Back to Journals » Journal of Inflammation Research » Volume 18
Deep Learning Models for Predicting the Recurrence of Idiopathic Granulomatous Mastitis
Received 23 October 2024
Accepted for publication 30 January 2025
Published 26 February 2025 Volume 2025:18 Pages 2943—2953
DOI https://doi.org/10.2147/JIR.S499512
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Lanying Li,1,2,* Wen Yang,3,* Haiming Jia1
1Vascular Surgery Breast Surgery Department, The Third Hospital of Mianyang, Sichuan Mental Health Center, Mianyang, 621000, People’s Republic of China; 2Clinical Medicine School, North Sichuan Medical College, Nanchong, Sichuan, 637000, People’s Republic of China; 3General Surgery Department, Lanzhou Second People’s Hospital, Lanzhou, Gansu, 730000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Haiming Jia, Breast Surgery Department of the Third Hospital of Mianyang, Sichuan Mental Health Center, Mianyang, Sichuan, 621000, People’s Republic of China, Email [email protected]
Background and Aim: Idiopathic granulomatous mastitis (IGM) is a rare chronic inflammatory breast disease that presents significant challenges in diagnosis and treatment. Predicting the recurrence of IGM is crucial for effective patient management and improved treatment outcomes. This study aims to evaluate and compare the performance of different machine learning models, including logistic regression, random forest, and neural networks, in predicting IGM recurrence using patient data.
Methods: A retrospective analysis was conducted on 212 patients diagnosed with IGM. Collected data included comprehensive serological markers, tumor characteristics, and treatment history. The dataset was divided into a training set (70%) and a testing set (30%). Data preprocessing involved normalization, feature selection, and data augmentation to ensure model robustness. Three predictive models were developed and compared: logistic regression, random forest, and neural networks. Performance metrics such as accuracy, sensitivity, specificity, and area under the ROC curve (AUC) were used to evaluate each model’s ability to predict IGM recurrence.
Results: The logistic regression model achieved an AUC of 0.837, 0.725 and 0.829 in the training cohort, validation cohort and test cohort. The random forest model showed improved performance with an AUC of 0.797, 0.755 and 0.793 in the training cohort, validation cohort and test cohort. The neural network model outperformed both the logistic regression and random forest models, with an AUC of 0.938, 0.880 and 0.913 and a better F1 score. Feature importance analysis indicated that variables such as smoking, surgery and a history of oral contraceptive use were most important in predicting recurrence.
Conclusion: This study demonstrates that, compared to logistic regression and random forest models, neural networks have superior performance in predicting the recurrence of granulomatous mastitis. The high accuracy and reliability of the neural network model highlight its potential clinical application in the early and accurate prediction of IGM recurrence.
Keywords: granulomatous mastitis, recurrence prediction, neural networks, machine learning models, serological markers
Introduction
Idiopathic granulomatous mastitis (IGM) is a rare chronic inflammatory breast disease that presents significant challenges in diagnosis and treatment. This condition is characterized by the presence of granulomas within the breast tissue, leading to severe discomfort and complications for patients.1–3 The etiology of IGM remains poorly understood, with potential links to autoimmune reactions, infections, and hormonal factors being explored.4,5 Despite its rarity, the recurrence of IGM poses a significant clinical problem, necessitating effective prediction methods to improve patient management and treatment outcomes.
Previous studies have employed various combined indicators or traditional statistical methods to predict the recurrence of IGM, with mixed results. Ciftci used the serum albumin to globulin ratio (AGR) as a predictive factor and developed an AGR-based scoring system, where a low AGR was identified as a risk factor for recurrence.6 Traditional methods, such as logistic regression, have provided foundational understanding but often fail to capture the complex, non-linear relationships within patient data. For instance, Lermi et al used logistic regression models to predict recurrence and found that surgical intervention and the presence of abscesses increased the recurrence rate in IGM treatment.7 However, the authors did not assess the model’s accuracy in prediction, highlighting the need for more sophisticated techniques.
In recent years, machine learning models such as random forests and neural networks have shown promise in medical predictive analytics due to their ability to handle large, complex datasets and uncover hidden patterns, thus offering superior predictive capabilities.8–10 Jin et al11 conducted a study applying random forest models to predict the recurrence of various breast diseases, demonstrating that their random forest model outperformed traditional logistic regression methods. However, the application of neural networks in this context has been relatively unexplored.
This study aims to fill this gap by evaluating and comparing the performance of logistic regression, random forest, and neural network models in predicting the recurrence of IGM using comprehensive patient data. By leveraging the strengths of these advanced machine learning models, we seek to provide a more accurate and reliable prediction tool for clinicians managing IGM, ultimately improving patient outcomes and reducing the burden of recurrent disease.
Materials and Methods
Study Design and Data Collection
A retrospective analysis was conducted on 212 patients diagnosed with idiopathic granulomatous mastitis (IGM) at Third Hospital of Mianyang between January 2020 and June 2022. The data collection process involved gathering comprehensive clinical information for each patient, including serological markers, basic demographic details, and treatment history. This comprehensive dataset was then used to develop and evaluate various predictive models for IGM recurrence. Inclusion criteria comprised (1) Pathologically confirmed diagnosis of idiopathic granulomatous mastitis, (2) Age over 18 years at the time of diagnosis, and (3) No diagnosis of malignant disease, while exclusion criteria included (1) Later diagnosed with tuberculous mastitis, (2) Later diagnosed with Wegener’s granulomatosis, (3) Male patients, (4) Insufficient follow-up information, and (5) Missing critical data. This study was conducted in accordance with the principles outlined in the Declaration of Helsinki and approved by the Institutional Review Board of the Ethics Committee of Third Hospital of Mianyang. Patient confidentiality and data privacy were strictly maintained throughout the study process.
Outcome Definition and Recurrence Monitoring
Definition of Recurrence
Recurrence of idiopathic granulomatous mastitis (IGM) was defined as the reappearance of clinical symptoms or imaging findings indicative of active disease following an initial resolution post-treatment. Recurrence was confirmed through a combination of clinical examination, imaging studies (including ultrasound and/or magnetic resonance imaging), and, if necessary, histopathological analysis of tissue biopsies.
Monitoring Protocol
All patients included in the study underwent a standardized follow-up protocol to monitor for recurrence. First 6 Months Post-Treatment: Patients were evaluated at 1-month intervals to closely monitor the early post-treatment phase. Each follow-up visit included a detailed clinical examination focusing on signs of inflammation (eg, erythema, tenderness, induration, or discharge), as well as a high-resolution breast ultrasound to detect subclinical disease. Laboratory tests, including inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), were performed if clinical or imaging findings raised suspicion of recurrence; 6–12 Months Post-Treatment: Patients were evaluated every 3 months. Clinical examination and breast ultrasound remained the mainstay of monitoring during this period. For patients with a history of higher disease severity or complex treatment courses, additional imaging (eg, MRI) was performed at 6 months; Beyond 12 Months: Follow-up visits were conducted at 6-month intervals for up to 24 months post-treatment to assess long-term outcomes. Clinical evaluation and breast ultrasound were performed at each visit, with imaging tailored based on clinical findings and risk factors for recurrence.
Data Preprocessing
To ensure all numerical features contributed equally to the model and to avoid dominance by variables with larger numerical ranges, continuous variables such as serological markers and imaging measurements were normalized to a common scale. This normalization step was crucial for maintaining consistency across features and enhancing model performance. Missing data, which accounted for less than 5% of the dataset, were addressed using imputation methods to minimize data loss and maintain dataset integrity. For continuous variables, missing values were replaced with the mean value calculated from the training dataset, ensuring the imputed data represented the central tendency of the feature. For categorical variables, missing entries were filled with the most frequently occurring value (mode) to preserve the original distribution of the data. To manage class imbalance between recurrent and non-recurrent cases, which could bias the model toward the majority class, the synthetic minority oversampling technique (SMOTE) was applied. This method generated synthetic samples for the minority class (recurrent cases) by creating new samples based on interpolations between existing samples within the same class. Importantly, SMOTE was applied only to the training dataset to prevent information leakage and ensure the evaluation of model performance on the testing dataset remained unbiased. These preprocessing steps collectively ensured that the dataset was well-prepared, balanced, and consistent for training, validation, and testing, supporting the development of robust predictive models.
Dataset Splitting
The Dataset Was Split Into Three Subsets to Ensure a Robust Evaluation of Model Performance
Training Set (70%): This subset (148 patients) was used to train the models by fitting the data to the algorithms and optimizing their weights.
Validation Set (15%): This subset (32 patients) was used for hyperparameter tuning and to validate model performance during training, ensuring that the models did not overfit to the training data.
Testing Set (15%): This subset (32 patients) was reserved for final evaluation to assess the generalization and predictive accuracy of the models on unseen data.
Model Training
Logistic regression, random forest, and neural networks were used as predictive models in this study.12,13 Each model was trained using the training dataset. During training, hyperparameter tuning was conducted using the validation set to optimize model parameters and ensure the models were well-calibrated. A grid search approach combined with cross-validation was employed to systematically explore the best combination of hyperparameters for each model, minimizing the risk of overfitting or underfitting.
Model Evaluation
After model training and tuning, the final performance of each model was evaluated using the testing dataset. This independent evaluation allowed for an unbiased assessment of the models’ ability to predict IGM recurrence. Performance metrics such as accuracy, sensitivity, specificity, F1-score, and area under the ROC curve (AUC) were calculated based on the predictions made on the testing set. The following metrics were used to assess the models’ ability to predict IGM recurrence:
Accuracy: The proportion of correctly predicted instances (both recurrent and non-recurrent) out of the total instances.
Sensitivity (Recall): The ability of the model to correctly identify patients with recurrence (true positive rate).
Specificity: The Ability of the Model to Correctly Identify Patients Without Recurrence (True Negative Rate)
Area Under the ROC Curve (AUC): A measure of the model’s ability to distinguish between recurrent and non-recurrent cases, with values closer to 1 indicating better performance.
Feature Importance Analysis
After training, feature importance analysis was conducted to identify which variables had the most significant impact on the prediction of IGM recurrence. This analysis provided insights into the underlying factors contributing to recurrence, helping to enhance the interpretability of the models.
By systematically evaluating and comparing the performance of logistic regression, random forest, and neural network models, this study aimed to identify the most effective approach for predicting IGM recurrence. The ultimate goal was to develop a reliable prediction tool that could aid clinicians in improving patient management and treatment outcomes for those suffering from IGM.
Results
Baseline Information Table for Idiopathic Granulomatous Mastitis Patients in Training Group and Validation Group
A total of 212 patients with idiopathic granulomatous mastitis receiving treatment in the hospital were randomly assigned to training, validation and test groups in a 0.7: 0.15: 0.15 ratio, with 148 patients in the training group, 32 patients in the validation group and 32 patients in the test group. The inclusion and exclusion process is shown in Figure 1. Among the patients in the training group, there were 89 patients over 30 years old, accounting for 60.1%. There were 49 patients with an ESR greater than 20 mm/hour, accounting for 33.1%, and 42 patients with a CRP greater than 5 mg/dL, accounting for 28.4%. In terms of the initial treatment regimen, there were 71 patients undergoing surgery, 40 patients receiving methotrexate, 50 patients receiving azathioprine, and 100 patients receiving antibiotics. The number of patients who eventually relapsed was 25, accounting for 16.7%. There were no statistically significant differences in various variables between the three groups (p > 0.05). Detailed data for the three groups are shown in Table 1.
 |
Table 1 Baseline Characteristics of Patients with Idiopathic Granulomatous Mastitis (IGM) in the Training and Validation Cohorts (N = 212) |
 |
Figure 1 Flow chart for inclusion and exclusion of patients with idiopathic granulomatous mastitis. |
Multivariate and Univariate Logistic Regression Analysis of Patients with Idiopathic Granulomatous Mastitis (IGM) in the Training Cohort
Univariate regression analysis showed that a history of oral contraceptive use, smoking, and surgical treatment were statistically significant (P < 0.05). These variables were included in the multivariate regression model. Multivariate Cox regression analysis indicated that a history of oral contraceptive use (HR = 1.246 [1.177–1.521], p = 0.031), smoking (HR = 1.649 [1.388–2.294], p < 0.001), and surgery (HR = 1.518 [1.408–1.931], p < 0.001) were significant (Table 2).
 |
Table 2 Multivariate and Univariate Logistic Regression Analysis of Patients with Idiopathic Granulomatous Mastitis (IGM) in the Training Cohort |
Model Characteristics of Different Predictors of Incisional Infections and the ROC Curve
Three variables derived from the multivariate logistics regression were used to construct a logistics model. The area under the curve (AUC) for the training cohort was 0.837, while the AUC for the validation cohort was 0.725 and 0.829 for test cohort (Figure 2). After multiple automated parameter adjustments using the random forest model, an optimal balance between performance on the training set and the test set was sought. Ultimately, 250 decision trees were selected, with a maximum depth of 12 and a minimum sample requirement of 2 for each split. The five most important variables in the model were identified as smoking, surgery, history of oral contraceptive use, and trauma to the breast, with importance coefficients of 0.25, 0.17, 0.13, 0.08, and 0.05, respectively (Figure 3A). ROC curve analysis showed an AUC of 0.797 for the training cohort,0.755 for the validation cohort and 0.793 for test cohort (Figure 3B).
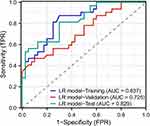 |
Figure 2 Performance of the Logistics Regression Model for ROC curve. |
For the deep learning model architecture, it starts with the input layer, which feeds into the first hidden layer consisting of 108 neurons. From this layer, the model splits into a main path and a side path. The main path extends to the second hidden layer with 96 neurons, while the side path leads to the third hidden layer with 72 neurons. These two paths converge at the fourth hidden layer, which contains 48 neurons, and then connect to the output layer. This structure illustrates the combination of sequential and parallel processing paths, enhancing the model’s ability to perform complex feature extraction and decision-making processes. The model architecture is shown in Figure 4A, with AUC values for the training, d validation and test sets of 0.938, 0.880 and 0.913, respectively (Figure 4B).
 |
Figure 4 Performance of the Deep Learning Model. A represents the model architecture diagram, and B represents the ROC curve of the model on the training, validation and test sets. |
Performance of Multiple Models
The overall best-performing model was the deep learning model, with F1-scores of 0.901, 0.871 and 0.894 on the training, validation and test sets, respectively, and AUCs of 0.938, 0.880 and 0.913, respectively. Additionally, the random forest model performed relatively poorly, with ROC-AUCs of 00.797, 0.755 and 0.793 for the training, validation and test sets, respectively (Table 3).
 |
Table 3 Evaluation Indicators for Each Model |
Discussion
The results of this study indicate that machine learning models, particularly neural networks, outperform traditional statistical methods such as logistic regression in predicting the recurrence of idiopathic granulomatous mastitis (IGM). The superior performance of neural networks can be attributed to their ability to capture complex nonlinear relationships in patient data, which are often overlooked by simpler models. Additionally, traditional models suffer from lower accuracy and the general inefficiency of conventional machine learning models. This finding aligns with the latest advancements in medical predictive analytics, where machine learning methods have been shown to significantly improve prediction accuracy and reliability.
Previous studies on IGM recurrence prediction mainly employed traditional statistical methods with varying degrees of success.6,7,14,15 For instance, a 10-year study by Co et al16 using a multi-center clinical database employed a logistic regression model to identify risk factors for IGM recurrence. It pointed out that smoking and the isolation of Corynebacterium were independent risk factors for disease recurrence (p < 0.05). However, their study did not evaluate the predictive accuracy of the model, leaving a gap in understanding the true efficacy of logistic regression in this context. In contrast, our study not only confirmed the importance of these factors but also demonstrated that while logistic regression is useful, it performs poorly compared to advanced machine learning models. The AUC of the logistic regression model in our study was 0.828, indicating moderate predictive ability.
Previous research also explored random forest models.17–20 Jin et al’s11 clinical trial showed that the random forest model outperformed logistic regression in predicting breast disease recurrence, with logistic regression’s AUC at 0.653 and random forest’s AUC at 0.829, and relatively high in the external training set at 0.779. Contrary to this study, our results showed that the performance of the random forest model did not surpass that of logistic regression, with the random forest AUC at 0.787. This discrepancy may be due to suboptimal parameter tuning; however, the top five variable importance rankings included three variables from the logistic regression model, indicating that variables such as history of oral contraceptive use, smoking, and surgical treatment are crucial for predicting IGM recurrence. Due to the ensemble nature of random forests, they combine multiple decision trees to enhance predictive accuracy and robustness.
However, the most significant improvement was seen in the neural network model, with an AUC of 0.921. The ability of neural networks to model complex interactions and their flexibility in handling various data types provides a distinct advantage over traditional methods and even other machine learning models.21–24 This finding is supported by recent literature on the application of deep learning in medical diagnostics, where neural networks consistently outperform other predictive models in various clinical settings. With the widespread adoption of artificial intelligence, for doctors, upon the admission of IGM patients, entering the patient’s examination data into a deep learning system allows the model to automatically calculate the probability of recurrence and guide doctors in early intervention to reduce recurrence rates. Accurate recurrence prediction can inform treatment decisions and subsequent strategies, ultimately improving patient outcomes. For example, patients identified as high-risk for recurrence may benefit from more aggressive treatment plans or closer monitoring, while low-risk patients can avoid unnecessary interventions.
The feature importance analysis in our study highlighted key variables, such as the presence of abscesses and a history of oral contraceptive use, as significant predictors of recurrence. These findings are consistent with previous research and provide actionable insights for clinicians. The international multidisciplinary consensus on IGM (2021 edition) mentions a recurrence rate of 15.4–24.8% for GLM, with Corynebacterium infection, breast skin lesions, and differences in prolactin (PRL) levels before and after treatment being important risk factors.25 Our feature importance analysis identified smoking, surgical history, and oral contraceptive use as the most significant predictors of IGM recurrence. These findings are consistent with prior studies in the field, further validating our model’s results. For example, Baron et al26 demonstrated that smoking contributes to chronic inflammation, increasing the risk of breast disease recurrence. Similarly, O’Connor et al27 highlighted the association between surgical intervention and increased recurrence rates, suggesting that tissue trauma and local inflammation play critical roles in disease progression. Moreover, Abbi et al28 identified hormonal factors, such as oral contraceptive use, as significant contributors to IGM recurrence, which aligns with our results. These consistent findings not only enhance the interpretability of our model but also underline the clinical relevance of the identified features. Targeted interventions, such as smoking cessation programs, cautious surgical planning, and hormonal monitoring, could potentially reduce the recurrence risk in high-risk patients. Future studies are warranted to validate these findings in larger, multicenter cohorts and to explore additional biomarkers that could further improve predictive accuracy.
Despite the encouraging results, our study has some limitations. The retrospective nature of the analysis and the relatively small sample size may limit the generalizability of the findings. Future research should aim to validate these models in larger prospective cohorts and across multiple institutions to ensure broader applicability. Additionally, while neural networks demonstrated superior performance, their interpretability remains a challenge. Clinicians may be reluctant to adopt models they do not fully understand, highlighting the need to develop interpretable AI techniques that can provide insights into the decision-making processes of these complex models.
Conclusion
This study demonstrates that neural networks significantly outperform logistic regression and random forest models in predicting the recurrence of IGM. The high accuracy and robustness of neural networks highlight their potential clinical utility in improving patient management and treatment outcomes. Moreover, deep machine learning-based methods provide researchers with powerful tools for uncovering complex patterns in data, offering significant advantages in advancing studies related to idiopathic granulomatous mastitis and similar medical conditions.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This retrospective study was approved by the Ethics Committee of Third Hospital of Mianyang and adhered to the ethical principles outlined in the Declaration of Helsinki. Informed consent forms were obtained from all patients.
Consent for Publication
The patient provided written informed consent for publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Mianyang Municipal Health Commission (No.202234), and Gansu Provincial Natural Science Foundation (No. 22JR5RA1070).
Disclosure
The authors declare that they have no competing interests.
References
1. Dilaveri C, Degnim A, Lee C, DeSimone D, Moldoveanu D, Ghosh K. Idiopathic granulomatous mastitis. Breast J. 2024;2024:6693720. doi:10.1155/2024/6693720
2. Ivanička V, Kaščák P. Idiopathic granulomatous mastitis. Ceska Gynekol. 2022;87(5):334–337. doi:10.48095/cccg2022334
3. Yin Y, Liu X, Meng Q, Han X, Zhang H, Lv Y. Idiopathic granulomatous mastitis: etiology, clinical manifestation, diagnosis and treatment. J Invest Surg. 2022;35(3):709–720. doi:10.1080/08941939.2021.1894516
4. Haitz K, Ly A, Smith G. Idiopathic granulomatous mastitis. Cutis. 2019;103(1):38–42.
5. Nguyen MH, Molland JG, Kennedy S, Gray TJ, Limaye S. Idiopathic granulomatous mastitis: case series and clinical review. Internal Med J. 2021;51(11):1791–1797. doi:10.1111/imj.15112
6. Ciftci AB, Bük ÖF, Yemez K, et al. Risk factors and the role of the albumin-to-globulin ratio in predicting recurrence among patients with idiopathic granulomatous mastitis. J Inflamm Res. 2022;15:5401–5412. doi:10.2147/JIR.S377804
7. Lermi N, Ekin A, Ocak T, et al. What predicts the recurrence in ıdiopathic granulomatous mastitis? Clin Rheumatol. 2023;42(9):2491–2500. doi:10.1007/s10067-023-06651-3
8. Fleuren LM, Klausch TLT, Zwager CL, et al. Machine learning for the prediction of sepsis: a systematic review and meta-analysis of diagnostic test accuracy. Intensive Care Med. 2020;46(3):383–400. doi:10.1007/s00134-019-05872-y
9. Ngiam KY, Khor IW. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019;20(5):e262–e273. doi:10.1016/S1470-2045(19)30149-4
10. Swanson K, Wu E, Zhang A, Alizadeh AA, Zou J. From patterns to patients: advances in clinical machine learning for cancer diagnosis, prognosis, and treatment. Cell. 2023;186(8):1772–1791. doi:10.1016/j.cell.2023.01.035
11. Jin Y, Lan A, Dai Y, Jiang L, Liu S. Development and testing of a random forest-based machine learning model for predicting events among breast cancer patients with a poor response to neoadjuvant chemotherapy. Eur J Med Res. 2023;28(1):394. doi:10.1186/s40001-023-01361-7
12. Elkahwagy D, Kiriacos CJ, Mansour M. Logistic regression and other statistical tools in diagnostic biomarker studies. Clin Transl Oncol. 2024;26(9):2172–2180. doi:10.1007/s12094-024-03413-8
13. Murmu A, Győrffy B. Artificial intelligence methods available for cancer research. Front Med. 2024;18(5):778–797. doi:10.1007/s11684-024-1085-3
14. Li Q, Wan J, Feng Z, Shi J, Wei W. Predictive significance of the preoperative neutrophil-lymphocyte ratio for recurrence in idiopathic granulomatous mastitis patients. Am Surg. 2023;89(12):5577–5583. doi:10.1177/00031348231161793
15. Yılmaz TU, Gürel B, Güler SA, et al. Scoring idiopathic granulomatous mastitis: an effective system for predicting recurrence? Eur J Breast Health. 2018;14(2):112–116. doi:10.5152/ejbh.2018.3709
16. Co M, Cheng VCC, Wei J, et al. Idiopathic granulomatous mastitis: a 10-year study from a multicentre clinical database. Pathology. 2018;50(7):742–747. doi:10.1016/j.pathol.2018.08.010
17. Heo J, Yoon JG, Park H, Kim YD, Nam HS, Heo JH. Machine learning-based model for prediction of outcomes in acute stroke. Stroke. 2019;50(5):1263–1265. doi:10.1161/STROKEAHA.118.024293
18. Wang Q, Qiao W, Zhang H, et al. Nomogram established on account of Lasso-Cox regression for predicting recurrence in patients with early-stage hepatocellular carcinoma. Front Immunol. 2022;13:1019638. doi:10.3389/fimmu.2022.1019638
19. Yang Y, Ma X, Wang Y, Ding X. Prognosis prediction of extremity and trunk wall soft-tissue sarcomas treated with surgical resection with radiomic analysis based on random survival forest. Updates Surg. 2022;74(1):355–365. doi:10.1007/s13304-021-01074-8
20. Zhang L, Huang T, Xu F, et al. Prediction of prognosis in elderly patients with sepsis based on machine learning (random survival forest). BMC Emerg Med. 2022;22(1):26. doi:10.1186/s12873-022-00582-z
21. Bhat M, Rabindranath M, Chara BS, Simonetto DA. Artificial intelligence, machine learning, and deep learning in liver transplantation. J Hepatol. 2023;78(6):1216–1233. doi:10.1016/j.jhep.2023.01.006
22. Gholipour K, Asghari-Jafarabadi M, Iezadi S, Jannati A, Keshavarz S. Modelling the prevalence of diabetes mellitus risk factors based on artificial neural network and multiple regression. East Med Health J. 2018;24(8):770–777. doi:10.26719/emhj.18.012
23. Jiang M, Ma Y, Guo S, et al. Using machine learning technologies in pressure injury management: systematic review. JMIR Med Inform. 2021;9(3):e25704. doi:10.2196/25704
24. Marchetti I, Koster EH, Sonuga-Barke EJ, De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychol Rev. 2012;22(3):229–251. doi:10.1007/s11065-012-9199-9
25. Yuan QQ, Xiao SY, Farouk O, et al. Management of granulomatous lobular mastitis: an international multidisciplinary consensus (2021 edition). Military Med Res. 2022;9(1):20. doi:10.1186/s40779-022-00380-5
26. Baron JA. Beneficial effects of nicotine and cigarette smoking: the real, the possible and the spurious. Br Med Bul. 1996;52(1):58–73. doi:10.1093/oxfordjournals.bmb.a011533
27. O’Connor R, Kiely PA, Dunne CP. The relationship between post-surgery infection and breast cancer recurrence. J Hosp Infect. 2020;106(3):522–535. doi:10.1016/j.jhin.2020.08.004
28. Abbi B, Sanghavi N, Lanjewar S, et al. Clinical, histological features, and predictors of relapse in patients with idiopathic granulomatous mastitis. Medicine. 2023;102(44):e35679. doi:10.1097/MD.0000000000035679
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


