Back to Journals » Research and Reports in Tropical Medicine » Volume 15
Demographic, Clinical, Radiological, and Surgical Outcome of Patients with Intestinal Tuberculosis: A Single-Center Retrospective Study
Authors Ghabisha S , Ahmed F , Almohtadi AM, Alghazali KA, Badheeb M , Al-Wageeh S
Received 9 April 2024
Accepted for publication 31 August 2024
Published 5 September 2024 Volume 2024:15 Pages 79—90
DOI https://doi.org/10.2147/RRTM.S465571
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mario A. Rodríguez-Pérez
Saif Ghabisha,1 Faisal Ahmed,2 Abdullatif Mothanna Almohtadi,3,4 Khairalah Abdulkarem Alghazali,4,5 Mohamed Badheeb,6 Saleh Al-Wageeh1
1Department of General Surgery, School of Medicine, Ibb University, Ibb, Yemen; 2Department of Urology, School of Medicine, Ibb University, Ibb, Yemen; 3Department of Radiology, School of Medicine, Ibb University, Ibb, Yemen; 4Department of Radiology, Ibb Scan Center, Ibb, Yemen; 5Department of Medical Immunology and Microbiology, School of Medicine, Jiblah University for Medical and Health Sciences, Ibb, Yemen; 6Internal Medicine, Yale New-Haven Health/Bridgeport Hospital, Bridgeport, CT, USA
Correspondence: Faisal Ahmed, Department of Urology, School of Medicine, Ibb University, Ibb, Yemen, Tel/Fax +9674428950, Email [email protected] Mohamed Badheeb, Internal Medicine, Yale New-Haven Health/Bridgeport Hospital, Bridgeport, CT, USA, Email [email protected]
Background: Intestinal tuberculosis (iTB) represents a potentially underrecognized clinical entity with limited clinical and radiological differentiating features. This study aims to assess the patterns of iTB clinical and radiological findings, along with the treatment approaches and the overall outcome.
Methods: This retrospective cross-sectional study included patients with histopathologically confirmed iTB who presented with acute abdomen and were surgically managed between September 2005 and October 2023. Clinical and sociodemographic variables, imaging features, surgical treatments, and overall outcomes were retrospectively analyzed.
Results: 96 patients with iTB were included, with a mean age of 36.1 ± 11.5 years and a relatively proportionate gender distribution. Abdominal pain was the most common presenting symptom (45.8%). The radiological features varied by the modality. Plain imaging showed non-specific findings, while ultrasonography showed loculated ascites (25%), and lymphadenopathy (22%). In computed tomography scans, multi-segmental symmetric intestinal thickening (53.1%) was the most prevalent finding. The most commonly performed surgical procedure was adhesiolysis (29.2%), with the ileocecal junction being the most commonly involved structure (39.6%). Histopathological examination of all the tissue biopsies revealed epithelioid granulomas. Postoperative complications occurred in 19 patients (19.8%), with surgical site infection being the most common complication (10.4%).
Conclusion: Intestinal obstruction is an underrecognized manifestation of tuberculosis, particularly in endemic regions. The non-specific clinical presentation, coupled with the limited utility of laboratory and radiological tests, often leads to delayed recognition and treatment. Maintaining a high index of suspicion is essential, especially in younger patients, inhabitants of endemic areas, or those with laboratory findings indicative of chronic inflammation. Prompt recognition is crucial to ensure the timely initiation of anti-tuberculosis therapy and to optimize patient outcomes through appropriate follow-up.
Keywords: Abdominal tuberculosis, bowel obstruction, acute abdomen, exploratory laparotomy, Yemen
Introduction
Intestinal tuberculosis (iTB) is a form of extrapulmonary TB, accounting for approximately 10% of the extrapulmonary TB cases.1 It can manifest alongside active pulmonary TB or independently without pulmonary involvement.2 Despite its relatively low incidence, iTB represents a severe variant of TB, that more frequently develops in patients with malnutrition, or immunosuppression (eg, HIV infection).3,4 In addition, iTB carries a poor prognosis with an increased risk for intestinal obstruction, perforation, or strictures.5 This can be attributed, in part, to the indolent nature of iTB, along with the non-specific presentation that can overlap with several clinical entities.3,6 Hence, it has been occasionally referred to as the “great mimicker” in the literature.7 Several reports have documented the invasive nature of iTB, involving the peritoneum, lymph nodes, or intestinal lumens.8
There has been a notable increase in the incidence and prevalence of TB, even in developed countries. This trend has been highlighted by the United Nations in its resolution on the fight against tuberculosis.9 While pulmonary involvement accounts for the majority of TB cases, reports from endemic regions, such as India, have observed extrapulmonary involvement in approximately 20% of cases.10,11 Prior studies from the United States and Europe have also noted a significant increase in extrapulmonary TB.10,11 Notably, iTB has a variable incidence, likely influenced by the endemicity of the region. For instance, iTB has been reported at rates as low as 6% and 9% in Europe and the United States, respectively, while higher rates, rising to 28%, have been observed in South Africa.11 It is unclear whether these figures represent an underdiagnosis of iTB or a possible underestimation of TB as a whole. Nonetheless, several reports have highlighted the concurrent pulmonary and intestinal involvement of TB, with incidence rates ranging from 13% to 67%.11
In Yemen, Al-Shehari et al investigated the incidence of TB from 2006 to 2018, focusing on the 13 years before the COVID-19 outbreak. A total of 92,482 patients were enrolled in the TB program records from 22 governorates.12 The incidence increased significantly in all age groups, with the sharpest increase in children under 15. Pediatric TB accounted for 9.6% of all cases. The incidence has more than doubled in the northern region of Yemen.12 Despite these alarming figures, they may still represent an underestimation of the true incidence and prevalence. The indolent nature of TB, combined with its prolonged inoculation period even in suspected cases, and the impact of the ongoing war in Yemen—resulting in limited access to healthcare facilities and restricted surveillance and monitoring program outreach—likely contribute to this underestimation.12,13 The effect of war seems to be noted in a recent report by the WHO indicating that there were 13,000 new cases of tuberculosis in Yemen in 2017, with 47 infections per 100,000 population including 2.4% of newly diagnosed and 19% of previously treated multidrug-resistant or rifampicin-resistant tuberculosis (MDR/RR-TB) patients.14 While iTB represents an aggressive form of TB infections, it is medically treatable, with most intestinal manifestations manageable with antitubercular treatment. However, due to the challenges in diagnosing iTB, delayed recognition and treatment can lead to complications such as intestinal obstruction, perforation, abscess, or fistula development that necessitate surgical interventions.6 This study aims to delve into the patterns of iTB presentation, imaging findings, surgical interventions, and overall outcomes in patients without a prior TB diagnosis.
Materials and Method
Study Design
This retrospective cross-sectional study included patients who presented with intestinal obstruction and underwent surgical exploration at Al-Nasar Hospital in IBB, Yemen, between September 2005 and October 2023. The Ibb University’s ethics committee approved the study protocol (ID: IBBUNI.AC.YEM. 2022.1–50) which was carried out in accordance with the Declaration of Helsinki and all patients provided informed consent before data capturing. The requirement for participant consent was waived due to the retrospective nature of the study and the absence of identifiable information.
Study Population
The study population comprised adult patients with a presumptive diagnosis of intestinal obstruction based on supportive clinical and radiological findings. Exclusion criteria were patients with a pre-established TB diagnosis, those managed conservatively, individuals with incomplete charts or lacking pre-operative imaging, HIV-infected or immunosuppressed patients, and those lacking post-operative histopathological confirmation of iTB.
Data Collection
Data were retrieved from the Al-Nasar Hospital registry, identifying cases meeting the study criteria. Collected information encompassed baseline sociodemographic characteristics (age, sex, educational level, and residence), clinical signs and symptoms blood laboratory results, plain radiography findings, abdominal ultrasound (US) results, abdominal computed tomography (CT) scan findings, surgical procedures performed, surgical findings, operative time, postoperative hospitalization duration, complications, and outcomes. The postoperative phase involved examining surgical complications, hospitalization, and death, with patients monitored for 12 months or until death. The Yemen National Tuberculosis and Leprosy Program initiated anti-tuberculosis therapy for tuberculosis patients, using Isoniazid, Rifampicin, Pyrazinamide, Ethambutol, and Streptomycin. All Patients were followed by medical (pulmonologists) and surgical (surgeon) teams providing care. The data were gathered via independent chart reviews conducted by the investigator. Following this, the collected data underwent a thorough assessment for accuracy, completeness, and consistency. Any contradictory findings or that lack the required information prompted a subsequent review and reevaluation of the charts.
Statistical Analysis
SPSS software for Windows (version 22; SPSS, Chicago, IL, USA) was used for statistical analyses. Categorical variables are displayed as absolute numbers and percentages, whereas continuous variables are displayed as mean ± SD and (range). Before statistical analysis, the normality of the data was assessed using the Shapiro–Wilk test.
Result
A total of 3417 patients diagnosed and operated on due to acute abdomen were identified, and 96 (2.6%) were diagnosed with abdominal tuberculosis. The mean age was 36.1 ± 11.5 years (range: 18–75 years), and 49 (51.0%) were female. The rates of Khat chewing, illiteracy, and family history of TB were 76 (79.2%), 42 (43.8%), and 9 (9.4%), respectively. Abdominal pain was the most common gastrointestinal symptom 44 (45.8%). Other symptoms such as fever, weight loss, night sweating, general weakness, and loss of appetite were reported in 65 (67.7%), 33 (34.4%), 17 (17.7%), 13 (13.5%), and 12 (12.5%) patients, respectively. The main physical examination finding was abdominal tenderness in 46 (47.9%) patients, followed by ascites in 42 (43.8%) patients (Table 1).
 |
Table 1 Baseline Characteristics and f Clinical Presentation of Patients |
Laboratory and Radiological Findings
The mean hemoglobin (g/dL) level was 11.2 ±1.9 (range: 7–15) and 56 (58.3%) of patients had anemia. Leukocytosis and elevated erythrocyte sedimentation rate (ESR) were presented in 45 (46.9%) and 68 (70.8%) of patients, respectively. On plain radiography, the most common finding was distended intestinal coils in 37 (38.5%) patients, with normal findings reported in 20.8% of patients. The most commonly seen ultrasonographic finding was loculated ascites (25.0%) (Table 2). In CT imaging, multi-segmental symmetric intestinal mural thickening (53.1%) was the most common sign. Other CT findings included solid masses (36.5%), ascites (26%), enlarged lymph nodes (19.8%), and non-enhancing central necrosis and rim enhancement (3.1%) (Figure 1).
 |
Table 2 Laboratory and Radiologic Findings of Patients |
Surgical Procedures/Findings
All patients underwent exploratory laparotomy, and the distribution of the intraoperative findings is shown in Table 3. The mean operative time was 76.5 ± 24.5 minutes (range: 40–125 min). The most common site of involvement was the ileocecal junction (39.6%), and the main operative finding was band adhesion in 20 (20.8%) patients, followed by multiple tubercles in 17 (17.7%) patients (Figure 2). The most commonly performed surgical procedures were adhesiolysis in 28 patients (29.2%), followed by diagnostic laparotomy and biopsy in 27 patients (28.1%). Strictureplasty, limited ileocolic resection with ileo-ascending colon anastomosis, resection and side-to-side anastomosis, right hemicolectomy, and ileostomy with ileostomy closure after 6 months were performed in 15 (15.6%), 14 (14.6%), 9 (9.4%), 2 (2.1%), and 1 (1.0%) patient, respectively. The length of hospital stay ranged from 1 to 27 days, with an average of 9 days.
 |
Table 3 Operative and Postoperative Characteristics of Patients |
Postoperative Complications and Outcome
Postoperative complications occurred in 19 (19.8%) patients, including surgical site infection, wound dehiscence/abdominal rupture, paralytic ileus, intra-abdominal abscess/peritonitis, enterocutaneous fistula, and septicemia in 10 (10.4%), 2 (2.1), 4 (4.2%), 1 (1.0%), 2 (2.1%), and 1 (1.0%) patient, respectively (Table 3). Patients with wound dehiscence/abdominal rupture, intra-abdominal abscess/peritonitis, enterocutaneous fistula, and septicemia were treated with a temporary ileostomy. All patients received anti-tuberculosis therapy, and after 9 months of treatment, clinical recovery without complications was achieved in all patients, except for one patient who expired during follow-up.
Microbiological Features
The postoperative Acid-fast bacilli test was positive in most cases 75 (78.1%). Histopathological examination of all the tissue biopsies revealed epithelioid granulomas (100%). Caseous necrosis and Langhans giant cells were also observed. The TB- polymerase chain reaction (PCR) result was available for 73 cases and was positive in most cases 66 (68.8%) (Table 4).
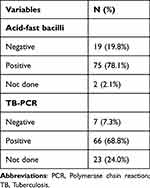 |
Table 4 Microbiological Features of Patients |
Discussion
The epidemiological trajectory of TB has exhibited fluctuations in recent years, with escalating incidence primarily observed in lower-income nations. This trend is influenced by several factors related to the economic regression since COVID-19 with diminished healthcare expenditure, along with inadequate social protection. Furthermore, the increased rates of immunosuppressive conditions (eg, HIV), introduction of several biological therapies, and increased prevalence of multi-drug-resistant TB compounded by suboptimal treatment have influenced the overall incidence and prevalence of TB.6,15
In this study, the mean age of affected individuals was 36.1 ± 11.5 years, in line with preceding national and regional assessments.8,16–18 Nevertheless, these findings deviate from prior observations which reported an older age group of overall small intestinal obstruction patients. For instance, a population-based analysis by Behman et al reported a mean age of 61.2 years.19 Similar to our findings were also reported by Cheng et al with a mean age of 37 years.20 While our investigation did not unveil a significant gender predilection, approximately 46.9% of patients hailed from economically disadvantaged backgrounds. These findings align with previous studies with socioeconomic backgrounds, where individuals from such strata encounter restricted access to healthcare amenities and are subjected to less targeted TB screening.8,20,21
The clinical diagnosis of iTB poses considerable challenges, as the majority of patients present with nonspecific symptoms such as weight loss, anemia, and fever with night sweats.21 In addition, the frequencies of these symptoms vary significantly in the literature. For instance, a study by Farina et al reported weight loss and night sweat in merely 9.7% and 1.8%, respectively.22 In our study, abdominal pain was the most frequently reported symptom (45.8%), accompanied by other nonspecific symptoms such as fever, weight loss, night sweats, general weakness, and loss of appetite. Dhali et al reported similar frequencies, with abdominal pain being the presenting symptom in (39.7%) of patients.23 The physical examination in iTB varies depending on the site of GI involvement and may include hepatosplenomegaly, abdominal tenderness, or palpable masses.24,25 However, these findings appear to be of limited utility, for instance, less than half of our patients exhibited abdominal tenderness, and splenomegaly or hepatomegaly was noted in 6.2% and 2.1% of our patients.
Similarly, the utility of laboratory testing in iTB is limited, as evidenced by the non-specific nature of findings such as normocytic anemia and elevated inflammatory markers, notably an elevated ESR.8 In our investigation, the mean hemoglobin value was recorded at 11.2 ± 1.9 g/dL, with 56 patients (58.3%) presenting with anemia, and 68 cases (70.8%) displaying elevated ESR levels. These findings lack the diagnostic specificity, however, can be suggestive of a chronic inflammatory process that would not be anticipated in acute abdomen.26,27 The radiological findings in iTB may mimic several gastrointestinal pathologies.28 For instance, plain X-ray imaging may reveal features such as mural thickening, distended bowel loops, or signs of perforation such as air under the diaphragm.29 Among our patients, distended bowel loops were the most prevalent finding in plain X-ray imaging in 38.5% of the patients.
Abdominal US is a common initial radiologic modality for detecting lymphadenopathy, peritoneal or omental thickening, ascites, mesenteric abnormalities, and gut wall thickening.30 Nevertheless, these findings were seen in the minority of the patients in our study (Table 2). Abdominal CT imaging was the most sensitive modality in our study, with (53.1%) of the patients having multi-segmental mural thickening, along solid masses (36.5%), ascites (26.0%), enlarged lymph nodes (19.8%), and non-enhancing-central-necrosis and rim enhancement (3.1%). These findings align relatively with prior reports, albeit with variations in reported rates across different studies.31 For example, Sinan et al reported the CT scan findings of 49 patients with proven iTB within 20 years and found that peritoneal involvement was the most common feature (77.5%) followed by ascites (55.2%). Other CT scan findings included diffuse lymphadenopathy (46.9%), bowel wall thickening (38%), and solid organ involvement (20.4%).32 In another report, Deshpande et al mentioned that the commonest pattern of iTB involvement was circumferential bowel wall thickening without bowel stratification with mild luminal narrowing.33 Nonetheless, the diagnostic utility of radiological studies in our investigation was limited, consistent with several reports indicating high rates of iTB misdiagnosis due to the lack of radiological specificity.33–35
The management of iTB predominantly relies on conventional anti-tuberculosis therapy, typically resulting in remission and complete recovery.36 Surgical interventions are reserved for advanced or complicated cases, such as bowel obstruction or perforation. Several surgical approaches are proposed, with the determination of appropriate intervention based on the underlying pathology and the involved gastrointestinal structures. In our study, intestinal adhesions were the most common intra-operative finding, with adhesiolysis being the most frequently performed intervention in our cohort (29.2%). Similar observations were reported by Chalya et al and Weledji et al, where adhesiolysis was frequently performed, likely attributable to the similar prevalence of intra-operative findings such as bands or adhesions.37,38 However, a more conservative approach, such as strictureplasty, has been advocated for localized strictures, as it offers advantages over repeat resections and entero-anastomoses by preserving the integrity of the small intestine and mitigating the risk of short bowel syndrome or blind loops.37 Nonetheless, segmental resection may be warranted in cases of extended strictures or concurrent perforations.6,39
In our study, nine patients (9.4%) developed perforations around strictures, necessitating excision with side-to-side anastomosis. Alternative approaches have been described in the literature including exteriorization of perforated loops, which present an appropriate approach for acute cases with perforative peritonitis.6
Mortality rates attributable to iTB exhibit considerable variability, ranging from 14% to 50% in low-income countries and 6% to 37% in high-income countries.11 Morbidities associated with iTB include delayed wound healing, incisional hernia, recurrent obstruction, and fecal fistula.36,37 Bowel perforation causes significant death rates and is thought to exacerbate iTB in up to 11% of adult cases, which might be attributed to the late hospital presentation.40 Our study identified surgical site infection as the primary postoperative complication, consistent with findings from prior investigations.38,41,42 In addition, one case expired during postoperative follow-up. Other complications reported in this study included wound dehiscence/abdominal rupture, intra-abdominal abscess/peritonitis, enterocutaneous fistula, and septicemia. In another report, Cheng et al found 43.2% of postoperative complications, with fistula (27%) and surgical site infections (21.6%) being the most prevalent postoperative complications.20
Study Limitations
The study has several limitations. The retrospective design limits generalizability and introduces potential selection bias, particularly due to the reliance on available medical records, which may be incomplete or inaccurately documented. Additionally, the absence of postoperative follow-up hindered further evaluation of tuberculosis cases, including the identification of concurrent pulmonary or extra-pulmonary involvement, as well as long-term follow-up to assess the initiation of anti-tuberculosis therapy. Moreover, the lack of a control group restricted our ability to conduct a comprehensive analysis of potential risk factors or prognostic indicators.
Conclusion
Intestinal obstruction is an underrecognized manifestation of tuberculosis, particularly in endemic regions. The non-specific clinical presentation, coupled with the limited utility of laboratory and radiological tests, often leads to delayed recognition and treatment. Maintaining a high index of suspicion is essential, especially in younger patients, inhabitants of endemic areas, or those with laboratory findings indicative of chronic inflammation. Prompt recognition is crucial to ensure the timely initiation of anti-tuberculosis therapy and to optimize patient outcomes through appropriate follow-up.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The Ibb University’s ethics committee approved the study protocol and all patients provided informed consent before data capturing.
Consent for Publication
All authors reviewed the manuscript and approved its submission.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Coccolini F, Kobayashi L, Kluger Y, et al. Duodeno-pancreatic and extrahepatic biliary tree trauma: WSES-AAST guidelines. World J Emerg Surg. 2019;14:56. PMID: 31867050. PMCID: PMC6907251. doi:10.1186/s13017-019-0278-6
2. Cho JK, Choi YM, Lee SS, et al. Clinical features and outcomes of abdominal tuberculosis in southeastern Korea: 12 years of experience. BMC Infect Dis. 2018;18(1):699. PMID: 30587154. PMCID: PMC6307147.. doi:10.1186/s12879-018-3635-2
3. Chakinala RC, Khatri AM. Gastrointestinal Tuberculosis. In: StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2024.
4. Sudcharoen A, Ruchikajorndech G, Srisajjakul S, et al. Clinical characteristics and diagnosis of intestinal tuberculosis in clinical practice at Thailand’s largest national tertiary referral center: an 11-year retrospective review. PLoS One. 2023;18(4):e0282392. PMID: 37053242 PMCID: PMC10101504.. doi:10.1371/journal.pone.0282392
5. Lowbridge C, Fadhil SAM, Krishnan GD, et al. How can gastro-intestinal tuberculosis diagnosis be improved? A prospective cohort study. BMC Infect Dis. 2020;20(1):255. PMID: 32228479. PMCID: PMC7106693.. doi:10.1186/s12879-020-04983-y
6. Pattanayak S, Behuria S. Is abdominal tuberculosis a surgical problem? Ann R Coll Surg Engl. 2015;97(6):414–419. PMID: 26274741. PMCID: PMC5126243. doi:10.1308/rcsann.2015.0010
7. Prapruttam D, Hedgire SS, Mani SE, Chandramohan A, Shyamkumar NK, Harisinghani M. Tuberculosis--the great mimicker. Semin Ultrasound CT MR. 2014;35(3):195–214. PMID: 24929261.. doi:10.1053/j.sult.2014.02.002
8. Barot M, Yagnik VD, Patel K, Dawka S. Surgical management of abdominal tuberculosis: a prospective single-center study. Tzu Chi Med J. 2021;33(3):282–287. PMID: 34386367. PMCID: PMC8323646.. doi:10.4103/tcmj.tcmj_206_20
9. Zumla A, Petersen E. The historic and unprecedented united nations general assembly high level meeting on tuberculosis (UNGA-HLM-TB)-’United to end TB: an urgent global response to a global epidemic’. Int J Infect Dis. 2018;75:118–120. PMID: 30244078.. doi:10.1016/j.ijid.2018.09.017
10. Cherian JJ, Lobo I, Sukhlecha A, et al. Treatment outcome of extrapulmonary tuberculosis under revised national tuberculosis control programme. Indian J Tuberc. 2017;64(2):104–108. PMID: 28410692.. doi:10.1016/j.ijtb.2016.11.028
11. Al-Zanbagi AB, Shariff MK. Gastrointestinal tuberculosis: a systematic review of epidemiology, presentation, diagnosis and treatment. Saudi J Gastroenterol. 2021;27(5):261–274. PMID: 34213424. PMCID: PMC8555774.. doi:10.4103/sjg.sjg_148_21
12. Al-Shehari WA, Yin YA, Wang X, et al. Prevalence and surveillance of tuberculosis in Yemen from 2006 to 2018. Epidemiol Infect. 2022;150:e146. PMID: 35856270. PMCID: PMC9354476.
13. Bagcchi S. WHO’s Global Tuberculosis Report 2022. Lancet Microbe. 2023;4(1):e20. PMID: 36521512.. doi:10.1016/S2666-5247(22)00359-7
14. Jaber AAS, Ibrahim B. Evaluation of risk factors associated with drug-resistant tuberculosis in Yemen: data from centres with high drug resistance. BMC Infect Dis. 2019;19(1):464. PMID: 31126246. PMCID: PMC6534925.. doi:10.1186/s12879-019-4069-1
15. Tanrikulu AC, Aldemir M, Gurkan F, Suner A, Dagli CE, Ece A. Clinical review of tuberculous peritonitis in 39 patients in Diyarbakir, Turkey. J Gastroenterol Hepatol. 2005;20(6):906–909. PMID: 15946139. doi:10.1111/j.1440-1746.2005.03778.x
16. Uzunkoy A, Harma M, Harma M. Diagnosis of abdominal tuberculosis: experience from 11 cases and review of the literature. World J Gastroenterol. 2004;10(24):3647–3649. PMID: 15534923. PMCID: PMC4612009.. doi:10.3748/wjg.v10.i24.3647
17. Abro A, Siddiqui FG, Akhtar S, Memon AS. Spectrum of clinical presentation and surgical management of intestinal tuberculosis at tertiary care hospital. J Ayub Med Coll Abbottabad. 2010;22(3):96–99. PMID: 22338429.
18. Jaskani S. Surgical management of acute presentation and outcome of patients with complicated abdominal tuberculosis. J Rawalpindi Med Coll. 2016;20(2):108—12.
19. Behman R, Nathens AB, Haas B, Look Hong N, Pechlivanoglou P, Karanicolas P. Surgery for adhesive small-bowel obstruction is associated with improved long-term survival mediated through recurrence prevention: a population-based, propensity-matched analysis. J Trauma Acute Care Surg. 2019;87(3):636–644. PMID: 31095068.. doi:10.1097/TA.0000000000002366
20. Cheng W, Zhang S, Li Y, Wang J, Li J. Intestinal tuberculosis: clinico-pathological profile and the importance of a high degree of suspicion. Trop Med Int Health. 2019;24(1):81–90. PMID: 30338607.. doi:10.1111/tmi.13169
21. Limpin ET, Lopez MPJ, Maglangit S, et al. Surgical Management of Patients With GI Tuberculosis. Dis Colon Rectum. 2023;66(1):106–112. PMID: 36515515.. doi:10.1097/DCR.0000000000002626
22. Farina E, D’Amore C, Lancella L, et al. Alert sign and symptoms for the early diagnosis of pulmonary tuberculosis: analysis of patients followed by a tertiary pediatric hospital. Ital J Pediatr. 2022;48(1):90. PMID: 35698090. PMCID: PMC9195307. doi:10.1186/s13052-022-01288-5
23. Dhali A, Das K, Dhali GK, Ghosh R, Sarkar A, Misra D. Abdominal tuberculosis: clinical profile and outcome. Int J Mycobacteriol. 2021;10(4):414–420. PMID: 34916461.. doi:10.4103/ijmy.ijmy_195_21
24. Wenting J, Yuyan M, Qingfeng S, et al. Clinical features and diagnostic approaches for abdominal tuberculosis: five-year experience from a non-tuberculosis-designated hospital in China. Rev Esp Enferm Dig. 2022;114(8):461–467. PMID: 34886676. doi:10.17235/reed.2021.8022/2021
25. Chen HL, Wu MS, Chang WH, Shih SC, Chi H, Bair MJ. Abdominal tuberculosis in southeastern Taiwan: 20 years of experience. J Formos Med Assoc. 2009;108(3):195–201. PMID: 19293034.. doi:10.1016/S0929-6646(09)60052-8
26. Sharma MP, Bhatia V. Abdominal tuberculosis. Indian J Med Res. 2004;120(4):305–315. PMID: 15520484.
27. Rana S, Farooqui MR, Rana S, Anees A, Ahmad Z, Jairajpuri ZS. The role of laboratory investigations in evaluating abdominal tuberculosis. J Family Community Med. 2015;22(3):152–157. PMID: 26392795. PMCID: PMC4558736.. doi:10.4103/2230-8229.163029
28. Ghabisha S, Ahmed F, Altam A, Hassan F, Badheeb M. Small bowel obstruction in virgin abdomen: predictors of surgical intervention need in resource-limited setting. J Multidiscip Healthc. 2023;16:4003–4014. PMID: 38107087. PMCID: PMC10725698.. doi:10.2147/JMDH.S441958
29. Donoghue HD, Holton J. Intestinal tuberculosis. Curr Opin Infect Dis. 2009;22(5):490–496. PMID: 19623062.. doi:10.1097/QCO.0b013e3283306712
30. Van Hoving DJ, Griesel R, Meintjes G, Takwoingi Y, Maartens G, Ochodo EA. Abdominal ultrasound for diagnosing abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in HIV-positive individuals. Cochrane Database Syst Rev. 2019;9(9):Cd012777. PMID: 31565799. PMC6766789. doi:10.1002/14651858.CD012777.pub2
31. da Rocha EL, Pedrassa BC, Bormann RL, Kierszenbaum ML, Torres LR, D’Ippolito G. Abdominal tuberculosis: a radiological review with emphasis on computed tomography and magnetic resonance imaging findings. Radiol Bras. 2015;48(3):181–191. PMID: 26185345. PMCID: PMC4492571.. doi:10.1590/0100-3984.2013.1801
32. Sinan T, Sheikh M, Ramadan S, Sahwney S, Behbehani A. CT features in abdominal tuberculosis: 20 years experience. BMC Med Imaging. 2002;2(1):3. PMID: 12427257. PMCID: PMC139990. doi:10.1186/1471-2342-2-3
33. Deshpande SS, Joshi AR, Deshpande SS, Phajlani SA. Computed tomographic features of abdominal tuberculosis: unmask the impersonator! Abdom Radiol. 2019;44(1):11–21. PMID: 30027495.. doi:10.1007/s00261-018-1700-3
34. Sato R, Nagai H, Matsui H, et al. Ten cases of intestinal tuberculosis which were initially misdiagnosed as inflammatory bowel disease. Intern Med. 2019;58(14):2003–2008. PMID: 30918188. PMCID: PMC6702022.. doi:10.2169/internalmedicine.2361-18
35. Patel B, Yagnik VD. Clinical and laboratory features of intestinal tuberculosis. Clin Exp Gastroenterol. 2018;11:97–103. PMID: 29559804. PMCID: PMC5856297.. doi:10.2147/CEG.S154235
36. Mandavdhare HS, Singh H, Dutta U, Sharma V. A real-world experience with 6 months of antitubercular therapy in abdominal tuberculosis. JGH Open. 2019;3(3):201–205. PMID: 31276036. PMCID: PMC6586575.. doi:10.1002/jgh3.12136
37. Weledji EP, Pokam BT. Abdominal tuberculosis: is there a role for surgery? World J Gastrointest Surg. 2017;9(8):174–181. PMID: 28932351. PMCID: PMC5583525.. doi:10.4240/wjgs.v9.i8.174
38. Chalya PL, McHembe MD, Mshana SE, Rambau P, Jaka H, Mabula JB. Tuberculous bowel obstruction at a university teaching hospital in Northwestern Tanzania: a surgical experience with 118 cases. World J Emerg Surg. 2013;8(1):12. PMID: 23497503. PMCID: PMC3608959.. doi:10.1186/1749-7922-8-12
39. Uygur-Bayramicli O, Dabak G, Dabak R. A clinical dilemma: abdominal tuberculosis. World J Gastroenterol. 2003;9(5):1098–1101. PMID: 12717865. PMCID: PMC4611381.. doi:10.3748/wjg.v9.i5.1098
40. Kentley J, Ooi JL, Potter J, et al. Intestinal tuberculosis: a diagnostic challenge. Trop Med Int Health. 2017;22(8):994–999. PMID: 28609809.. doi:10.1111/tmi.12908
41. Akbar M, Haider IZ, Naveed D, et al. Surgical management of tuberculous small bowel obstruction. J Ayub Med Coll Abbottabad. 2010;22(2):171–175. PMID: 21702296.
42. Ethiraj S, Sahoo AK, Das BM Spectrum of abdominal tuberculosis presenting as acute surgical emergency: Relevance in 21st century, a case series. Indian J Tuberc. 2023;70(4):422–9. PMID: 37968048.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



