some cultivated species and their wild relatives in Egypt using phytochemical screening, nutritive value, antioxidant activity, and GC-MS analysis. Catrina Int J Environ Sci. 2023;28(1):19. doi:10.21608/cat.2023.202700.1167
5. Bioversity International. (2006) Crop wild relatives. Available from: http://www.bioversityinternational.org/nc/publications/publication/issue/crop_wild_relatives.html.
6. Bohra A, Kilian B, Sivasankar S, et al. Reap the crop wild relatives for breeding future crops. Trends Biotechnol. 2022;40(4):412–431. doi:10.1016/j.tibtech.2021.08.009
7. Tyack N, Dempewolf H, Khoury CK. The potential of payment for ecosystem services for crop wild relative conservation. Plants. 2020;9(10):1305. doi:10.3390/plants9101305
8. Pimentel D, Wilson C, McCullum C, et al. Economic and environmental benefits of biodiversity. BioScience. 1997;47(11):747–757. doi:10.2307/1313097
9. Brozynska M, Furtado A, Henry RJ. Genomics of crop wild relatives: expanding the gene pool for crop improvement. Plant Biotechnol J. 2016;14(4):1070–1085. doi:10.1111/pbi.12454
10. Magos Brehm J, Maxted N, Ford-Lloyd BV, Martins-Loução MA. National inventories of crop wild relatives and wild harvested plants: case-study for Portugal. Genet Resour Crop Evol. 2008;55:779–796. doi:10.1007/s10722-007-9283-9
11. Khoury CK, Carver D, Greene SL, et al. Crop wild relatives of the United States require urgent conservation action. Proc Natl Acad Sci U S A. 2020;117(52):33351–33357. doi:10.1073/pnas.2007029117
12. Castañeda-álvarez NP, Khoury CK, Achicanoy HA, et al. Global conservation priorities for crop wild relatives. Nat Plants. 2016;2(4):1–6. doi:10.1038/nplants.2016.22
13. Perrino EV, Wagensommer RP. Crop wild relatives (CWRs) threatened and endemic to Italy: urgent actions for protection and use. Biol. 2022;11(2):193. doi:10.3390/biology11020193
14. Ghazanfar SA, Fisher M, eds.. Vegetation of the Arabian Peninsula. Springer Science & Business Media; 1998.
15. Miller AG, Cope TA, Nyberg JA. Flora of the Arabian Peninsula and Socotra. 1996.
16. Kotwicki V, Al Sulaimani Z. Climates of the Arabian Peninsula—past, present, future. Int J Climate Change Strat Manag. 2009;1(3):297–310. doi:10.1108/17568690910977500
17. Alyafei M, Alzayadneh W, Alyafei MAS. CO2 concentration and UV-B radiation changes the photosynthetic pigments and biochemical concentrations in UAE native plants grown in open top chambers. Innov Agri. 2019;2:e32816. doi:10.25081/ia.2019.v2.1979
18. Quezada-Martinez D, Addo Nyarko CP, Schiessl SV, Mason AS. Using wild relatives and related species to build climate resilience in Brassica crops. Theor Appl Genet. 2021;134(6):1711–1728. doi:10.1007/s00122-021-03793-3
19. Kashyap A, Garg P, Tanwar K, et al. Strategies for utilization of crop wild relatives in plant breeding programs. Theor Appl Genet. 2022;135(12):4151–4167. doi:10.1007/s00122-022-04220-x
20. Kameswara Rao N. Crop wild relatives from the Arabian Peninsula. Genet Resour Crop Evol. 2013;60:1709–1725. doi:10.1007/s10722-013-9972-5
21. Fischer I, Steige KA, Stephan W, Mboup M. Sequence evolution and expression regulation of stress-responsive genes in natural populations of wild tomato. PLoS One. 2013;8(10):e78182. doi:10.1371/journal.pone.0078182
22. Barone A, Chiusano ML, Ercolano MR, Giuliano G, Grandillo S, Frusciante L. Structural and functional genomics of tomato. Int J Plant Genomics. 2008;2008:1–12. doi:10.1155/2008/820274
23. Yadav SS. Crop Wild Relatives and Climate Change. Redden R, Maxted N, Dulloo ME, Guarino L, Smith P, eds.. Hoboken, NJ: Wiley-Blackwell; 2015. doi:10.1002/9781118854396
24. Razali R, Bougouffa S, Morton MJ, et al. The genome sequence of the wild tomato Solanum pimpinellifolium provides insights into salinity tolerance. Front Plant Sci. 2018;9:1402. doi:10.3389/fpls.2018.01402
25. Foolad MR, Chen FQ, Lin GY. RFLP mapping of QTLs conferring salt tolerance during germination in an interspecific cross of tomato. Theor Appl Genet. 1998;97(7–8):1133–1144. doi:10.1007/s001220051002
26. Foolad MR, Subbiah P, Zhang L. Common QTL affect the rate of tomato seed germination under different stress and nonstress conditions. Int J Plant Genomics. 2008;2007. doi:10.1155/2007/97386.
27. Breto MP, Aśins MJ, Carbonell EA. Salt tolerance in Lycopersicon species. III. Detection of quantitative trait loci by means of molecular markers. Theor Appl Genet. 1994;88(3–4):395–401. doi:10.1007/BF00223650
28. Foolad MR, Lin GY, Chen FQ. Comparison of QTLs for seed germination under non-stress, cold stress, and salt stress in tomato. Plant Breed. 1999;118(2):167–173. doi:10.1046/j.1439-0523.1999.118002167.x
29. Kashyap SP, Kumari N, Mishra P, Moharana DP, Aamir M. Tapping the potential of Solanum lycopersicum L. pertaining to salinity tolerance: perspectives and challenges. Genet Resour Crop Evol. 2021;68(8):2207–2233. doi:10.1007/s10722-021-01174-9
30. Chaudhry S, Sidhu GP. Climate change regulated abiotic stress mechanisms in plants: a comprehensive review. Plant Cell Rep. 2022;41(1):1–31. doi:10.1007/s00299-021-02759-5
31. Elkelish A, Qari SH, Mazrou YS, et al. Exogenous ascorbic acid induced chilling tolerance in tomato plants through modulating metabolism, osmolytes, antioxidants, and transcriptional regulation of catalase and heat shock proteins. Plants. 2020;9(4):431. doi:10.3390/plants9040431
32. Anjum SA, Tanveer M, Hussain S, et al. Cadmium toxicity in maize (Zea mays L.): consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ Sci Pollut Res. 2015;22(20):17022–17030. doi:10.1007/s11356-015-4882-z
33. Debouza NE, Ksiksi T. The impact of elevated temperatures and CO2 on seed germination and early plant morphology: the case of native Fabaceae plants in the UAE. Innov Agri. 2024;7:1–9. doi:10.3897/ia.2024.135233
34. Bouton JH. Breeding lucerne for persistence. Crop Pasture Sci. 2012;63(2):95–106. doi:10.1071/CP12009
35. Zhang XX, Ren XL, Qi XT, et al. Evolution of the CBL and CIPK gene families in Medicago: genome-wide characterization, pervasive duplication, and expression pattern under salt and drought stress. BMC Plant Biol. 2022;22(1):1–6. doi:10.1186/s12870-022-03884-3
36. Wang T, Ren L, Li C, et al. The genome of a wild Medicago species provides insights into the tolerant mechanisms of legume forage to environmental stress. BMC Biol. 2021;19(1):1–7. doi:10.1186/s12915-021-01033-0
37. Small E, Jomphe M. A synopsis of the genus Medicago (Leguminosae). Can J Bot. 1989;67(11):3260–3294. doi:10.1139/b89-405
38. Young ND, Debellé F, Oldroyd GE, et al. The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature. 2011;480(7378):520–524. doi:10.1038/nature10625
39. Jenczewski E, Prosperi JM, Ronfort JL. Evidence for gene flow between wild and cultivated Medicago sativa (Leguminosae) based on allozyme markers and quantitative traits. Am J Bot. 1999;86(5):677–687. doi:10.2307/2656577
40. Wu R, Xu B, MrERF SF. MrbZIP, and MrSURNod of Medicago ruthenica are involved in plant growth and abiotic stress response. Front Plant Sci. 2022;13:907674. doi:10.3389/fpls.2022.907674
41. Quan W, Liu X, Wang L, et al. Ectopic expression of Medicago truncatula homeodomain finger protein, MtPHD6, enhances drought tolerance in Arabidopsis. BMC Genomics. 2019;20(1):1–6. doi:10.1186/s12864-019-6350-5
42. Stagnari F, Maggio A, Galieni A, Pisante M. Multiple benefits of legumes for agriculture sustainability: an overview. Chem Biol Technol Agric. 2017;4(1):1–3. doi:10.1186/s40538-016-0085-1
43. Yin M, Zhang S, Du X, et al. Genomic analysis of Medicago ruthenica provides insights into its tolerance to abiotic stress and demographic history. Mol Ecol Resour. 2021;21(5):1641–1657. doi:10.1111/1755-0998.13363
44. Campbell J, Pecaut M, Luttges M. Prevalence and arrangement of lignified vascular elements in 6-day-old alfalfa (Medicago sativa L.) seedlings raised in reduced gravity. J Plant Physiol. 1996;149(5):539–547. doi:10.1016/S0176-1617(96)80331-2
45. Van Zonneveld M, Rakha M, Tan SY, et al. Mapping patterns of abiotic and biotic stress resilience uncovers conservation gaps and breeding potential of Vigna wild relatives. Sci Rep. 2020;10(1):2111. doi:10.1038/s41598-020-58646-8
46. Naito K, Wakatake T, Shibata TF, et al. Genome sequence of 12 Vigna species as a knowledge base of stress tolerance and resistance. bioRxiv. 2022. doi:10.1101/2022.03.28.486085
47. Iseki K, Takahashi Y, Muto C, Naito K, Tomooka N. Diversity of drought tolerance in the genus Vigna. Front Plant Sci. 2018;9:729. doi:10.3389/fpls.2018.00729
48. Yoshida Y, Marubodee R, Ogiso-Tanaka E, et al. Salt tolerance in wild relatives of adzuki bean, Vigna angularis (Willd.) Ohwi et Ohashi. Genet Resour Crop Evol. 2016;63:627–637. doi:10.1007/s10722-015-0272-0
49. Iseki K, Takahashi Y, Muto C, Naito K, Tomooka N. Diversity and evolution of salt tolerance in the genus Vigna. PLoS One. 2016;11(10):e0164711. doi:10.1371/journal.pone.0164711
50. Tomooka N, Naito K, Kaga A, et al. Evolution, domestication and neo-domestication of the genus Vigna. Plant Genet Resour. 2014;12(S1):S168–S171. doi:10.1017/S1479262114000483
51. Chankaew S, Isemura T, Naito K, et al. QTL mapping for salt tolerance and domestication-related traits in Vigna marina subsp. oblonga, a halophytic species. Theor Appl Genet. 2014;127:691–702. doi:10.1007/s00122-013-2251-1
52. Takahashi Y, Somta P, Muto C, et al. Novel genetic resources in the genus Vigna unveiled from gene bank accessions. PLoS One. 2016;11(1):e0147568. doi:10.1371/journal.pone.0147568
53. Abady S, Shimelis H, Janila P, et al. Assessment of the genetic diversity and population structure of groundnut germplasm collections using phenotypic traits and SNP markers: implications for drought tolerance breeding. PLoS One. 2021;16(11):e0259883. doi:10.1371/journal.pone.0259883
54. Harouna DV, Venkataramana PB, Matemu AO, Ndakidemi PA. Agro-morphological exploration of some unexplored wild Vigna legumes for domestication. Agronomy. 2020;10(1):111. doi:10.3390/agronomy10010111
55. Amorim LL, Ferreira-Neto JR, Bezerra-Neto JP, et al. Cowpea and abiotic stresses: identification of reference genes for transcriptional profiling by qPCR. Plant Methods. 2018;14(1):1–7. doi:10.1186/s13007-018-0354-z
56. Sakkir S, Kabshawi M, Mehairbi M. Medicinal plants diversity and their conservation status in the United Arab Emirates (UAE). J Med Plants Res. 2012;6(7):1304–1322. doi:10.5897/JMPR11.1412
57. Ahmed SA, Hanif S, Iftkhar T. Phytochemical profiling with antioxidant and antimicrobial screening of Amaranthus viridis L. leaf and seed extracts. Open J Med Microbiol. 2013;2013:1–6. doi:10.4236/ojmm.2013.33025
58. Sahib AS, Mohammed IH, Sloo SA. Antigiardial effect of Anethum graveolens aqueous extract in children. J Intercult Ethnopharmacol. 2014;3(3):109–113. doi:10.5455/jice.20140523104104
59. Egamberdieva D, Mamadalieva N, Khodjimatov O, Tiezzi A. Medicinal plants from Chatkal Biosphere Reserve used for folk medicine in Uzbekistan. Med Aromat Plant Sci Biotechnol. 2013;7:56–64.
60. Bouchmaa N, Mrid RB, Kabach I, et al. Beta vulgaris subsp. maritima: a valuable food with high added health benefits. Appl Sci. 2022;12(4):1866. doi:10.3390/app12041866
61. Baban MM, Ahmad SA, Abu-Odeh AM, Baban M, Talib WH. Anticancer, immunomodulatory, and phytochemical screening of Carthamus oxyacantha M.Bieb growing in the North of Iraq. Plants. 2024;13(1):42. doi:10.3390/plants13010042
62. Nawaz I, Tahir A, Iqbal SM, et al. Anti-inflammatory, anti-nociceptive, and anti-pyretic activities of Cenchrus ciliaris L. J Ethnopharmacol. 2023;309:116332. doi:10.1016/j.jep.2023.116332
63. Khurshid S, Javaid A, Shoaib A, Javed S, Qaisar U. Antifungal activity of aerial parts of Cenchrus pennisetiformis against Fusarium oxysporum f. sp. lycopersici Planta Daninha. 2018;36:e018166627. doi:10.1590/S0100-83582018360100023
64. Abdel-Aziz MS, Shaheen MS, El-Nekeety AA, Abdel-Wahhab MA. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J Saudi Chem Soc. 2014;18(4):356–363. doi:10.1016/j.jscs.2013.09.011
65. Janda K, Gutowska I, Geszke-Moritz M, Jakubczyk K. The common cichory (Cichorium intybus L.) as a source of extracts with health-promoting properties—a review. Molecules. 2021;26(6):1814. doi:10.3390/molecules26061814
66. Li QY, Munawar M, Saeed M, et al. Citrullus colocynthis (L.) Schrad (Bitter Apple Fruit): promising traditional uses, pharmacological effects, aspects, and potential applications. Front Pharmacol. 2022;12:791049. doi:10.3389/fphar.2021.791049
67. Ali MM, Elgindi MR, Abd El Kader MA, Mahmoud II, Atia HG. Antioxidant, anti-inflammatory, and analgesic activities of the chloroform extract of Crotalaria aegyptiaca. Bull Fac Pharm Cairo Univ. 2004;42(2):137–145.
68. Choudhary SS, Panigrahi PN, Dhara SK, et al. Cucumis callosus (Rottl.) Cogn. fruit extract ameliorates calcium oxalate urolithiasis in ethylene glycol induced hyperoxaluric rat model. Heliyon. 2023;9(3):e13718. doi:10.1016/j.heliyon.2023.e14043
69. Joshi AK, et al. Ijppr. Hum. 2024;30:189–213.
70. Hassan I, Haq AU, Shah SM, et al. Qualitative phytochemical screening and anti-inflammatory evaluation of aerial part extracts of Medicago laciniata (L.) mill. Mill Phytopharmacol Commun. 2021;1(1):9–16. doi:10.55627/ppc.001.01.0099
71. Zeb A, Ahmad K. Variations of carotenoids, total bioactive contents, and antioxidant activity in leaves of Medicago polymorpha. J Food Process Preserv. 2024;2024:1–7. doi:10.1155/2024/8721888
72. Bashir A, Asif M, Saadullah M, et al. Therapeutic potential of standardized extract of Melilotus indicus (L.) All. and its phytochemicals against skin cancer in animal model: in vitro, in vivo, and in silico studies. ACS Omega. 2022;7(29):25772–25782. doi:10.1021/acsomega.2c03053
73. Singh KP, Daboriya V, Kumar S, Singh S. Antibacterial activity and phytochemical investigations on Nicotiana plumbaginifolia Viv. (wild tobacco). Rom J Biol Plant Biol. 2010;55(2):135–142.
74. Sarwar I, Asif M, Jamshaid T, et al. Phytochemical and biological studies of Panicum antidotale aerial parts ethanol extract supported by molecular docking study. Front Pharmacol. 2024;14:1243742. doi:10.3389/fphar.2023.1243742
75. Jafaar HJ, Isbilen O, Volkan E, Sariyar G. Alkaloid profiling and antimicrobial activities of Papaver glaucum and P. decaisnei. BMC Res Notes. 2021;14:1–7. doi:10.1186/s13104-021-05762-x
76. Janbaz KH, Aslam N, Imran I, Jabeen Q. Evaluation of anti-inflammatory, analgesic and antipyretic activities of Salsola imbricata Forssk in rats. J Anim Plant Sci. 2021;31(3):842–846. doi:10.36899/JAPS.2021.3.0276
77. Shah MA, Khan RA, Ahmed M. Sorghum halepense (L.) Pers rhizomes inhibitory potential against diabetes and free radicals. Clin Phytosci. 2021;7:1–6. doi:10.1186/s40816-021-00259-3
78. Sheweita SA, ElHady SA, Hammoda HM. Trigonella stellata reduced the deleterious effects of diabetes mellitus through alleviation of oxidative stress, antioxidant-and drug-metabolizing enzymes activities. J Ethnopharmacol. 2020;256:112821. doi:10.1016/j.jep.2020.112821
79. Lynch RC, Padgitt-Cobb LK, Garfinkel AR, et al. Domesticated cannabinoid synthases amid a wild mosaic cannabis pangenome. bioRxiv. 2024. doi:10.1101/2024.05.21.595196
80. Hou M, Wang R, Zhao S, Wang Z. Ginsenosides in Panax genus and their biosynthesis. Acta Pharm Sin B. 2021;11(7):1813–1834. doi:10.1016/j.apsb.2020.12.017
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

