Back to Journals » Journal of Inflammation Research » Volume 18
Danshensu Ethyl Ester Alleviates LPS-Induced Acute Lung Injury by Targeting the NLRP3 Inflammasome
Authors Ding W, Xu S, Xie S , Dong Y, Jiang Y, Xie N, Wang P , Feng J , Qu G
Received 25 January 2025
Accepted for publication 19 June 2025
Published 4 July 2025 Volume 2025:18 Pages 8767—8785
DOI https://doi.org/10.2147/JIR.S517701
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Tara Strutt
Wensi Ding,1,2,* Sen Xu,1,* Shuyang Xie,1,3,* Yao Dong,4 Yujie Jiang,4 Ning Xie,5 Pingyu Wang,6 Jiankai Feng,2 Guiwu Qu7
1Department of Biochemistry and Molecular Biology, Binzhou Medical University, Yantai, People’s Republic of China; 2Department of Laboratory Medicine, Yantai Affiliated Hospital of Binzhou Medical University, Yantai, People’s Republic of China; 3Shandong Laboratory of Advanced Materials and Green Manufacturing, Yantai, People’s Republic of China; 4Binzhou Medical University, Yantai, People’s Republic of China; 5Yantaishan Hospital, Yantai, People’s Republic of China; 6Department of Epidemiology, Binzhou Medical University, YanTai, People’s Republic of China; 7Department of Traditional Chinese Medicine, School of Pharmacy, Binzhou Medical University, Yantai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiankai Feng, Department of Laboratory Medicine, Yantai Affiliated Hospital of Binzhou Medical University, No. 717, Jinbu Avenue, Yantai, 264100, People’s Republic of China, Email [email protected] Guiwu Qu, Department of Traditional Chinese Medicine, School of Pharmacy, Binzhou Medical University, No. 346, Guanhai Road, Yantai, 264003, People’s Republic of China, Email [email protected]
Purpose: To investigate whether Danshensu ethyl ester (DEE) can attenuate acute lung injury (ALI) and explore the detailed mechanism.
Methods: The ALI model was induced in mice using LPS. The effects of DEE on lung wet-to-dry weight ratio (W/D), bronchoalveolar lavage fluid (BALF) protein levels, and neutrophil infiltration (neutrophils) were assessed. In addition, molecular docking and molecular dynamics simulations were also carried out to determine the binding situation between DEE and NLRP3. We evaluated in both in vivo and in vitro models the expression of NLRP3-related proteins as well as the release of cytokines. The generation of reactive oxygen species (ROS) and the formation of ASC fluorescent specks in cells were also observed.
Results: The results demonstrated that DEE significantly alleviated pulmonary edema and lung injury of mice. Molecular docking and simulations revealed that DEE directly targets and tightly binds to the NLRP3 protein. Furthermore, both in vivo and in vitro experiments showed that DEE suppressed activation of the NF-κB signaling pathway induced by LPS, and decreased the expression of NLRP3, ASC, and cleaved caspase-1, inhibiting the release of cytokines such as IL-1β, IL-6, and TNF-α. Additionally, DEE suppressed ROS generation and ASC specks formation, thereby inhibiting the assembly and activation of the NLRP3 inflammasome.
Conclusion: DEE exerts an inhibitory influence on the LPS-induced inflammatory response by suppressing the activation of the NLRP3 inflammasome. This study provides the potential application of DEE in NLRP3-driven ALI therapy.
Keywords: acute lung injury ALI, danshensu ethyl ester DEE, lipopolysaccharide LPS, NLRP3 inflammasome, inflammation, neutrophil
Introduction
Acute lung injury (ALI), marked by severe and uncontrolled inflammation in the lungs, can lead to respiratory failure and, in severe instances, even fatality.1,2 ALI can result from a variety of factors, such as infection, inhalation of toxic gases or particles, severe trauma, etc.3 Diffuse alveolar and microvascular injury caused by ALI can lead to edema and restricted gas exchange after hypoxia.1 Its pathological and physiological features include leukocyte infiltration, episodes of pulmonary edema, and impaired oxygenation, even leading to pulmonary fibrosis.4 Due to the complexity of pathogenic factors and pathogenesis of ALI, there is no specific drug for ALI, so it is crucial to investigate the underlying pathogenesis of ALI and develop therapeutic medications.
Danshensu, with cardiovascular protective, anti-inflammatory, and antitumor effects, is the primary water-soluble active ingredient in the commonly used traditional Chinese medicine danshen(Salvia miltiorrhiza Bge).5,6 However, danshensu possesses drawbacks such as chemical instability, susceptibility to oxidation, and discoloration, rendering it unsuitable for clinical application.7 To overcome the instability of danshensu, our laboratory synthesized danshensu methyl ester(DME), which exerts anti-inflammatory and antioxidant properties by inhibiting the TLR4/NF-κB pathway and oxidative stress.8 In order to better study the anti-inflammatory properties of danshensu derivatives, our laboratory further synthesized danshensu ethyl ester (DEE), another ester derivative of danshensu. DEE is characterized by higher chemical stability, stronger liposolubility, and resistance to oxidation, making it easier to cross cell membranes and exert its antibacterial, anti-inflammatory, and immune-enhancing effects. Recent studies have also shown that danshensu and its derivatives have powerful anti-inflammatory effects and play significant roles in improving arthritis, colitis, reducing cerebral ischemia/reperfusion injury, and stabilizing atherosclerotic vulnerable plaques.9–12
The NLRP3 inflammasome, a multiprotein complex, is found to be crucial in the development and pathogenesis of ALI.13 It is composed of the innate immune receptor NLRP3, the adapter ASC (apoptosis-associated speck-like protein containing a CARD), and the effector caspase-1.14 Activating the NLRP3 inflammasome involves a two-stage procedure: priming and activation. Pathogen-associated molecular patterns (PAMPs, eg, LPS) or damage-associated molecular patterns (DAMPs) trigger the activation of NF-κB, upregulating NLRP3 and pro-IL-1β expression (priming step). After that, various stimuli like ATP or Nigericin (Nig) prompt the assembly and complete activation of the NLRP3 inflammasome.15 Once activated, NLRP3 attracts ASC and pro-caspase-1 to form the NLRP3 inflammasome, leading to the activation of cleaved caspase-1.16 This activated cleaved caspase-1 then converts pro-IL-1β and pro-IL-18 into their active forms and releases them.17 These cytokines not only increase the activity of phagocytes, but are also responsible for recruiting other immune cells like macrophages and neutrophils, which subsequently give rise to a more intense inflammatory response.18 Abnormal activation of the NLRP3 inflammasome may play a role in the development and worsening of many chronic inflammatory conditions.19 Such as gout,20 sepsis,21 chronic liver disease,22 atherosclerosis,23 and type 2 diabetes.24 Recent research suggests that NLRP3 inflammasome activation can result in excessive production of IL-1β, which intensifies the severity of ALI.25
Given the crucial role of the NLRP3 inflammasome in the occurrence and development of ALI, and the impact of DEE on the NLRP3 inflammasome in ALI remains unclear, this study focuses on exploring the mechanism of DEE in ameliorating the LPS-induced mouse model of ALI and interaction with NLRP3. Results showed that DEE exerts an inhibitory influence on the LPS-induced inflammatory response by suppressing the activation of the NLRP3 inflammasome.
Materials and Methods
Synthesis and Structural Analysis of DEE
The synthesis steps of DEE include alkaline degradation treatment of traditional Chinese medicine Danshen (Salvia miltiorrhiza Bge.,Beijing Tongrentang Co.,Ltd., Beijing, China), which degrades salvianolic acid B in Danshen into danshensu; Chromatography of degradation solution combined with salinization and crystallization to obtain pure Sodium danshensu; Esterification of Sodium danshensu by reacting with ethanol to form DEE under the catalysis of acid. The specific operation steps are as follows:
Danshen was crushed into coarse powder and decocted in a mass of 0.4% sodium hydroxide solution for 2h. The pH was adjusted to 2–3 using hydrochloric acid, and then filtered. The filtrate was separated by chromatography using AB-8 macroporous adsorption resin as the medium. The elution steps are as follows: First, wash away the impurities with acidic water (pH = 2), then rinse with pure water and collect the solution to obtain the danshensu fraction. After the danshensu solution is concentrated under reduced pressure to an appropriate volume, we add activated carbon, stir and filter. Adjust the pH value to 6.0–6.5 with NaOH, and store the crystals at 0–5°C. The crystals were rinsed with an 80% ethanol solution for decolorization. Recrystallize using the same method, and dried under reduced pressure to obtain colorless sodium danshensu crystals.
About 10g sodium danshensu was dissolved in absolute alcohol, and added hydrochloric acid as a catalyst at 60°C for 4 h. Upon completion of the reaction, the methanol and hydrochloric acid were removed. Dissolve the residue in ethyl acetate and wash with water. High Performance Liquid Chromatography(HPLC) was used to monitor the reaction process and detect product purity:C18 reverse phase chromatography column, detection wavelength of 286nm, mobile phase of 2% formic acid (A) – acetonitrile (B). The gradient elution program: 0–15 min, 10% B → 100% B; 15–20 min, 100% B; 20–25 min, 100% B → 10% B; 25–30 min, 10% B. Subsequently, the obtained sample was dissolved in DMSO-d6 for nuclear magnetic resonance (NMR)(Bruker Advance Neo 600, Billerica, MA, USA) analysis.
Animals
Female (C57BL/6) mice, aged 6 to 8 weeks and weighing between 18 and 24 grams, were acquired from Jinan Pengyue Laboratory Animal Breeding Co., Ltd. The mice were kept in a pathogen-free laminar flow hood environment, which had ventilation and temperature control (22–26°C), relative humidity ranging from 40–80%, and a 12-hour light/dark cycle.
Mouse ALI Model and Intervention
Thirty-six mice of the C57BL/6 were randomly assigned into 6 different groups: Control, ALI, ALI + DEE, and ALI + Dexamethasone (DEX, Solarbio, Beijing, China). In DEE and DEX intervention groups, mice were intraperitoneally injected with different concentrations of DEE(10,20,30mg/kg) or DEX(5mg/kg) and ALI group and control group were given 10mL/kg normal saline. One hour after the injection of DEE or DEX, the mice in the ALI group, ALI+DEE group, and ALI+DEX group received an intratracheal instillation of 0.5 mg/kg of Lipopolysaccharide (LPS, Solarbio, Beijing, China). Control mice were given normal saline administration. After intratracheal infusion of LPS 12 h, mice were injected intraperitoneally with 0.12–0.14mL 1% sodium pentobarbital solution, euthanized under anesthesia, and subsequently bronchoalveolar lavage fluid (BALF) and lung tissue samples were collected.
Cell Culture and Treatment
J774A.1 cells(Cell Bank of the Chinese Academy of Sciences, Shanghai, China) were incubated in DMEM medium (Hyclone, Logan, UT, USA) with 10% fetal bovine serum(AusGenex, Gold Coast, Australia) at 37 °C in a 5% CO2 incubator. To investigate the roles of NLRP3 inflammasome, J774A.1 cells (2× 106 /mL) were seeded in 6-well plates. After being treated with DEE for 1 hour, they were further incubated with LPS (1 μg/mL) for another 3 hours. Then, Nigericin (Nig, 10 μM, InvivoGen, San Diego, CA, USA) was added and incubated for 45 min, and the samples were collected for subsequent experiments.
Histopathological Evaluation
Mouse single-leaf right lung tissues were harvested and placed in 4% paraformaldehyde (Biosharp, Shanghai, China) for a duration of 24 hours. Subsequently, the tissues were dehydrated using alcohol of varying concentrations, embedded in paraffin, and then sectioned. Finally, they were stained using HE staining solution (Beyotime, Shanghai, China).
Lung Wet/Dry (W/D) Ratios
Upon the successful establishment of the model, the W/D ratios were determined by using the entire left lung of the mice. The surface moisture of the lung tissue was wiped dry and immediately weighed to obtain a wet weight. Then the lung tissue was placed in an oven at 65°C for 72 h. Tissue edema was evaluated by measuring the ratio of W/D.
The Detection Method and Related Analysis of Bronchoalveolar Lavage Fluid (BALF)
After mice were anesthetized, the trachea was exposed and injected with 0.8 mL PBS intratracheal. Subsequently, BALF was aspirated repeatedly twice. The protein concentration in untreated BALF was determined using a BCA kit (Beyotime, Shanghai, China). The expressions of inflammatory factors IL-1β, IL-6 and TNF-α in BALF were detected using an ELISA kit (Absin, Shanghai, China). The BALF was then centrifuged at 4°C for 10 minutes at 1300 rpm to obtain the supernatant. Red cell lysate (Solarbio, Beijing, China) was added to the cell precipitate, then centrifuged at 1000rpm, 5min. The total number of cells was calculated using a cell count plate. Wright-Giemsa staining (Solarbio) was used to count neutrophils and macrophages.
Western Blot
The extraction of total protein from cells and around 30–40 mg of right lung tissue was carried out with RIPA (Beyotime, Shanghai,China). Then, the protein samples were separated via 8–12% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and subsequently transferred onto a PVDF membrane(Millipore, Billerica, Massachusetts, USA). The membranes with the desired target bands were immersed in Tris buffer, which consisted of 5% skim milk and 0.05% Tween 20, for a blocking duration of 2 hours. Later, they were incubated with primary antibodies at 4°C throughout the night. Afterwards, they were incubated with the secondary antibody at 4°C for 2 hours. Finally, the proteins were analyzed using enhanced chemiluminescence substrates (Beyotime, Shanghai, China) and were quantified with software (ImageJ, Bethesda, Maryland, USA). The antibodies were as follows: Anti-NLRP3 (1:1000, A5652, ABclonal, San Mateo, CA, USA), Anti-caspase-1 (1:400, 645101, Biolegend, San Diego, CA, USA), Anti-ASC (1:1000, 67824, CST, Danvers MA, USA), Anti-NF-κB p65 (1:1000, 3033S, CST), Anti-phospho-NF-κB p65 (1:1000, 8242S, CST), Anti-β-actin (1:3000, AP0060, Bioworld Nanjing, China).
Immunohistochemistry
The lung tissue sections were dewaxed, immersed in EDTA antigen retrieval buffer at 100°C for 10 minutes, followed by three washes with PBS. They were then incubated with 3% H2O2 for 15 min at room temperature and blocked using a solution (5% BSA, 0.2% Tween-20 in PBS) at 37°C for 30 minutes. After drying, they were incubated overnight with primary antibody NLRP3(1:100) at 4°C. After rinsing with PBS, the tissue sections were treated with the secondary antibody (Biotin-labeled, anti-rabbit). Then, SABC (Boster, Wuhan, China) was added and the mixture was incubated at 37°C for 30 minutes. Then, staining was performed using DAB and hematoxylin(Boster). Images were captured and observed using a microscope(EVOSTM M7000, Thermo Fisher Scientific, Waltham, Massachusetts, USA).
Cell Viability Assay
J774A.1 cells were plated in 96-well plates(1×104/well) and exposed to diverse concentrations of DEE (0, 5, 10, 20, 40 and 80 μg/mL). After 24 hours, 10 μL of CCK-8(Biosharp, China) was added to each well, followed by further incubation at 37°C for 2 hours in a dark setting. Absorbance values were determined at 450nm using a microplate reader(INFINITE M PLEX, Tecan, Männedorf, Zürich, Switzerland).
Real-Time qPCR
RNA was extracted from lung tissues or J774A.1 cells with the Trizol reagent (Vazyme, Nanjing, China). Then, the RNA was reverse transcribed into cDNA with the reverse transcription reagent (Vazyme). SYBR qPCR Master Mix (Vazyme) was used for qPCR analysis. The following thermocycling conditions were performed: pre-denaturation at 95°C for 30 sec; followed by 40 cycles of denaturation at 95°C for 10 sec, annealing at 60°C for 30 sec and extension at 72°C for 30 sec. β-actin served as the reference, and data analysis was conducted applying the 2-ΔΔCT method. The primer sequences are shown in Table 1.
 |
Table 1 Primer Sequences for Real-Time qPCR |
ELISA Detection
Following the treatment of J774A.1 cells with different drugs, the levels of IL-1β, IL-6, and TNF-α released were determined in the cell culture supernatant, according to ELISA kit instructions. Mouse enzyme-linked immunosorbent assay (ELISA) kits for IL-1β (abs520001), IL-6 (abs520004), TNF- α (abs520010) were acquired from Absin (Shanghai, China). Additionally, these inflammatory factors were also measured in the BALF of C57BL/6 mice.
Immunofluorescence Staining
J774A.1 cells were inoculated on sterile slides at a density of 1 × 105/mL and incubated overnight. After drugs treatment, the cells were fixed with paraformaldehyde for 20min at room temperature. The slides were then permeabilized with Triton-100 (0.2%, Biofroxx, Germany) for 10min, and blocked with 1% BSA solution (SolarbiO) for 30min. Cell slides were incubated with rabbit anti-ASC overnight (4°C, 12–14 h) and reacted with donkey anti-rabbit IgG (Alexa Fluor 488-labeled antibody, 1:400, Absin) at 37°C for 2 h. Eventually, the cells were observed with a fluorescence microscope (LEICA Stellaris 5, Leica Microsystems, Wetzlar, Germany).
ROS Content Analysis
The J774A.1 cells were incubated with DEE (5,10,20 μg/mL) or DMSO (D2650, Sigma-Aldrich, St. Louis, Missouri, USA) for 1 h. The cells underwent stimulation with LPS (1 μg/mL) for 3 h and Nig (10 μ M) for another 45min. A solution of DCFH-DA (Beyotime, Shanghai, China) was diluted in serum-free DMEM at a ratio of 1:1000 and subsequently added to the cells. The cells were then incubated in this solution for 20 minutes at a temperature of 37°C. Images were analyzed within 30 minutes using a fluorescence microscope(Thermo Fisher Scientific, Waltham, Massachusetts, USA).
After J774A.1 cells were treated with drugs, the DCFH-DA probe was diluted at a ratio of 1:1000 with serum-free DMEM and incubated at 37°C for 20 min. Subsequently, the cells were collected and washed twice with PBS. Finally, the cells were resuspended in PBS, and the ROS levels were detected at 525 nm using a flow cytometer (Beckman Coulter, Lane Cove West, NSW, Australia).
Molecular Docking
The NLRP3 crystal structure (PDB: 7ALV) was retrieved from the Protein Data Bank (PDB). Protein preparation was carried out by removing water molecules, adding missing atoms, and optimizing the receptor structure. Subsequently, the three-dimensional conformation of the ligand was generated and its geometric structure was optimized. Then, molecular docking was carried out using the software Autodock Vina 1.1.2 (Scripps Research Institute, USA). Visualization analysis was performed using PyMOL (Schrodinger Inc.,USA).
Molecular Dynamics Simulations (MDs)
After molecular docking, Gromacs 2022 was utilized to conduct molecular dynamics (MD) simulations. For the small molecule DEE, the GAFF force field was adopted, while for the protein NLRP3, the AMBER14SB force field and the TIP3P water model were used for modeling. The files of NLRP3 and DEE ligands were combined to build the simulation system of the complex. With the temperature controlled at 298 K, 100 ps of NVT and NPT equilibrium simulations were carried out, and then 100 ns of MD simulations were performed, saving conformations every 10 ps. Post-simulation, the trajectories were analyzed using VMD(University of Illinois at Urbana - Champaign, USA) and PyMOL(Schrodinger Inc.,USA).
Statistical Analysis
Results are expressed as mean ± standard deviation. The differences between groups were assessed using One-way Analysis of Variance (ANOVA). If the ANOVA results indicated significant differences, comparisons between groups were further conducted using Tukey’s Honestly Significant Difference test. A p-value < 0.05 was considered statistically significant. All calculated results were statistically analyzed using GraphPad Prism 9.1 software.
Results
The Preparation and Structural Verification of Danshensu Ethyl Ester
By using HPLC, we obtained 10.3 g of a waxy solid with a purity of 98.57%, and the yield was approximately 100%.(Figure 1A) The obtained sample was dissolved in DMSO-d6 and subjected to NMR analysis. The NMR data are as follows:1H NMR (600 MHz, DMSO) δ 8.70 (s, 1H), 8.62 (s, 1H), 6.62–6.57 (m, 2H), 6.42 (dd, J = 8.0, 2.1 Hz, 1H), 5.41 (d, J = 6.1 Hz, 1H), 4.10 (dt, J = 7.2, 5.7 Hz, 1H), 4.04 (q, J = 7.1 Hz, 2H), 2.73 (dd, J = 13.7, 5.5 Hz, 1H), 2.64 (dd, 1H), 1.14 (t, J = 7.1 Hz, 3H). 13C NMR (151 MHz, DMSO) δ 174.03, 145.22, 144.15, 128.66, 120.48, 117.27, 115.67, 72.16, 60.37, 40.12, 14.55.(Figure 1B–D) Based on the above NMR data, the compound was confirmed to be Danshensu ethyl ester, and its structure was obtained. (Figure 1E)
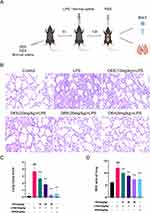
DEE Improved LPS-Induced ALI
After the mouse model was established and samples were collected, lung tissues were embedded and lung histomorphological changes were observed by HE staining. Compared with controls, the alveolar wall was thickened, alveolar space narrowed, inflammatory infiltrating cells and pulmonary hemorrhage increased in ALI mice, indicating the successful production of the ALI model (Figure 2A and B). In contrast to the LPS-induced ALI model group, DEE recovered alveolar structure, reduced pulmonary edema, and significantly improved the damage to the alveolar wall. The higher the concentration of DEE (from 10 to 30 mg/kg), the better improvement effects were achieved, indicating that DEE can effectively reduce LPS-induced ALI. Comparable results were detected in the positive control group (DEX) (Figure 2B–D).
DEE Inhibited the Levels of Inflammation in ALI Mice
BALF was collected and the initial total cells were counted using a cell counting plate. The quantification of neutrophils and macrophages was carried out by means of Wright-Giemsa staining. Compared with the control group, LPS stimulation induced an increase in protein content and a rise in the total cell count, including neutrophils and macrophages in the BALF of the ALI model. Whereas, both the protein content and the overall cell count, which encompassed neutrophils and macrophages, were decreased in the DEE or DEX-treated groups (Figure 3A–D). These results indicate that DEE has an anti-inflammatory role as DEX does in treatment of ALI mice.
Subsequently, the alterations in the expression of principal inflammatory markers, namely IL-1β, IL-6 and TNF-α, were inspected to explore the mechanism of DEE ameliorating ALI of mice. The results indicated elevated levels of IL-1β, TNF-α, and IL-6 in the BALF of model mice treated with LPS in comparison to those of healthy control mice. Compared with the LPS-induced ALI model group, both DEE and the positive drug DEX significantly reduced the levels of these inflammatory factors in the BALF (Figure 3E–G). Moreover, with the elevation of DEE concentration, the levels of inflammatory factors declined progressively, suggesting that DEE possesses a remarkable anti-inflammatory effect and displays a dose-dependent pattern.
Molecular Docking Study and Molecular Dynamics
Danshensu and its derivatives can alleviate osteoarthritis and improve colitis by suppressing NLRP3 inflammasome activation.9,10 We next explored whether DEE could inhibit the inflammatory response in ALI mice through NLRP3 inflammasome. Firstly, we hypothesized that DEE might bind to the NLRP3 protein. To confirm this hypothesis, we carried out molecular docking. The possible binding mode of DEE with NLRP3 was demonstrated using molecular docking methods.(Figure 4A) Then we performed MD to determine the potential binding mode of the small molecule with NLRP3. The Root Mean Square Deviation (RMSD) analysis results showed that the complex structure fluctuated synchronously with the protein, indicating that the RMSD fluctuation of the complex was caused by the protein itself and the complex structure gradually stabilized.(Figure 4B) At the same time, we analyzed the number of hydrogen bonds generated within 100 ns. The number of hydrogen bonds fluctuated between 2 and 4, and there was a relatively stable hydrogen bond pair 381TYR: Ligand between DEE and NLRP3.(Figure 4C and D) Finally, we selected the conformation at the end of the simulation to analyze its structure and interactions. The amino acids PRO-412 and THR-169 in the NLRP3 protein form hydrogen bonds with DEE, while TYR-381, ILE-151, ILE-234, TRP-416, and ILE-521 formed Pi-Pi T-shaped, Alkyl, and Pi-Alkyl hydrophobic interactions with DEE. Amino acids such as PHE-373 and HIE-522 formed van der Waals interactions with DEE.(Figure 4E) These results indicate that DEE stably binds to NLRP3.
DEE Can Improve Inflammatory Responses in Mice by Inhibiting the NLRP3 Inflammasome
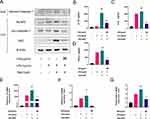
Western blot analysis revealed that, compared with healthy control group, the expression of NLRP3 in the lung tissues of LPS-induced ALI mice was significantly upregulated. However, with the increase in DEE treatment concentration, the expression of NLRP3 gradually decreased, which is similar to the roles of DEX. The DEE concentration of 30 mg/kg greatly reduced the NLRP3 expression levels, close to the healthy control (Figure 5A and B). Moreover, DEE treatment also significantly reduced the high expression levels of p-p65, pro-caspase-1, cleaved caspase-1, and ASC induced by LPS stimulation (Figure 5B). Moreover, DEE treatment significantly reduced the levels of IL-1β, IL-6, and TNF-α in the lung tissues, with a dose-dependent effect in vivo (Figure 5C–E). Collectively, these findings suggest that DEE ameliorates the inflammation by inhibiting the NLRP3 inflammasome-related pathway.
DEE Inhibited the Activation NF-kB Pathway and the Priming Stage of NLRP3 Inflammasome
LPS triggers the activation of NF-κB, resulting in elevated expression levels of NLRP3 and IL-1β.16 The murine J774A.1 macrophage cell line were then chosen to explore whether DEE plays a protective role in ameliorating inflammation. First, CCK-8 experiments showed up to 80 µ g/mL of DEE had toxic effects on cells (Figure 6A) and 20 µg/mL of DEE was selected for the subsequent experiments in vitro. The results showed that DEE significantly decreased p65 phosphorylation, NLRP3 expression, and restrained the production of IL-1β, TNF-α, and IL-6 (Figure 6B–E). These results indicate that DEE is capable of suppressing the activation of the NF-kB pathway and the priming stage of NLRP3 inflammasome.
DEE Attenuated Inflammatory Cytokine Levels by Inhibiting NLRP3 Inflammasome Activation
To delve deeper into DEE’s inhibitory action on the NLRP3 inflammasome, LPS+Nig was used to activate the NLRP3 inflammasome in cells. Western blot analysis revealed that DEE inhibited the elevated expression of NLRP3, ASC, pro-caspase-1 and cleaved caspase-1 in LPS+Nig-stimulated J774A.1 cells (Figure 7A). Moreover, ELISA showed that DEE treatment decreased the secretion of cellular IL-1β, TNF-α, and IL-6 induced by LPS+Nig stimulation (Figure 7B–D). The RT-qPCR results further confirmed that DEE inhibited the expression of these inflammatory cytokines within the cells (Figure 7E–G). These results supported that DEE inhibited NLRP3 inflammasome activation and slowed down IL- 1β production, aligning with in vivo observations and indicating that DEE exerts an anti-inflammatory effect by inhibiting NLRP3 inflammasome activation.
DEE Inhibited ROS Production and ASC Specks Formation
To investigate the mechanism by which DEE inhibited the NLRP3 inflammasome activation, we examined the effect of DEE on ROS production and ASC specks formation. We found that DEE treatment could inhibit the LPS +Nig-induced ROS production, confirming the antioxidant properties of DEE. At the same time, ROS production is a common upstream signaling event for the activation of the NLRP3 inflammasome.26,27 Our results showed that 20 μg/mL of DEE can inhibit ROS generation (Figure 8A–C). Formation of ASC fluorescent specks promotes the assembly and activation of the NLRP3 inflammasome.28 The immunofluorescence detection of ASC further indicated that 20 μg/mL of DEE treatment significantly reduced ASC specks formation caused by LPS+Nig stimulation(Figure 8D–E). These outcomes imply that DEE is capable of restraining the assembly and activation of the NLRP3 inflammasome complex.
Discussion
ALI represents a clinical syndrome marked by severe hypoxemia and bilateral pulmonary infiltrates.29 At present, there exist no specific drugs for the treatment of ALI, so new therapeutic agents need to be developed to ameliorate clinical symptoms. A multitude of danshensu derivatives have exhibited powerful anti-inflammatory effects both in ALI models and on macrophages.30 Previous studies have shown that danshensu methyl ester (DME) can alleviate LPS-induced ALI in mice by inhibiting the TLR4/NF-κB signaling pathway and oxidative stress.8 DEE is another derivative of danshensu synthesized by us, which overcomes the drawbacks of danshensu’s instability and poor liposolubility. Moreover, DEE can not only inhibit the priming stage of the NLRP3 inflammasome by suppressing the activation of NF-κB, but also exert significant anti-inflammatory effects by directly inhibiting the assembly and activation of the NLRP3 inflammasome, thereby reducing acute lung injury in mice.
In the present study, we explored the potential effect of DEE on improving ALI. DEX, as the positive control, has anti-inflammatory and immunosuppressive effects, which can rapidly and potently inhibit the systemic inflammatory response. It has significant curative effects in acute scenarios such as severe sepsis or cytokine storms.31,32 However, its long-term application is limited by dose-dependent side effects, including weight gain, osteoporosis, cataracts, and an increased risk of infection due to broad-spectrum immunosuppression.33 In contrast, as a derivative of danshensu, DEE inherits the significant anti-inflammatory activity of its parent compound and the advantages of natural safety. Moreover, danshensu has a history of hundreds of years of application in traditional Chinese medicine formulas. It has significant anti-inflammatory effects, and there are no reports of serious adverse reactions.5,34 Therefore, danshensu derivatives, including DEE, may provide a safer long-term treatment option for chronic inflammatory disease, but the clinical translation of DEE still requires further verification of its therapeutic window and long-term safety.
DEE exhibited anti-inflammatory effects in LPS-induced ALI mice, which typically show pulmonary hemorrhage, edema, thickened alveolar walls, narrowed alveolar cavities, and inflammatory cell accumulation. We found that both DEE and DEX significantly improved the pathological changes of ALI lung tissues in mice, as well as improved pulmonary hemorrhage and inflammatory cell infiltration. DEE treatment showed a dose-dependent effect, with higher doses yielding more pronounced therapeutic outcomes. Inflammatory cells and cytokines play crucial roles in ALI development and severity assessment. Neutrophil infiltration is considered as a factor in the progression of ALI.35 Due to the massive release of proinflammatory cytokines, neutrophils and monocytes reach the alveoli, causing harm to the endothelial cells and lung epithelial cells.36 Retrieving BALF via the respiratory tract for testing the levels of inflammation-related factors enables a more precise reflection of lung inflammation.37 IL-1β, IL-6 and TNF-α are important inflammatory factors, which play a crucial role in the occurrence of inflammatory response and can be utilized as biomarkers to gauge the degree of the inflammatory reaction within the organism. The synthesis of these inflammatory mediators at the site of injury represents one of the characteristic features of ALI.38 Our results indicated that DEE, at all doses, significantly lowered these cytokine levels in BALF compared to the LPS-induced ALI group. Additionally, the DEE group showed a decrease in neutrophils and macrophages, as well as a reduction in total protein content. This suggests that DEE significantly suppressed lung inflammation in ALI mice.
IL-1β activates inflammatory cells, triggers a cytokine release and inflammatory cascade, amplifying the response.25 It also enhances capillary permeability and promotes inflammation, leading to pulmonary edema by increasing alveolar and vascular endothelium permeability.39 The release of IL-1β was associated with the activation of NLRP3, inspiring us to focus on studying the NLRP3 inflammasome. Moreover, through molecular docking and molecular dynamics simulation experiments, results demonstrate that DEE can directly target the NLRP3 protein and stably bind to it, which indicates that DEE might act on the NLRP3 protein, thereby inhibiting the assembly and activation of the NLRP3 inflammasome. NLRP3 inflammasome is rapidly activated in response to various infection and stress signals, and triggers a strong inflammatory response.40 The NLRP3 inflammasome activation is involved in ALI progression, and the multiple inflammatory mediators mediated by it play a vital role in the pathogenesis of ALI.41 Both in vivo and in vitro, DEE demonstrated inhibitory effects on NLRP3 inflammasome activation. In in vivo experiments, DEE reduced the LPS-induced increases in p-p65 and NLRP3 expression, inhibited the cleavage of caspase-1, suppressed NLRP3-dependent IL-1β secretion, and suppressed the secretion of IL-6 and TNF-α.
Macrophages, as a crucial immune system component, are essential in inflammation research.42 Therefore, we chose J774A.1 mouse monocytes/macrophages to further study the role of DEE in ameliorating inflammation in vitro. The outcomes demonstrated that administering DEE before LPS induction could inhibit the activation of the NF-kB pathway induced by LPS, thereby suppressing the expression of NLRP3. This suggested that DEE suppressed the priming stage of NLRP3 inflammasome activation.16 DEE could also inhibit the expression of ASC and cleaved caspase-1, along with the secretion of IL-1β, IL-6, and TNF-α, indicating that DEE inhibited multiple stimulation-induced activation of the NLRP3 inflammasome in J774A.1 cells.
The production of ROS is one of the most important signals for the activation of the NLRP3 inflammasome.26 We found that DEE treatment inhibited the production of ROS caused by LPS+Nig induction, which may further affect NLRP3 levels. ASC serves as the pivotal element of the NLRP3 inflammasome complex, comprising an N-terminal pyrin domain (PYD) and the C-terminal CARD. ASC would aggregate via PYD-PYD interactions and condenses into specks through CARD-CARD interactions, thereby promoting the assembly and activation of the NLRP3 inflammasome.28 We found that DEE inhibited ASC specks formation, suggesting that DEE is able to impede the assembly and activation of the NLRP3 inflammasome complex. However, AIM2 and NLRC4 in the inflammasome family also play crucial roles in inflammation regulation.43 Future studies can explore the impact of DEE on the AIM2 and NLRC4 inflammasomes to expand the anti-inflammatory mechanism of DEE.
Recent studies have revealed the crucial role of the NLRP3 inflammasome in adipose tissue metabolism and inflammation44,45, and adipose tissue can affect ALI through pathways such as extracellular vesicles.46 Natural compounds targeting the NLRP3 inflammasome also have great potential in alleviating ALI. For example, salidroside and astragaloside IV can alleviate pulmonary inflammation and injury by inhibiting the NLRP3 inflammasome through regulating the upstream NF-κB signaling pathway.47,48 This study further revealed that DEE can not only block the initiation of the NLRP3 inflammasome by inhibiting the activation of NF-κB, but also may act on NLRP3 to inhibit the assembly and activation of the NLRP3 inflammasome. Molecular docking and kinetic simulations also showed the binding mode between DEE and the key domain of NLRP3, indicating the possible mechanism of DEE in ameliorating ALI.
Although this study has revealed the mechanism by which DEE alleviates ALI by inhibiting the NLRP3 inflammasome, several limitations still exist. Firstly, in future research, cell models that more closely mimic the real lung microenvironment, such as air-liquid interface cell cultures, lung organoids, or primary cells, can be employed to enhance physiological relevance.49 Meanwhile, exploring the broader application potential of DEE using human specimens will also be an important direction for future studies. Secondly, although a safe concentration of 20 µg/mL was selected in vitro experiments, the complex physiological environment may affect the distribution and safety of the drug. Therefore, it is necessary to further explore the therapeutic window of DEE in the vivo study. In addition, this study indicated the potential value of DEE in the treatment of ALI through DEE-pre-treatment model, and future research will further focus on the clinical translation of DEE for ALI therapy.
Conclusion
DEE can inhibit the activation of the NLRP3 inflammasome and exhibit a significant protective effect against LPS-induced ALI. This study not only reveals the potential value of DEE in the treatment of ALI but also broadens its application in anti-inflammatory therapy. In the future, the anti-inflammatory mechanism and clinical transformation potential of DEE need to be further explored.
Abbreviations
ALI, acute lung injury; LPS, lipopolysaccharide; DEE, danshensu ethyl ester; DEX, dexamethasone; NLRP3, nucleotide-binding domain leucine-rich repeat containing family, pyrin domain-containing 3; NF-κB, nuclear factor-κB; STAT3, signal transducer and activator of transcription 3; BALF, bronchoalveolar lavage fluid; ROS, reactive oxygen species; Nig, nigericin; HPLC, high performance liquid chromatography; W/D, wet/dry; IL-1β, interleukin-1 beta; IL-6, interleukin-6; TNF-α, tumor necrosis factor alpha.
Data Sharing Statement
The data used in this study are available from the corresponding author and the first author upon reasonable request.
Ethics Statement
All animal experiments took place at the Binzhou Medical College Animal Experiment Center, adhering to National Research Council guidelines and received approval from the Ethics Committee of Binzhou Medical College. (No. 2022-374) Alexander von Bunge (1803–1890) is the formal identifier and name of Salvia miltiorrhiza. The name was first published in Bunge’s work Enumeratio Plantarum quas in China Boreali Collegit (1833, p. 50).50 Holotype: Deposited in the Komarov Botanical Institute Herbarium (LE), St. Petersburg, Russia.
Acknowledgments
The authors sincerely thank all participants for their contributions.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the Natural Science Foundation of Shandong (No. ZR2022LSW002, ZR2024MH228), the National Natural Science Foundation of China (No.81772281, 31371321), Shandong Province Medical and Health Technology Development Plan Project (202304020786), and the Science Fund of Shandong Laboratory of Advanced Materials and Green Manufacturing (Yantai) (AMGM2023F16).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698–710. doi:10.1001/jama.2017.21907
2. Ortiz-Diaz E, Festic E, Gajic O, Levitt JE. Emerging pharmacological therapies for prevention and early treatment of acute lung injury. Semin Resp Crit Care Med. 2013;34(4):448–458. doi:10.1055/s-0033-1351118
3. Mokrá D. Acute lung injury - from pathophysiology to treatment. Physiol Res. 2020;69(Suppl 3):S353–s366. doi:10.33549/physiolres.934602
4. Mowery NT, Terzian WTH, Nelson AC. Acute lung injury. Curr Problems Surg. 2020;57(5):100777. doi:10.1016/j.cpsurg.2020.100777
5. Zhang J, Zhang Q, Liu G, Zhang N. Therapeutic potentials and mechanisms of the Chinese traditional medicine Danshensu. Eur J Pharmacol. 2019;864:172710. doi:10.1016/j.ejphar.2019.172710
6. Ai F, Chen M, Li W, et al. Danshen improves damaged cardiac angiogenesis and cardiac function induced by myocardial infarction by modulating HIF1α/VEGFA signaling pathway. Int J Clin Exp Med. 2015;8(10):18311–18318.
7. Yang Y, Du L, Zhao H, et al. Danshensu derivatives: a series of promising drugs with protective effects against cardiocerebrovascular diseases and cancers. Med Res Rev. 2025. doi:10.1002/med.22102
8. Han X, Ding W, Qu G, et al. Danshensu methyl ester attenuated LPS-induced acute lung injury by inhibiting TLR4/NF-κB pathway. Respir Physiol Neurobiol. 2024;322:104219. doi:10.1016/j.resp.2024.104219
9. Wu D, Xu J, Jiao W, et al. Suppression of macrophage activation by sodium danshensu via HIF-1α/STAT3/NLRP3 pathway ameliorated collagen-induced arthritis in mice. Molecules. 2023;28(4). doi:10.3390/molecules28041551
10. Pan LL, Ren Z, Liu Y, et al. A novel danshensu derivative ameliorates experimental colitis by modulating NADPH oxidase 4-dependent NLRP3 inflammasome activation. J Cell Mol Med. 2020;24(22):12955–12969. doi:10.1111/jcmm.15890
11. Zhang X, Yang Q, Zhang R, et al. Sodium Danshensu ameliorates cerebral ischemia/reperfusion injury by inhibiting CLIC4 / NLRP3 inflammasome-mediated endothelial cell pyroptosis. BioFactors. 2024;50(1):74–88. doi:10.1002/biof.1991
12. Zeng M, Zhang X, Lv N, et al. Sodium Danshensu stabilizes atherosclerotic vulnerable plaques by targeting IKKβ mediated inflammation in macrophages. Biomed Pharmacothe. 2023;165:115153. doi:10.1016/j.biopha.2023.115153
13. Gu W, Zeng Q, Wang X, Jasem H, Ma L. Acute lung injury and the NLRP3 inflammasome. J Inflamm Res. 2024;17:3801–3813. doi:10.2147/jir.S464838
14. Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Ann Rev Immunol. 2011;29:707–735. doi:10.1146/annurev-immunol-031210-101405
15. Xu X, Li J, Long X, et al. C646 protects against DSS-induced colitis model by targeting NLRP3 inflammasome. Front Pharmacol. 2021;12:707610. doi:10.3389/fphar.2021.707610
16. Paik S, Kim JK, Silwal P, Sasakawa C, Jo EK. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol Immunol. 2021;18(5):1141–1160. doi:10.1038/s41423-021-00670-3
17. Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18(9):2114–2127. doi:10.1038/s41423-021-00740-6
18. Guarda G, Braun M, Staehli F, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34(2):213–223. doi:10.1016/j.immuni.2011.02.006
19. Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17(8):588–606. doi:10.1038/nrd.2018.97
20. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:7081):237–41. doi:10.1038/nature04516
21. Mao K, Chen S, Chen M, et al. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Research. 2013;23(2):201–212. doi:10.1038/cr.2013.6
22. Frissen M, Liao L, Schneider KM, et al. Bidirectional role of NLRP3 during acute and chronic cholestatic liver injury. Hepatology. 2021;73(5):1836–1854. doi:10.1002/hep.31494
23. Orecchioni M, Kobiyama K, Winkels H, et al. Olfactory receptor 2 in vascular macrophages drives atherosclerosis by NLRP3-dependent IL-1 production. Science. 2022;375:6577):214–221. doi:10.1126/science.abg3067
24. Kim SR, Lee SG, Kim SH, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11(1):2127. doi:10.1038/s41467-020-15983-6
25. Li H, Li Y, Song C, et al. Neutrophil extracellular traps augmented alveolar macrophage pyroptosis via AIM2 inflammasome activation in LPS-induced ALI/ARDS. J Inflamm Res. 2021;14:4839–4858. doi:10.2147/jir.S321513
26. Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13). doi:10.3390/ijms20133328
27. Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38(6):1142–1153. doi:10.1016/j.immuni.2013.05.016
28. Dick MS, Sborgi L, Rühl S, Hiller S, Broz P. ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat Commun. 2016;7:11929. doi:10.1038/ncomms11929
29. Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nature Reviews Disease Primers. 2019;5(1):18. doi:10.1038/s41572-019-0069-0
30. XD ME, Cao YF, Che YY, et al. Danshen: a phytochemical and pharmacological overview. Chinese J Nat Med. 2019;17(1):59–80. doi:10.1016/s1875-5364(19)30010-x
31. Giles AJ, Hutchinson MND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51. doi:10.1186/s40425-018-0371-5
32. Dahabiyeh LA, Malkawi AK, Wang X, et al. Dexamethasone-induced perturbations in tissue metabolomics revealed by chemical isotope labeling LC-MS analysis. Metabolites. 2020;10(2). doi:10.3390/metabo10020042
33. Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457–465. doi:10.1517/14740338.2016.1140743
34. Li ZM, Xu SW, Liu PQ. Salvia miltiorrhizaBurge (Danshen): a golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol Sin. 2018;39(5):802–824. doi:10.1038/aps.2017.193
35. Kang ZY, Huang QY, Zhen NX, et al. Heterogeneity of immune cells and their communications unveiled by transcriptome profiling in acute inflammatory lung injury. Front Immunol. 2024;15:1382449. doi:10.3389/fimmu.2024.1382449
36. Kumar V. Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front Immunol. 2020;11:1722. doi:10.3389/fimmu.2020.01722
37. Hogea SP, Tudorache E, Pescaru C, Marc M, Oancea C. Bronchoalveolar lavage: role in the evaluation of pulmonary interstitial disease. Expert Rev Respir Med. 2020;14(11):1117–1130. doi:10.1080/17476348.2020.1806063
38. Fairweather D, Rose NR. Inflammatory heart disease: a role for cytokines. Lupus. 2005;14(9):646–651. doi:10.1191/0961203305lu2192oa
39. Eskandarian Boroujeni M, Sekrecka A, Antonczyk A, et al. Dysregulated interferon response and immune hyperactivation in severe COVID-19: targeting STATs as a novel therapeutic strategy. Front Immunol. 2022;13:888897. doi:10.3389/fimmu.2022.888897
40. Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407–420. doi:10.1038/nri.2016.58
41. Ying Y, Mao Y, Yao M. NLRP3 inflammasome activation by MicroRNA-495 promoter methylation may contribute to the progression of acute lung injury. Mol Ther Nucleic Acids. 2019;18:801–814. doi:10.1016/j.omtn.2019.08.028
42. Liu J, Yu L, Mo N, et al. Supercritical fluid extract of angelica sinensis and Zingiber officinale roscoe ameliorates TNBS-induced colitis in rats. Int J Mol Sci. 2019;20(15). doi:10.3390/ijms20153816
43. Oh S, Lee J, Oh J, et al. Integrated NLRP3, AIM2, NLRC4, pyrin inflammasome activation and assembly drive PANoptosis. Cell Mol Immunol. 2023;20(12):1513–1526. doi:10.1038/s41423-023-01107-9
44. Unamuno X, Gómez-Ambrosi J, Ramírez B, et al. NLRP3 inflammasome blockade reduces adipose tissue inflammation and extracellular matrix remodeling. Cell Mol Immunol. 2021;18(4):1045–1057. doi:10.1038/s41423-019-0296-z
45. Barra NG, Henriksbo BD, Anhê FF, Schertzer JD. The NLRP3 inflammasome regulates adipose tissue metabolism. Biochem J. 2020;477(6):1089–1107. doi:10.1042/bcj20190472
46. Miethe S, Potaczek DP, Bazan-Socha S, et al. The emerging role of extracellular vesicles as communicators between adipose tissue and pathologic lungs with a special focus on asthma. Am J Physiol Cell Physiol. 2023;324(5):C1119–c1125. doi:10.1152/ajpcell.00057.2023
47. Shi S, Huang D, Wu Y, et al. Salidroside pretreatment alleviates PM(2.5) caused lung injury via inhibition of apoptosis and pyroptosis through regulating NLRP3 Inflammasome. Food Chem Toxicol. 2023;177:113858. doi:10.1016/j.fct.2023.113858
48. Huang D, Shi S, Wang Y, et al. Astragaloside IV alleviates PM2.5-caused lung toxicity by inhibiting inflammasome-mediated pyroptosis via NLRP3/caspase-1 axis inhibition in mice. Biomed Pharmacothe. 2022;150:112978. doi:10.1016/j.biopha.2022.112978
49. Kühl L, Graichen P, von Daacke N, et al. Human lung organoids-a novel experimental and precision medicine approach. Cells. 2023;12(16). doi:10.3390/cells12162067
50. von Bunge A. Enumeratio Plantarum Quas in China Boreali Collegit. St. Petersburg: Academia Scientiarum Imp. Rossica; 1833:50.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
P2X7R-NEK7-NLRP3 Inflammasome Activation: A Novel Therapeutic Pathway of Qishen Granule in the Treatment of Acute Myocardial Ischemia
Li Y, Sun X, Liu X, Li J, Li X, Wang G, Liu Y, Lu X, Cui L, Shao M, Wang Y, Wang W, Li C
Journal of Inflammation Research 2022, 15:5309-5326
Published Date: 13 September 2022
Berberine Alleviates Acute Lung Injury in Septic Mice by Modulating Treg/Th17 Homeostasis and Downregulating NF-κB Signaling

Chen L, Liu X, Wang X, Lu Z, Ye Y
Drug Design, Development and Therapy 2023, 17:1139-1151
Published Date: 13 April 2023
Melatonin Attenuates Sepsis-Induced Acute Lung Injury via Inhibiting Excessive Mitophagy
Ling J, Yu S, Xiong F, Xu T, Li S
Drug Design, Development and Therapy 2023, 17:2775-2786
Published Date: 11 September 2023
Capsaicin Attenuates LPS-Induced Acute Lung Injury by Inhibiting Inflammation and Autophagy Through Regulation of the TRPV1/AKT Pathway
Hu Q, Liu H, Wang R, Yao L, Chen S, Wang Y, Lv C
Journal of Inflammation Research 2024, 17:153-170
Published Date: 9 January 2024
Quercetin: A Flavonoid with Potential for Treating Acute Lung Injury

Huang M, Liu X, Ren Y, Huang Q, Shi Y, Yuan P, Chen M
Drug Design, Development and Therapy 2024, 18:5709-5728
Published Date: 6 December 2024