Back to Journals » Drug Design, Development and Therapy » Volume 19
Design and Synthesis of Novel 5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidine Derivatives as VCP/p97 Inhibitors for the Treatment of Acute Myeloid Leukemia (AML)
Authors Wang X, Long Z, Wen T, Miao H, Ye X, Lei M, Zhu Y
Received 19 December 2024
Accepted for publication 12 May 2025
Published 27 May 2025 Volume 2025:19 Pages 4457—4479
DOI https://doi.org/10.2147/DDDT.S509036
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Muzammal Hussain
Xueyuan Wang,1 Zebo Long,1 Tiantian Wen,1 Hang Miao,1 Xinran Ye,2 Meng Lei,2,3 Yongqiang Zhu1,3
1College of Life Science, Nanjing Normal University, Nanjing, 210037, People’s Republic of China; 2College of Science, Nanjing Forestry University, Nanjing, 210037, People’s Republic of China; 3Jiangsu Chia Tai Fenghai Pharmaceutical Co. Ltd., Nanjing, 210046, People’s Republic of China
Correspondence: Meng Lei, Email [email protected] Yongqiang Zhu, Email [email protected]
Background: VCP/p97 plays an important role in endoplasmic reticulum related degradation pathways, and inhibition of p97 was shown to induce ER stress and subsequently cell death in a variety of solid tumors and hematoma. For acute myeloid leukemia (AML) cells, inhibition of p97 activity leads to the accumulation of ubiquitylated proteins, activation of unfolded protein response (UPR) and apoptosis.
Methods: We have designed and synthesized a series of novel 5,6,7,8-tetrahydropyridine[2,3-d]pyrimidine derivatives. After synthesizing all the target compounds, the optimal lead compound was identified through screening for enzyme inhibitory activity and anti-tumor cell proliferation activity. Subsequently, the liver microsomal stability and pharmacokinetics of the lead compound was investigated. Finally, the in vivo antitumor efficacy of the lead compound was evaluated to assess its potential for the treatment of acute myeloid leukemia (AML).
Results: Compound V12 and metabolite V13, which was screened by enzyme inhibition activity, showed strong inhibitory activities against a variety of cell lines with IC50 values less than 1 μM. In pharmacokinetic studies, after intragastric administration of V12 (10 mg/kg) in SD rats, V12 was rapidly metabolized toV13. The oral half-life of V13 in plasma was 3.5 h, and the Cmax and AUC0-inf values of V13 reached 1070 ng/mL and 1412 ng•h/mL, respectively, showing good pharmacokinetic properties. In addition, compound V12 showed a strong anti-tumor therapeutic effect in vivo and lower toxic side effects in the human AML (Molm-13) mouse xenograft model.
Conclusion: These results indicate that compound V12 is a potent p97 inhibitor with excellent in vitro and in vivo antitumor efficacy, which might provide a new therapeutic strategy for the treatment of AML.
Keywords: VCP/p97, enzyme inhibitory activity, AML, in vivo efficacy
Graphical Abstract:

Introduction
Acute myeloid leukemia (AML) is the most aggressive adult acute leukemia, characterised by clonal expansion of abnormally differentiated blasts of myeloid lineage. AML is one of the most common types of leukemia and has a poor prognosis. It causes more than 80,000 deaths worldwide each year, a number that is expected to double in the next 20 years.1 With the continuous research, people have a better understanding of the molecular pathophysiology of AML. In recent years, multiple targeted therapeutic drugs were approved by FDA for the treatment of newly diagnosed or relapsed/refractory AML.2–9 Contrary to traditional broad-spectrum cytotoxic chemotherapy drugs, the treatment of AML is becoming increasingly personalized.10,11 However, despite significant progress in the treatment of AML over the past few decades and the approval of various new drugs, most patients still faced a dismal prognosis. In particular, for AML patients aged 60 years and older, current treatment strategies can only induce remission, and frequent recurrence results in a 2-year survival rate of only 10%–50%, which is still a huge unmet clinical need.12
The ubiquitin proteasome system (UPS) is the main protein degradation system that play an important role in regulating cell cycle progression, transcriptional regulation, apoptosis, genome integrity, immune response, tumorigenesis, angiogenesis, and chemotherapy resistance.13–17 The uncontrolled rapid growth of tumor cells produces a large number of misfolded proteins, which are more dependent on UPS. Due to the poor prognosis of leukemia patients, targeting the components of the ubiquitin proteasome system for the treatment of leukemia has also received attention. For example, the MDM2 (E3 ligase) antagonist RG7112 exerts anti leukemia effects by stabilizing p53.18 Pevonedistat (TAK-924 or MLN4924) inhibits Cullin RING E3 ligase by inhibiting the stability of developmental downregulated protein 8 activating enzyme, leading to inhibition of ubiquitin activation and reduced AML growth.19
Valosin-containing protein (VCP/p97) is an ATPase that converts chemical energy into mechanical energy to perform a series of functions. The most extensively studied role of p97 is in endoplasmic reticulum associated degradation (ERAD).20,21 In this process, misfolded polyubiquitinated proteins in the endoplasmic reticulum must first be transferred to the cytoplasm under the action of p97 before being delivered to proteasomes for degradation.22,23 Since p97 also plays an important role in the UPS, researchers used the p97 inhibitor CB-5083 to screen a total of 131 human cancer cell lines from 16 cancer types. Compared with other cancer subtypes, AML cell lines were more sensitive to CB-5083, indicating that AML cell lines were highly dependent on p97.24 In addition, p97 inhibition leads to the accumulation of ubiquitinated proteins in AML cells, activates the unfolded protein response, induces apoptosis, and inhibit the proliferation of various AML cells at nanomolar concentrations.25
Although multiple p97 inhibitors have been developed (Figure 1), only CB-5083 and CB-5339 have conducted Phase I clinical trials. However, the clinical trial of CB-5083 has been terminated due to off-target effects causing vision problems.26 CB-5339, as a second-generation p97 inhibitor, has higher selectivity and has been validated in multiple AML models, making it the only p97 inhibitor currently under clinical development. Herein, we report our design and synthesis of a series of novel 5,6,7, 8-tetrahydropyridine [2,3-d] pyrimidine derivatives as p97 inhibitors, and then biologically evaluated in vitro and in vivo. Among the inhibitors, compared to compound CB-5339, compounds V12 and V13 exhibited better p97 inhibitory activities. In the screening of 11 kinds of tumor cells, compound V12 and its metabolite V13 showed strong proliferative inhibitory activities against a variety of cell lines, especially leukemia cell lines. In vivo studies have shown that compound V12 showed good pharmacokinetic properties, good anti-tumor efficacy and lower toxicity. These data further indicated that compound V12 had a good therapeutic effect on leukemia.
 |
Figure 1 Representative P97 small molecule inhibitor. |
Results and Discussion
Design Strategy of Novel p97 Inhibitors
Since compound CB-5083 showed off-target toxicity in clinical trials, replacing the 7,8-dihydro-5H-pyran[4,3-d]pyrimidine core with 5,6,7,8-tetrahydropyrido[2,3-d]pyrimidine to obtain the second-generation p97 inhibitor CB-5339 can avoid off-target toxicity.24 Therefore, we have retained the 5,6,7,8-tetrahydropyrido[2,3-d]pyrimidine core in this paper. The specific design strategy of novel p97 inhibitors were shown in Figure 2. Molecular docking studies showed that the end of the cavity where the benzylamine substituent bound to p97 was the residue Cys522. So, we introduced hydroxyl groups into the benzene ring or replace benzylamine N atoms with O and S atoms to investigate the effect on the activity. The amide substituent of indole forms a strong hydrogen bond with the hydroxyl group of threonine (T688). According to the principle of electron iso-platoon, we introduced different polar substituents to the indole ring in the hope of obtaining compounds with better activity. In addition, substituting the aromatic C atom with N atom could improve the water solubility of the compounds, and may also improve the biological activity of the compounds. Therefore, we introduced N atom at the A-F position of benzylamine benzene ring or indole ring. Herein, we present the discovery of compound V12, a potent and orally bioavailable p97 inhibitor.
 |
Figure 2 Design of VCP/p97 inhibitors. |
Chemistry
The syntheses of key intermediates 5, 6a-6c, 12 and 18a-18j have been summarized in Scheme 1. 1a-1e reacted with benzenesulfonyl chloride to give compounds 2a-2e (yield 85–93%). Intermediate 2 reacted with iodomethane to introduce a methyl at the 2-position of the indole, and then deprotected to obtain the intermediate 4a-4e (yield 47–66%). Compound 5 was prepared from 4b via palladium-catalyzed carbonyl intercalation reaction (yield 79%). 4c-4e reacted with zinc cyanide under the catalysis of Pd2(dba)3 to obtain 6a-6c (yield 53–78%). Intermediate 7 reacted with benzenesulfonyl chloride under the action of NaH to obtain 8 (yield 95%). Compound 8 was oxidized by m-CPBA to obtain 9, and then chlorinated with indole at position 4 under the action of phosphorus oxychloride to obtain intermediate 10 (with a total yield of 55% in two steps). A methyl group was introduced, and then deprotected to obtain the intermediate 11 (with a two-step total yield of 51%). 11 reacted with zinc cyanide under the catalysis of Pd2(dba)3 to obtain 12 (yield 53%). Commercially available 13a-13b reacted with urea and then produced 14a-14b in the presence of sodium hydroxide (yield 76–81%). Compound 14a-14b were reduced to obtain 15a-15b, followed by chlorination to obtain the intermediates 16a-16b. The introduction of Boc protection group on the amino group and replacement of 4-chloride with amines, alcohol or sulfhydryl group to give 18a-18j (yield 45–79%).
The syntheses of target molecules V1-V13 have been summarized in Scheme 2. The intermediates 18a-18j were coupled with 4a under palladium catalysis to obtain compound 19a-19j (yield 62–0%). The nitrile group was then hydrolyzed to formamide, and then deprotected to obtain the target molecules V1-V8 and V10-V11 (yield 52–68%). Target molecule V1 reacted with Lawesson’s reagent to give molecule V9 (yield 80%). Intermediate 18a was coupled with compound 5 to give methyl ester 20, saponification of which afforded carboxylic acid 21 (yield 70%). Amide-coupling reactions were performed on acid 21, and then deprotected to give inhibitors V12 (yield 62%). In addition, the Boc protective group of carboxylic acid 21 was directly removed to give the target molecule V13 (yield 85%).
The syntheses of target molecules V14-V20 have been summarized in Scheme 3. Similar to the synthesis method of molecules V1, an identical synthetic sequence was performed with 18a and indole 6a-c or 12 as starting materials, leading to compounds V14-V17. Finally, we chose three indoles with substituents at position 3 as raw materials. Similar to the synthesis route of compounds V1 or V12, intermediate 18a and indole 22a-22c were used as starting materials to obtain the target molecules V18-V20.
Biological Evaluation
Enzymatic
We first tested the inhibitory activities of all the target compounds against human p97 enzyme. CB-5339 was used as the standard. IC50 values of the target compounds V2-V20 and CB-5339 were shown in Table 1.
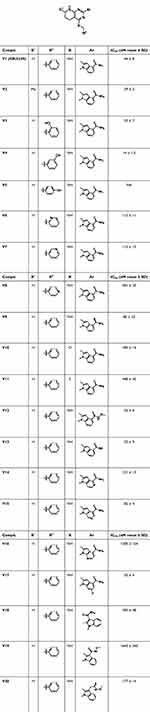 |
Table 1 Inhibitory Activities of Target Compounds on p97 Enzyme |
When the H atom at the R1 position (CB-5339, 44 nM) was replaced by a methyl group (2, 39 nM) showed potent p97 activity. Subsequently, a hydroxyl group was introduced into the benzene ring of the benzyl amino groups, and the activity of 2-hydroxybenzyl (3, 53 nM) was slightly reduced. When the hydroxyl group was located at position 4, the activity of the compound 5 was completely lost. Interestingly, 3-hydroxybenzyl (4, 14 nM) showed better inhibitory activity to CB-5339. Subsequently, substituting benzene ring with pyridine group (6–8, 115 nM-381 nM) did not improve the inhibitory activity of p97. In addition, we replace the N atom on benzylamine with an O atom (10, 185.2 nM) or S atom (11, 448.2 nM), the activity was reduced greatly. These results indicated that the benzylamine structure was necessary for maintaining activity. Attention was next focused on indole. When formamide (CB-5339) was replaced with thioformamide (V9, 80 nM), its activity decreased. Continuing to improve the activity, we introduce methoxy on formamide to obtain compound 12 (26 nM), which showed good inhibitory activity. In addition, replacing formamide with carboxylic acid (V13, 32 nM) also has good inhibitory activity. Subsequently, the effect of introducing N atoms into the indole benzene ring on the biological activity was investigated. The results show that the inhibitory activities of compounds 14 (121 nM) and 15 (82 nM) were decreased potencies in a certain extent, and the activity of compound 16 (1200 nM) was reduced by more than 25 times. Compared with compound CB-5339, the introduction of F atom at the 6-position of indole (V17, 35 nM) does not affect the inhibitory activity against p97. Finally, we transferred the indole 4 substituent of compound CB-5339 and V12 to the indole 3 position to obtain compounds V18 (393 nM) and V19 (1643 nM), and the activity decreased sharply. However, when we extended one carbon atom between the indole 3 substituent of compound V19 and the benzene ring to obtain compound V20 (177 nM), the inhibitory activity against p97 was enhanced, but still lower than V12. This may be due to the transfer of the 4-position substituent of indole to the 3-position, increasing the distance between the substituents and the amino acid residues at the p97 binding site, leading to a decrease in activity.
Cell
We selected compounds with IC50 value less than 200 nM for p97 inhibition to further evaluate their anti-proliferation activity against multiple myeloma cell line RPMI-8226 and colorectal cancer cell line HCT-116 (Table 2). The results showed that multiple compounds exhibited strong inhibitory effects on HCT-116 cells and RPMI8226 cells. In the proliferation inhibition results of multiple myeloma cell line RPMI-8226, compounds V4 (IC50, 0.3 μM), V12 (IC50, 0.5 μM) and V13 (IC50, 0.8 μM) showed better inhibitory activity, superior to the positive control CB-5339 (IC50, 0.9 μM). And the proliferation inhibition experiment results of colorectal cancer cell line HCT-116, the inhibitory activities of compounds V4 (0.7 μM) and V12 (0.7 μM) were the same as CB-5339 (0.7 μM), while compound V13 exhibits better inhibitory activity, with an IC50 value of only 0.4 μM.
 |
Table 2 Representative Compounds in vitro Anti-Proliferative Activities (IC50, μM, Mean ± SD) |
In subsequent PK studies, we found that compound V12 will rapidly metabolized to V13 in SD rats. Therefore, we further tested the proliferative inhibitory activity of compounds V12 and V13 on various tumor cell lines (Table 3). The results showed that compound V13 showed strong inhibitory activities against all tested hematoma cell lines and pancreatic cancer cell line BXPC-3, with IC50 value less than 1 μM. Furthermore, it exhibits comparable or even better cell inhibitory activity than CB-5339. So, based on the above results, compound V12 and V13 were selected to be further evaluated the drug availability.
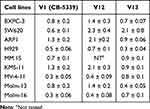 |
Table 3 Antiproliferative Activities of Compounds V12 and V13 on Various Cell Lines (IC50, μM, Mean ± SD) |
Binding Mode of Compound V12 and V13
To provide a structural basis for the competitive inhibition mechanism of p97 inhibitors, we attached V12 and V13 pairs to the p97 protein (PDB 6mck). As shown in Figure 3. The docking results suggested that the carbonyl group on indole 4 formamide formed strong hydrogen bond with the Thr688. In addition, the N atom at the benzylamine position forms a strong hydrogen bond with the Asp478. In addition, the Ile656 forms hydrophobic interactions with the indole-phenyl ring. Amino acid residues of Leu526, Leu527, Leu482, and Ile479 form a hydrophobic pocket, which forms a hydrophobic interaction with the benzene ring of benzylamine.
Microsome Stabilities
Based on the above results, we further tested the stability of the compounds V12 and V13 in five liver microsomes (Table 4). Compound V12 undergoes rapid metabolism in five species of liver microsomes, the T1/2 was only 13.3 minutes in human liver microsomes and even less than 10 minutes in the mouse, rat, and monkey liver microsomes. The T1/2 of compound V13 in the human, dog, and monkey liver microsomes were greater than 120 minutes, demonstrating good stability.
 |
Table 4 The Microsomal Stability Profiles of Compound V12 and V13a |
Pharmacokinetic
In order to investigate the oral absorption and metabolism of compound V12 in vivo, we further investigated the pharmacokinetics of V12 in SD rats, and detected both V12 and V13 in plasma (Table 5). The results showed that after gavage or tail vein injection of V12, the Cmax and AUC0-inf values in plasma were very low. We speculate that the indole 4 substituent of compound V12 may be metabolized into carboxylic acid (V13) in rats. Therefore, we then tested the plasma concentration of V13 in the same batch of plasma samples. The results indicate that after gavage or tail vein injection of V12, V12 rapidly metabolizes into V13. The oral T1/2 of compound V12 reached 3.52 h, almost three times that of the CB-5339. The oral Tmax, and Cmax of V13 were 0.25 h and 1070 ng/mL, respectively, and the plasma AUC0-inf value reached 1412 ng•h/mL, showed good PK properties. In addition, compound V12 had an oral absorption bioavailability of 29.7%, which has the potential to be developed as an oral drug.
 |
Table 5 Single-Dose Pharmacokinetic Data of Compounds V12 and CB-5339 in SD Rats |
Because of its potent activity and good pharmacokinetic properties, compound V12 was further evaluated for in vivo antitumor efficacy. We first examined the effects of compound V12 in nude mice weight (Figure 4a). In our previous study, the oral administration of CB-5339 at 100 mg/kg showed serious side effects in nude mice, and death occurred on day 7.27 Therefore, we chose an oral dosage of 50 mg/kg for compound CB-5339. The oral doses of compound V12 were 100 mg/kg and 300 mg/kg. The body weight of the nude mice in the administration group was comparable to that of the control group, and the nude mice were in good condition, even at a dose of 300 mg/kg, indicating better safety. Subsequently, we evaluated the in vivo antitumor efficacy of compound V12 in the Molm-13 nude mouse xenograft model, and the results were shown in Figure 4b. The CB-5339 was used as the standard. The results showed that compound V12 could significantly inhibit tumor growth, and the efficacy in vivo was comparable to that of the CB-5339. These results indicate that compound V12 has lower toxicity and a wider treatment window.
Conclusion
In summary, in order to develop novel AML therapeutics, we designed and synthesized a series of novel p97 inhibitors based on core 5,6,7,8-tetrahydropyrido[2,3-d]pyrimidine. Then, all target compounds were subsequently biologically investigated in vitro and in vivo. Among them, compound V12 and its metabolite V13 showed excellent in vitro antitumor activities against a variety of solid tumor and hematoma cell lines. Subsequently, we further investigated the liver microsomal metabolism of compound V12 and its absorption and metabolism after oral administration in rats. The results showed that compound V12 was metabolized rapidly in liver microsomes while its metabolite V13 was almost not metabolized. The pharmacokinetic results of SD rats showed that compound V12 was rapidly metabolized into V13 in the body, and the oral bioavailability was 29.7%. In addition, in the Molm-13 nude mouse xenograft model, compound V12 showed good in vivo efficacy and safety. These results showed that compound V12 could be developed as an oral drug for the treatment of AML.
Experimental Section
General Methods
Purchased reagents were used directly without treatment. The reaction was monitored by silica gel chromatography plate or LCMS (Shimadzu, LCMS-2020). Intermediates and target compounds were purified by silica gel column chromatography (300–400 mesh). The purity of the target compounds was detected by HPLC (Agilent, 1100 Series). NMR spectra were acquired on a Bruker Avance 400 spectrometer (1H NMR at 400 MHz, 13C NMR at 100 MHz). High-resolution mass spectra (HRMS) were recorded on a Bruker Solarix Fourier transform ion cyclotron resonance mass spectrometer.
Chemistry
2-Methyl-1H-Indole-4-Carbonitrile (4a)
Under ice bath conditions, intermediate 1a obtained by purchase (5 g, 35.17 mmol) was dissolved in THF (200 mL). The NaH (1.3 g, 52.75 mmol) was added slowly, and stirred for 30 min under the condition of ice bath. The 4-Benzenesulfonic acid chloride (6.5 g, 42.21 mmol) was added. The reaction was then transferred to room temperature and continued for 1 h. The reaction was quenched with 5% ammonium chloride solution and then extracted by EA. The organic phase was dried and concentrated, and the product 2a was purified by recrystallization (EtOAc/PE) (7.4 g, yield 75%). 1H NMR (400 MHz, Chloroform-d) δ 8.26 (d, J = 8.4 hz, 1H), 7.94–7.88 (m, 2H), 7.78 (d, J = 3.7 hz, 1H), 7.64–7.57 (m, 2H), 7.51 (dd, J = 8.5, 7.2 hz, 2H), 7.41 (t, J = 8.0 hz, 1H), 6.92 (d, J = 3.7 hz, 1H). MS (ESI): m/z 283.1 (MH+).
In a three-mouth flask, DIPA (5.9 g, 58.3 mmol) was dissolved in anhydrous THF (50 mL), then cooled to −78°C and n-Butyllithium solution (2.5 M in THF, 23.3 mL, 58.3 mmol) was slowly added. The reaction continued at −78°C for 1 h. The intermediate 2a (9.8 g, 29.2 mmol) was dissolved in anhydrous THF (10 mL) and slowly added to the reaction solution. The reaction continued at −78°C for 1 h and iodomethane (8.3 g, 58.3 mmol) was added. The reaction was then transferred to ambient temperature and continued for 2 h. The reaction was quenched with 5% ammonium chloride solution and then extracted by EA. The organic phase was dried and concentrated, and the product 3a was purified by recrystallization (EtOAc/PE), (6.8 g, yield 66%), white solid. MS (ESI): m/z 297.4 (MH+).
The intermediate 3a (5.4 g, 18.19 mmol) was dissolved in ethanol (100 mL) and then 4 mol/L of sodium hydroxide aqueous solution (18.2 mL) was added. Concentration to remove ethanol after reaction at 40°C for 12 hours, then added water (100 mL) and extracted with ethyl acetate. The organic phase was dried and concentrated, and the product 4a was purified by column chromatography (2.6 g, yield 90%), white solid. 1H NMR (400 MHz, Chloroform-d) δ 8.29 (s, 1H), 7.52 (dt, J = 8.1, 1.0 hz, 1H), 7.43 (dd, J = 7.5, 0.9 hz, 1H), 7.15 (t, J = 7.8 hz, 1H), 6.47 (dt, J = 2.1, 1.0 hz, 1H), 2.53 (s, 1H). MS (ESI): m/z 157.3 (MH+).
Methyl 2-Methyl-1H-Indole-4-Carboxylate (5)
In a high-pressure reactor, 4b (25.6 g, 121.8 mmol) was dissolved in methanol (200 mL), and palladium acetate (2.7 g, 12.2 mmol), 1,3-Bis (diphenylphosphino) propane (5.0 g, 12.2 mmol) and triethylamine (24.6 g, 243.6 mmol) was added. The reaction was degassed and reacted in carbon monoxide atmosphere at 80°C for 24 h. The reaction solution was concentrated and re-dissolved in EA. The organic phase was washed with water, dried and concentrated. And then, the intermediate 5 was purified by silica gel column (18.5 g, yield 80%), yellow solid. 1H NMR (400 MHz, Chloroform-d) δ 8.17 (s, 1H), 7.88 (d, J = 7.6 hz, 1H), 7.49 (d, J = 7.9 hz, 1H), 7.17 (t, J = 7.8 hz, 1H), 6.92–6.86 (m, 1H), 4.00 (s, 3H), 2.51 (s, 3H). MS (ESI): m/z 190.3 (MH+).
2-Methyl-1H-Pyrrolo[2,3-C]pyridine-4-Carbonitrile (6b)
At ambient temperature, to a solution of intermediate (4d) (280 mg, 1.3 mmol) in DMF (10 mL) was added ultrapure water (0.3 mL), and then exhaust with ultrasound. Subsequently, dppf (72 mg, 0.13 mmol), Pd2(dba)3 (60 mg, 0.065 mmol), and zinc cyanide (160 mg, 1.36 mmol) were added, and then stirred at 120°C for 1.5 h. 50 mL of water was added to the reaction solution, and extracted with ethyl acetate. The organic phase was washed with brine, dried and concentrated. And then, the intermediate 6b was purified by silica gel column chromatography (160 mg, yield 78%). MS ESI: m/z 158.1 (MH+).
2-Methyl-1H-Pyrrolo[3,2-C]pyridine-4-Carbonitrile (12)
According to the synthesis method of intermediate 2a, intermediate 8 was synthesized from commercially available 1H-pyrrolo[3, 2-C]pyridine (yield 95%, white solid). 1H NMR (400 MHz, CDCl3) δ 8.90 (s, 1H), 8.51 (d, J = 5.8 hz, 1H), 7.92 (m, 3H), 7.65–7.58 (m, 2H), 7.51 (dd, J = 8.5, 7.0 hz, 2H), 6.77 (d, J = 3.7 hz, 1H). MS ESI: m/z 259.0 (MH+)。
Compound 8 (4.2 g, 16.1 mmol) was dissolved in dioxane (40 mL) and m-CPBA (3.1 g, 17.7 mmol) was slowly added, then stirred for 5 h at ambient temperature. The saturated solution of sodium thiosulfate was added to quench the reaction, followed by the addition of an appropriate amount of water, and then extracted with DCM. The organic phase were washed with brine, dried and evaporated in vacuo. The intermediate 9 was purified by silica gel column chromatography (3 g, yield 68%), yellow solid. 1H NMR (400 MHz, CDCl3) δ 8.52 (dd, J = 1.7, 0.7 hz, 1H), 8.19 (dd, J = 7.2, 1.8 hz, 1H), 7.90 (m, 3H), 7.71–7.62 (m, 2H), 7.55 (m, 2H), 6.66 (dd, J = 3.7, 0.8 hz, 1H). MS ESI: m/z 275.0 (MH+).
At room temperature, compound 9 (3 g, 10.9 mmol) was dissolved in MeCN/dioxane (30/30, v/v) and POCl3 (1.8 g, 12 mmol) was slowly added. The solution was stirred for 18 h at 90°C. The reaction mixture was concentrated to remove POCl3, and extracted with ethyl acetate. The organic phase were washed with brine, dried and concentrated. Purification by column chromatography to provide intermediate 10 (2.6 g, yield 81%), yellow solid. 1H NMR (400 MHz, CDCl3) δ 8.27 (d, J = 5.8 hz, 1H), 7.92 (d, J = 1.4 hz, 1H), 7.87 (dd, J = 5.8, 0.9 hz, 1H), 7.68–7.60 (m, 2H), 7.53 (m, 2H), 6.82 (dd, J = 3.7, 0.9 hz, 1H). MS ESI: m/z 292.0 (MH+)。
In accordance with the synthesis of 4a in Scheme 1., intermediate 10 reacted with iodomethane and was then deprotected to give target compound 11. Whereafter, compound 11 was dissolved in NMP (10 mL) and then dppf (134 mg, 0.24 mmol), Pd2(dba)3 (114 mg, 0.13 mmol), zinc cyanide (78 mg, 0.66 mmol) and zinc powder were added. After the reaction displacement of nitrogen for 3 times, the reaction was conducted at 120°C for 18 h. Water (10 mL) was then added to the reaction solution, which was then filtered, and the filtrate was extracted with EA. The organic phase were washed with brine, dried with Na2SO4, filtered and concentrated. Purification by silica gel column chromatography to provide compound 12 (50 mg, yield 53%). MS ESI: m/z 158.1 (MH+).
Tert-butyl 4-(Benzylamino)-2-Chloro-6,7-Dihydropyrido[2,3-D]pyrimidine-8(5H)- Carboxylate (18a)
The urea (150 g, 2497 mmol) was added to the eggplant shaped bottle, heated to 195°C and melted, and then 13a (69 g, 499 mmol) was slowly added. After the reaction continued for 1.5 hours, the reaction temperature was lowered to 100°C. The NaOH aqueous solution was added (600 mL, 499 mmol) and stirred at 100°C for 1 h. The reaction mixture was then cooled down to ambient temperature and the pH of the mixture was adjusted to 4 with the addition of hydrochloric acid aqueous solution (1 mol/L), then filtered. The filter cake was dried to obtain intermediate 14a (66 g, 81% yield). 1H NMR (400 MHz, DMSO-d6) δ 11.68 (s, 1H), 11.47 (s, 1H), 8.60 (dd, J = 4.8, 1.9 hz, 1H), 8.26 (dd, J = 7.8, 1.9 hz, 1H), 7.25 (dd, J = 7.7, 4.8 hz, 1H). MS ESI: m/z 164.1 (MH+).
To a solution of intermediate 14a (66 g, 6 mmol) in acetic acid (900 mL) and water (600 mL), Pd(OH)2/C (6.6 g, 10% wt) was added. After the reaction displacement of H2 for 3 times, the reaction was refluxed at 70°C for 18 h under hydrogen atmosphere. Filtered and concentrated, and then dried under vacuum at 60°C to provide intermediate 15a (54 g, crude). 15a was used directly in the next step. 1H NMR (400 MHz, DMSO-d6) δ 10.10 (d, J = 6.1 hz, 2H), 5.97 (d, J = 2.7 hz, 1H), 3.17 (m, 2H), 2.17 (t, J = 6.2 hz, 2H), 1.74–1.56 (m, 2H). MS ESI: m/z 168.1 (MH+).
The intermediate 15a (30 g, 179.4 mmoL) and PCl5 (18.7 g, 89.7 mmoL) were dissolved in POCl3 (300 mL). The reaction mixture was then reacted at 130°C for 12 hours. Then, the reaction solution was concentrated to remove POCl3 and quenched by adding ice water (300 mm), extracted with EA. The organic phase were washed with brine, dried with Na2SO4, filtered and concentrated. Purification by silica gel column chromatography to give intermediate 16a (13.5 g, 36.8% yield). 1H NMR (400 MHz, CDCl3) δ 7.01 (s, 1H), 3.50 (m, 2H), 2.74 (t, J = 6.4 hz, 2H), 2.04–1.87 (m, 2H). MS ESI: m/z 204.1 (MH+).
At ambient temperature, intermediate 16a (13.5 g, 66.2 mmol), DMAP (1.6 g, 13.2 mmoL) and (Boc)2O (15.9 g, 72.8 mmol) were dissolved in DCM (200 mL). Then the reaction was stirred for 3 h. The reaction solution was washed with water and brine, dried and concentrated. Purification by silica gel column chromatography to give intermediate 17a (18 g, 89.4% yield). 1H NMR (400 MHz, CDCl3) δ 3.83–3.76 (m, 2H), 2.79 (t, J = 6.7 hz, 2H), 2.08–1.96 (m, 2H), 1.58 (s, 9H). MS ESI: m/z 305.3 (MH+).
TEA (34.9 g, 345 mm) was added to a solution of intermediate 17a (35 g, 115 mmoL) and benzylamine (18.5 g, 173 mm) in isopropyl alcohol (500 mL) and the reaction solution was refluxed at 70°C for 12 h. Then concentrated, and the crude product was dissolved with ethyl acetate and the organic phase was washed with water and brine, dried and concentrated. The crude product was purified by silica gel column chromatography to obtain the key intermediate 18a (27 g, 62.8% yield). 1H NMR (400 MHz, CDCl3) δ 7.43–7.31 (m, 5H), 4.81 (t, J = 5.4 hz, 1H), 4.71 (d, J = 5.3 hz, 2H), 3.78–3.69 (m, 2H), 2.33 (t, J = 6.8 hz, 2H), 2.05–1.94 (m, 2H), 1.57 (s, 9H). MS ESI: m/z 375.1 (MH+).
Tert-Butyl 4-(Benzyloxy)-2-Chloro-6,7-Dihydropyrido[2,3-D]pyrimidine-8(5H)- Carboxylate (18i)
At 0°C, NaH (40 mg, 0.99 mm) and benzyl alcohol (78 mg, 0.73 mm) were added to a solution of intermediate 17a (200 mg, 0.66 mmol) in DMF (5 mL). Then reacted at ambient temperature for 3 h. Water (50 mL) was added to quench the reaction, and extracted 3 times with EA. The organic phase were washed with brine, dried with Na2SO4. Concentrated and purification by column chromatography to provide intermediate 18i (112 mg, yield 45%), white solid. 1H NMR (400 MHz, Chloroform-d) δ 7.55–7.33 (m, 5H), 5.44 (s, 2H), 3.79–3.70 (m, 2H), 2.62 (m, 2H), 1.98–1.89 (m, 2H), 1.57 (s, 9H). MS ESI: m/z 376.1 (MH+)。
Tert-Butyl 4-(Benzylthio)-2-Chloro-6,7-Dihydropyrido[2,3-D]pyrimidine-8(5H)- Carboxylate (18j)
At ambient temperature, to a solution of intermediate 17a (200 mg, 0.66 mmol) in DMF (5 mL) were added benzyl mercaptan (98 mg, 0.79 mm) and K2CO3 (182 mg, 1.32 mm). Then the reaction was stirred at ambient temperature for 3 h. Water (50 mL) was added to quench the reaction, and extracted 3 times with EA. The organic phase were washed with brine, dried with Na2SO4. Concentrated and purification by column chromatography to provide intermediate 18j (310 mg, yield 79%), yellow solid. 1H NMR (400 MHz, CDCl3) δ 7.46–7.43 (m, 2H), 7.37–7.31 (m, 2H), 7.28 (s, 1H), 4.47 (s, 2H), 3.80–3.68 (m, 2H), 2.52 (t, J = 6.7 hz, 2H), 2.01–1.88 (m, 2H), 1.57 (s, 9H). MS ESI: m/z 392.1 (MH+)。
1-(4-(Benzylamino)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl)-2-Methyl-1H -Indole-4-Carboxamide (V1, CB-5339)
18a (200 mg, 0.53 mmol), 4a (83 mg, 1.8 mmol), Cs2CO3 (261 mg, 0.8 mmol), Pd2(dba)3 (73 mg, 0.08 mmol) and X-Phos (38 mg, 0.08 mmol) were dissolved in 1,4-dioxane (10 mL). Then the mixture was replaced with nitrogen for 3 times, and refluxed at 105°C for 4 h. The reaction solution was filtered, concentrated and then dissolved in EA, organic phase washed with water and brine, dried with Na2SO4. Concentrated and purification by column chromatography to provide intermediate 19a (235 mg, yield 89.7%). MS ESI: m/z 495.2 (MH+).
Urea hydrogen peroxide (219 mg, 2.38 mmol) and K2CO3 (66 mg, 0.48 mmol) were dissolved in water and then added to a DMSO solution of intermediate 19a (235 mg, 0.48 mmol), and reacted at 40°C for 4 h. Water (50 mL) was then added to the reaction solution, and extracted 3 times with EA. The organic phase were washed with brine, dried with Na2SO4. Concentrated and purification by column chromatography to provide intermediate (220 mg, 89.3% yield). MS ESI: m/z 513.2 (MH+).
The intermediate of the previous step (220 mg, 0.43 mmol) was dissolved in DCM (5 mL), TFA (1 mL) was added under ice bath conditions, and then the reaction continues at ambient temperature for 4 h. The reaction solution was concentrated to remove most of the TFA, then water was added, the pH was adjusted to 9 with 5% Na2CO3, and extracted with EA. The organic phase were washed with brine, dried with Na2SO4. Concentrated and purification by column chromatography to provide compound V1 (CB-5339) (120 mg, yield 67.6%). 1H NMR (400 MHz, DMSO-d6) δ 7.83 (d, J = 8.2 hz, 1H), 7.73 (s, 1H), 7.42 (d, J = 7.4 hz, 1H), 7.31 (p, J = 7.4 hz, 4H), 7.23 (d, J = 6.3 hz, 2H), 7.09 (d, J = 6.1 hz, 1H), 6.93–6.82 (m, 2H), 6.79 (s, 1H), 4.58 (s, 2H), 3.39 (s, 2H), 3.25 (d, J = 6.7 hz, 2H), 2.46 (s, 3H), 1.96–1.81 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 170.02, 160.13, 159.83, 154.49, 141.53, 138.81, 137.38, 128.66, 127.14, 126.92, 126.80, 125.44, 120.57, 116.69, 104.76, 87.57, 44.06 (d, J = 10.6 hz), 21.14, 20.18, 16.10.HRMS calcd for C24H24N6O [M + Na]+ 435.1904, found 435.1909。
1-(4-(Benzylamino)-7-Methyl-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl)-2- Methyl-1H-Indole-4-Carboxamide (V2)
Using intermediates 18b and 4a as raw materials, compound V2 was synthesized according to the preparation method of compound V1. 1H NMR (400 MHz, DMSO-d6) δ 7.88 (d, J = 8.2 hz, 1H), 7.71 (s, 1H), 7.42 (d, J = 7.4 hz, 1H), 7.36–7.26 (m, 5H), 7.23 (dd, J = 11.3, 4.7 hz, 2H), 7.06 (q, J = 4.7, 3.2 hz, 1H), 6.90 (t, J = 7.9 hz, 1H), 6.78 (s, 1H), 4.59 (d, J = 6.1 hz, 2H), 3.49–3.42 (m, 2H), 2.74–2.60 (m, 1H), 2.47 (s, 3H), 2.05–1.88 (m, 1H), 1.53 (m, 1H), 1.20 (d, J = 6.3 hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 170.05, 160.20, 159.85, 154.64, 141.51, 138.84, 137.40, 128.65, 127.16, 126.95, 126.80, 125.46, 120.57, 116.79, 104.79, 87.39, 55.39, 46.07, 44.14, 21.82, 19.35, 16.16. HRMS calcd for C25H26N6O [M + Na]+ 449.2060, found 449.2076.
1-(4-((2-Hydroxybenzyl)amino)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl) −2-Methyl-1H-Indole-4-Carboxamide (V3)
Using intermediates 18c and 4a as raw materials, compound V3 was synthesized according to the preparation method of compound V1. 1H NMR (400 MHz, DMSO-d6) δ 7.89 (d, J = 8.2 hz, 1H), 7.70 (s, 1H), 7.41 (d, J = 7.4 hz, 1H), 7.19 (s, 1H), 7.09 (d, J = 7.6 hz,1H), 7.04 (t, J = 7.7 hz, 1H), 6.94–6.69 (m, 6H), 4.51 (d, J = 5.3 hz, 2H), 3.26 (s, 2H), 2.47 (s, 3H), 1.88 (d, J = 7.7 hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 170.01, 160.15, 159.82, 155.12, 154.42, 138.79, 137.43, 127.74, 127.12, 125.46, 120.59 (d, J = 6.5 hz), 119.14, 116.67, 115.25, 104.81, 87.52, 21.14, 20.13, 16.01. HRMS calcd for C24H24N6O2 [M + Na]+ 451.1853, found 451.1860.
1-(4-((3-Hydroxybenzyl)amino)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl) −2-Methyl-1H-Indole-4-Carboxamide (V4)
Using intermediates 18d and 4a as raw materials, compound V4 was synthesized according to the preparation method of compound V1. 1H NMR (400 MHz, DMSO-d6) δ 7.87 (d, J = 8.2 hz, 1H), 7.70 (s, 1H), 7.42 (d, J = 7.4 hz, 1H), 7.18 (s, 1H), 7.10 (t, J = 7.9 hz, 1H), 7.00 (t, J = 6.2 hz, 1H), 6.91 (t, J = 7.9 hz, 1H), 6.79 (d, J = 8.5 hz, 2H), 6.71 (d, J = 6.4 hz, 2H), 6.65–6.58 (m, 1H), 5.76 (s, 1H), 4.51 (d, J = 5.5 hz, 2H), 3.26 (t, J = 5.3 hz, 3H), 2.49 (s, 6H), 1.89 (p, J = 6.1 hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 160.19, 159.85, 157.78, 154.55, 143.09, 138.83, 137.41, 129.59, 127.14, 125.42, 120.58 (d, J = 5.4 hz), 117.50, 116.82, 113.65 (d, J = 3.9 hz), 104.77, 87.52, 55.38, 44.01, 21.18, 20.19, 16.14. HRMS calcd for C24H24N6O2 [M + Na]+ 451.1853, found 451.1861.
1-(4-((4-Hydroxybenzyl)amino)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl) −2-Methyl-1H-Indole-4-Carboxamide (V5)
Using intermediates 18e and 4a as raw materials, compound V5 was synthesized according to the preparation method of compound V1. 1H NMR (400 MHz, DMSO-d6) δ 8.18 (s, 1H), 7.95 (dd, J = 8.0, 1.3 hz, 1H), 7.70 (s, 1H), 7.26 (d, J = 2.3 hz, 1H), 7.12–6.99 (m, 2H), 6.97–6.92 (m, 2H), 6.88–6.71 (m, 2H), 6.59–6.52 (m, 2H), 6.22–6.18 (m, 1H), 5.76 (s, 1H), 4.09 (s, 2H), 3.23 (dt, J = 7.3, 3.6 hz, 2H), 2.47 (s, 3H), 2.38 (t, J = 6.3 hz, 2H), 1.83 (p, J = 6.3 hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 172.05, 161.55, 160.48, 155.27, 154.51, 136.74, 135.32, 132.93, 129.84, 129.63, 126.58, 124.50, 121.19, 120.12 (d, J = 10.6 hz), 115.14, 114.42, 113.94, 87.38 (d, J = 3.6 hz), 55.38, 29.33, 21.14, 20.33, 13.12. HRMS calcd for C24H24N6O2 [M + Na]+ 451.1853, found 451.1860.
2-Methyl-1-(4-((Pyridin-2-Ylmethyl)amino)-5,6,7,8-Tetrahydropyrido[2,3-D] Pyrimidin-2-Yl)-1H-Indole-4-Carboxamide (V6)
Using intermediates 18f and 4a as raw materials, compound V6 was synthesized according to the preparation method of compound V1. 1H NMR (400 MHz, DMSO-d6) δ 8.52 (dd, J = 5.3, 1.8 hz, 1H), 7.80 (d, J = 8.3 hz, 1H), 7.75 (td, J = 7.7, 1.8 hz, 1H), 7.70 (s, 1H), 7.41 (dd, J = 7.5, 0.9 hz, 1H), 7.30 (d, J = 7.9 hz, 1H), 7.26 (m, 1H), 7.19 (s, 1H), 7.10 (t, J = 6.1 hz, 1H), 6.87 (t, J = 7.9 hz, 2H), 6.76 (s, 1H), 4.65 (d, J = 5.3 hz, 2H), 3.36 (s, 2H), 3.27 (dd, J = 7.1, 3.8 hz, 2H), 2.40–2.36 (s, 3H), 1.90 (p, J = 6.0 hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 169.98, 160.78, 160.11, 159.89, 154.52, 149.27, 138.80, 137.36, 137.05, 127.14, 125.46, 122.20, 120.72, 120.56, 116.61, 104.77, 88.08–86.71, 46.29, 21.14, 20.15, 15.99. HRMS calcd for C23H23N7O [M + Na]+ 436.1856, found 436.1862.
2-Methyl-1-(4-((Pyridin-3-Ylmethyl)amino)-5,6,7,8-Tetrahydropyrido[2,3-D] Pyrimidin-2-Yl)-1H-Indole-4-Carboxamide (V7)
Using intermediates 18g and 4a as raw materials, compound V7 was synthesized according to the preparation method of compound V1. 1H NMR (400 MHz, DMSO-d6) δ 8.51 (d, J = 2.2 hz, 1H), 8.45 (dd, J = 4.8, 1.6 hz, 1H), 7.81 (d, J = 8.3 hz, 1H), 7.74–7.72 (m, 1H), 7.68 (dd, J = 6.0, 4.0 hz, 1H), 7.43 (d, J = 7.4 hz, 1H), 7.35 (dd, J = 7.9, 4.8 hz, 1H), 7.21 (s, 1H), 7.11 (t, J = 6.1 hz, 1H), 6.91 (t, J = 7.9 hz, 1H), 6.88 (d, J = 2.8 hz, 1H), 6.79 (s, 1H), 4.60 (d, J = 5.5 hz, 2H), 3.26 (p, J = 3.1 hz, 2H), 2.47 (d, J = 6.6 hz, 2H), 2.45 (s, 3H), 1.88 (p, J = 6.0 hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 170.03, 159.95, 154.49, 148.70, 148.11, 138.75, 137.35, 136.89, 134.91, 127.14, 125.53, 123.90, 120.59, 116.53, 104.78, 87.80, 55.39, 42.02, 21.09, 20.14, 16.06. HRMS calcd for C23H23N7O [M + Na]+ 436.1856, found 436.1864.
2-Methyl-1-(4-((Pyridin-4-Ylmethyl)amino)-5,6,7,8-Tetrahydropyrido[2,3-D] Pyrimidin-2-Yl)-1H-Indole-4-Carboxamide (V8)
Using intermediates 18h and 4a as raw materials, compound V8 was synthesized according to the preparation method of compound V1. 1H NMR (400 MHz, DMSO-d6) δ 8.54–8.48 (m, 2H), 7.74–7.68 (m, 2H), 7.44–7.38 (m, 1H), 7.32–7.27 (m, 2H), 7.20 (s, 1H), 7.14 (t, J = 6.1 hz, 1H), 6.90 (d, J = 2.5 hz, 1H), 6.84 (t, J = 7.9 hz, 1H), 6.77 (s, 1H), 4.58 (d, J = 5.5 hz, 2H), 3.27 (m, 2H), 2.52 (d, J = 6.5 hz, 2H), 2.39 (d, J = 1.0 hz, 3H), 1.90 (t, J = 5.9 hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 170.02, 159.98, 154.47, 151.02, 149.82, 138.73, 137.33, 127.13, 125.48, 122.15, 120.53, 116.42, 104.76, 87.85, 67.49, 55.38, 43.45, 25.59, 21.09, 20.15, 15.94. HRMS calcd for C23H23N7O [M + Na]+ 436.1856, found 436.1863.
1-(4-(Benzylamino)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl)-2-Methyl- 1H-Indole-4-Carbothioamide (V9)
Dissolve the target product V1 (100 mg, 0.24 mmol) in anhydrous THF (20 mL) and then Lawesson’s reagent (146.7 mg, 0.36 mmol) was added. The mixture was refluxed for 12 h and concentrated. The crude product was extracted with DCM after adding water. The organic phase were washed with brine, dried and concentrated. The crude product was purified by silica gel column chromatography to provide compound V9 (83 mg, yield 80%). 1H NMR (400 MHz, DMSO-d6) δ 9.81 (s, 1H,), 9.27 (s, 1H), 7.78 (d, J = 8.3 hz, 1H), 7.37–7.19 (m, 6H), 7.07 (t, J = 6.1 hz, 1H), 6.92–6.80 (m, 2H), 6.61 (s, 1H), 4.58 (d, J = 6.0 hz, 2H), 3.29–3.23 (m, 2H), 2.45 (s, 3H), 1.88 (p, J = 6.1, 5.7 hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 201.99, 160.16, 159.86, 154.48, 141.54, 138.47, 136.96, 133.07, 128.65, 126.93, 126.78, 125.16, 120.61, 120.18, 115.88, 104.02, 87.64, 44.13, 21.14, 20.19, 16.08. HRMS calcd for C24H24N6S [M + H]+ 429.1856, found 429.1872.
1-(4-(Benzyloxy)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl)-2-Methyl-1H -Indole-4-Carboxamide (V10)
Using intermediates 18i and 4a as raw materials, compound V10 was synthesized according to the preparation method of compound V1. 1H NMR (400 MHz, DMSO-d6) δ 8.11 (d, J = 8.3 hz, 1H), 7.78 (s, 1H), 7.50 (d, J = 7.5 hz, 2H), 7.45–7.36 (m, 4H), 7.35–7.30 (m, 1H), 7.26 (s, 1H), 7.07 (t, J = 7.9 hz, 1H), 6.90 (s, 1H), 5.42 (s, 2H), 3.30 (q, J = 3.9, 3.4 hz, 2H), 2.61 (s, 3H), 2.58 (s, 2H), 1.83 (p, J = 5.7 hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 169.94, 165.40, 162.03, 154.12, 138.87, 137.85, 137.36, 128.92, 128.13, 127.54, 127.46, 125.74, 121.09 (d, J = 10.3 hz), 116.80, 105.71, 91.35, 67.51, 20.40, 19.41, 16.44. HRMS calcd for C24H23N5O2 [M + Na]+ 436.1744, found 436.1748.
1-(4-(Benzylthio)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl)-2-Methyl-1H-Indole-4-Carboxamide (V11)
Using intermediates 18j and 4a as raw materials, compound V11 was synthesized according to the preparation method of compound V1. 1H NMR (400 MHz, DMSO-d6) δ 8.10 (d, J = 8.2 hz, 1H), 7.78 (s, 1H), 7.73 (d, J = 2.6 hz, 1H), 7.50 (dd, J = 7.5, 0.9 hz, 1H), 7.41–7.36 (m, 2H), 7.35–7.28 (m, 2H), 7.27–7.22 (m, 2H), 7.07 (t, J = 7.9 hz, 1H), 6.91 (t, J = 0.9 hz, 1H), 4.48 (s, 2H), 3.30 (dd, J = 7.1, 4.0 hz, 2H), 2.62 (d, J = 1.0 hz, 3H), 1.86 (p, J = 5.7, 5.1 hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 169.92, 162.95, 159.71, 154.44, 138.74, 137.96, 137.41, 129.26, 128.97, 127.53 (d, J = 10.8 hz), 125.82 (d, J = 3.3 hz), 121.07 (d, J = 6.1 hz), 116.51, 105.61, 104.67, 33.09, 22.09, 20.38, 16.26. HRMS calcd for C24H23N5OS [M + Na]+ 452.1515, found 452.1524.
1-(4-(Benzylamino)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl)-N-Methoxy-2-Methyl-1H-Indole-4-Carboxamide (V12)
Using intermediates 18a and 5 as raw materials, compound V12 was synthesized according to the preparation method of intermediate 19a. 1H NMR (400 MHz, DMSO-d6) δ 8.28 (d, J = 8.3 hz, 1H), 7.71 (ddd, J = 8.6, 5.9, 3.4 hz, 2H), 7.38–7.29 (m, 4H), 7.25 (td, J = 6.0, 2.6 hz, 1H), 6.99 (t, J = 7.9 hz, 1H), 6.89–6.86 (m, 1H), 4.67 (d, J = 5.9 hz, 2H), 3.88 (s, 3H), 3.77–3.69 (m, 2H), 2.56 (s, 2H), 2.54–2.54 (m, 3H), 2.04–1.91 (m, 2H), 1.43 (s, 9H). MS ESI: m/z 528.3 (MH+).
The LiOH⋅H2O (2.38 g, 56.9 mmol) was dissolved in water (5 mL) and added to the THF/MeOH (15 mL/5 mL) solution of intermediate 20 (5 g, 9.48 mmol). The solution was refluxed at 60°C for 6 h, and then concentrated. The crude product was extracted with methyl tert-butyl ether after adding water. Then the pH of the water phase was adjusted to 4 and extracted with EA. The organic phase were washed with brine, dried with Na2SO4, and then concentrated. The crude product 21 was used directly for the next step without purification (4.4 g, yield 90%).
21 (200 mg, 0.39 mmol) was dissolved in THF (10 mL), and then methoxyammonium chloride (33 mg, 0.39 mmol), DIPEA (202 mg, 1.56 mmol), EDCI (112 mg, 0.59 mmol) and HOBt (80 mg, 0.59 mmol) were added, and then stirred at ambient temperature overnight. The reaction was quenched with water, extracted with ethyl acetate, dried with Na2SO4, and concentrated. The residue was purified by column chromatography (160 mg, yield 75%). The resulting product was deprotected with TFA and purified to give compound V12 (80 mg, yield 62%). 1H NMR (400 MHz, DMSO-d6) δ 11.45 (s, 1H), 7.84 (d, J = 8.3 hz, 1H), 7.30 (dq, J = 16.4, 8.2 hz, 5H), 7.10 (t, J = 6.3 hz, 1H), 6.89 (t, J = 8.0 hz, 1H), 6.86 (s, 1H), 6.65 (s, 1H), 5.77 (d, J = 1.5 hz, 1H), 4.59 (d, J = 5.6 hz, 2H), 3.72 (d, J = 1.6 hz, 3H), 3.37 (dd, J = 9.5, 1.6 hz, 2H), 3.26 (t, J = 5.3 hz, 2H), 2.47 (s, 3H), 1.88 (p, J = 5.8 hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 166.06, 160.15, 159.82, 154.42, 141.52, 139.17, 137.23, 128.66, 126.85, 123.22, 120.69, 120.00, 116.98, 104.09, 87.63, 63.63, 55.40, 44.12, 21.13, 20.18, 16.08. HRMS calcd for C25H26N6O2 [M + H]+ 443.2190, found 443.2202。
1-(4-(Benzylamino)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl)-2-Methyl- 1H-Indole-4-Carboxylic Acid (V13)
Intermediate 21 was deprotected under TFA to give the target product V13, brown powder. 1H NMR (400 MHz, DMSO-d6) δ 7.91 (d, J = 8.2 hz, 1H), 7.67 (d, J = 7.5 hz, 1H), 7.39–7.27 (m, 4H), 7.23 (m, 1H), 6.93 (t, J = 7.9 hz, 1H), 6.85 (s, 1H), 4.59 (d, J = 5.4 hz, 2H), 3.35 (s, 2H), 3.26 (t, J = 3.6 hz, 2H), 2.48 (s, 3H), 1.89 (t, J = 5.8 hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 168.80, 160.15 (d, J = 6.8 hz), 159.84 (d, J = 5.5 hz), 154.39, 141.50, 139.92, 137.51, 128.67, 128.35, 126.90, 126.81, 123.75, 120.68, 118.51, 104.89, 44.06 (d, J = 10.5 hz), 21.12, 20.17, 16.05. HRMS calcd for C24H23N5O2 [M + Na]+ 436.1744, found 436.1759.
1-(4-(Benzylamino)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl)-2-Methyl-1H-Pyrrolo[3,2-C]pyridine-4-Carboxamide (V14)
Using intermediates 18a and 12 as raw materials, compound V14 was synthesized according to the preparation method of compound V1. 1H NMR (400 MHz, DMSO-d6) δ 8.06 (d, J = 3.1 hz, 1H), 7.99 (d, J = 5.6 hz, 1H), 7.70 (d, J = 5.6 hz, 1H), 7.47 (d, J = 3.0 hz, 1H), 7.37–7.28 (m, 4H), 7.26–7.21 (m, 1H), 7.15 (t, J = 6.1 hz, 1H), 7.05 (s, 1H), 6.92 (d, J = 2.8 hz, 1H), 4.59 (d, J = 5.5 hz, 2H), 3.27 (p, J = 3.3 hz, 2H), 2.52–2.51 (m, 3H), 1.89 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 168.19, 160.13, 159.81, 154.01, 142.43, 141.43, 141.25, 141.15, 139.49, 128.73, 126.88, 123.82, 111.55, 104.52, 88.78–87.48 (m), 44.16, 21.05, 20.17, 16.02. HRMS calcd for C23H23N7O [M + Na]+ 436.1856, found 436.1876.
1-(4-(Benzylamino)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl)-2-Methyl-1H-Pyrrolo[2,3-C]pyridine-4-Carboxamide (V15)
Using intermediates 18a and 6b as raw materials, compound V15 was synthesized according to the preparation method of compound V1. 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.56 (s, 1H), 7.96 (s, 1H), 7.46 (s, 1H), 7.30 (d, J = 4.5 hz, 4H), 7.23–7.15 (m, 1H), 7.11 (t, J = 6.2 hz, 1H), 7.00 (d, J = 2.6 hz, 1H), 6.85 (s, 1H), 4.62 (d, J = 5.5 hz, 2H), 3.27 (q, J = 3.7 hz, 2H), 2.54 (s, 3H), 2.47 (d, J = 7.7 hz, 2H), 1.88 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 168.35, 160.12, 159.81, 154.13, 144.13, 141.28, 138.82, 137.87, 134.04, 132.42, 128.66, 126.93, 120.80, 104.77, 87.75, 44.22, 21.07, 20.17, 16.65. HRMS calcd for C23H23N7O [M + Na]+ 436.1856, found 436.1848.
1-(4-(Benzylamino)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl)-2-Methyl- 1H-Pyrrolo[2,3-B]pyridine-4-Carboxamide (V16)
Using intermediates 18a and 6c as raw materials, compound V16 was synthesized according to the preparation method of compound V1. 1H NMR (400 MHz, DMSO-d6) δ 8.20 (s, 1H), 8.15 (d, J = 4.9 hz, 1H), 7.99 (s, 1H), 7.57 (s, 1H), 7.39 (d, J = 5.0 hz, 1H), 7.33–7.26 (m, 4H), 7.19 (m, 1H), 7.03 (t, J = 6.1 hz, 1H), 6.84 (d, J = 2.6 hz, 1H), 6.65 (d, J = 1.2 hz, 1H), 4.51 (d, J = 5.3 hz, 2H), 3.25 (dt, J = 6.9, 3.5 hz, 2H), 2.46 (d, J = 6.3 hz, 2H), 2.18 (d, J = 1.0 hz, 3H), 1.88 (t, J = 5.7 hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 168.50, 160.78–159.34 (m), 153.16, 150.17, 141.58, 141.40, 139.64, 132.47, 128.52, 127.43, 126.81, 118.30, 114.72, 99.89, 88.94, 44.00, 20.97, 20.19, 13.78. HRMS calcd for C23H23N7O [M + Na]+ 436.1856, found 436.1748.
1-(4-(Benzylamino)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl)-6-Fluoro- 2-Methyl-1H-Indole-4-Carboxamide (V17)
Using intermediates 18a and 6a as raw materials, compound V17 was synthesized according to the preparation method of compound V1. 1H NMR (400 MHz, DMSO-d6) δ 7.90 (dd, J = 10.7, 2.4 hz, 1H), 7.83 (d, J = 5.3 hz, 1H), 7.44–7.24 (m, 5H), 7.20 (dp, J = 6.4, 4.2 hz, 1H), 7.05 (dt, J = 13.2, 6.2 hz, 1H), 6.92 (d, J = 2.9 hz, 1H), 6.79 (s, 1H), 5.76 (s, 1H), 4.61 (d, J = 6.0 hz, 2H), 3.31–3.22 (m, 2H), 2.48 (s, 2H), 2.45 (s, 3H), 1.88 (t, J = 5.8 hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 168.76, 160.15, 159.81, 159.10, 156.78, 154.48, 141.36, 139.58, 137.27, 128.65, 126.88, 125.96, 124.04, 108.40, 104.78, 103.64, 87.55, 55.38, 44.12, 21.13, 20.18, 16.46. HRMS calcd for C24H23FN6O [M + H]+ 431.1990, found 431.1924.
1-(4-(Benzylamino)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl)-2-Methyl-1H-Indole-3-Carboxamide (V18)
Using intermediates 18a and 22a as raw materials, compound V18 was synthesized according to the preparation method of compound V1. 1H NMR (400 MHz, DMSO-d6) δ 7.70 (d, J = 7.9 hz, 1H), 7.49 (d, J = 8.3 hz, 1H), 7.31 (m, 4H), 7.25–7.20 (m, 1H), 7.18 (s, 1H), 7.11 (t, J = 6.1 hz, 1H), 7.08–7.04 (m, 1H), 6.92 (m, 1H), 6.86 (d, J = 2.6 hz, 1H), 4.57 (d, J = 5.4 hz, 2H), 3.27 (p, J = 3.4 hz, 2H), 2.54 (s, 3H), 1.90 (q, J = 5.9 hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 167.47, 160.23, 159.98, 153.88, 141.43, 139.62, 135.67, 128.65, 126.99, 126.80, 126.22, 122.10, 121.30, 119.75, 113.41, 111.41, 88.21, 44.03, 21.03, 20.18, 14.11. HRMS calcd for C24H24N6O [M + Na]+ 435.1904, found 435.1913.
1-(4-(Benzylamino)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl)-N-Methoxy-2-Methyl-1H-Indole-3-Carboxamide (V19)
Using intermediates 18a and 22b as raw materials, compound V19 was synthesized according to the preparation method of compound V12. 1H NMR (400 MHz, DMSO-d6) δ 11.01 (s, 1H), 7.64 (d, J = 7.9 hz, 1H), 7.51 (d, J = 8.3 hz, 1H), 7.36–7.26 (m, 4H), 7.25–7.20 (m, 1H), 7.14 (t, J = 6.1 hz, 1H), 7.08 (t, J = 7.5 hz, 1H), 6.94 (t, J = 7.7 hz, 1H), 6.88 (s, 1H), 4.57 (d, J = 5.3 hz, 2H), 3.72 (s, 3H), 3.27 (t, J = 5.6 hz, 2H), 2.51 (s, 3H), 2.51 (s, 2H), 1.89 (t, J = 5.8 hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 160.22, 159.90, 153.74, 141.39, 139.76, 135.71, 128.66, 126.98, 126.82, 125.90, 122.40, 121.49, 119.69, 113.56, 88.27, 63.67, 43.99 (d, J = 10.0 hz), 21.00, 20.17, 14.17. HRMS calcd for C25H26N6O2 [M + Na]+ 465.2009, found 465.2016。
2-(1-(4-(Benzylamino)-5,6,7,8-Tetrahydropyrido[2,3-D]pyrimidin-2-Yl)-2- Methyl-1H-Indol-3-Yl)-N-Methoxyacetamide (V20)
Using intermediates 18a and 22c as raw materials, compound V20 was synthesized according to the preparation method of compound V12. 1H NMR (400 MHz, DMSO-d6) δ 11.25 (s, 1H), 7.68 (d, J = 8.2 hz, 1H), 7.47 (d, J = 7.8 hz, 1H), 7.32 (h, J = 6.2 hz, 4H), 7.23 (td, J = 6.6, 2.3 hz, 1H), 7.05–6.95 (m, 2H), 6.91–6.84 (m, 1H), 6.77 (d, J = 2.4 hz, 1H), 4.60 (d, J = 6.0 hz, 2H), 3.56 (s, 3H), 3.35 (s, 2H), 3.31–3.20 (m, 2H), 2.47 (s, 2H), 2.40 (s, 3H), 1.89 (p, J = 6.0 hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 167.59, 160.19, 159.89, 154.67, 141.57, 135.82, 134.96, 128.63, 126.95, 126.77, 121.61, 120.31, 117.94, 113.84, 108.18, 87.42, 63.61, 44.06, 28.96, 21.21, 20.20, 13.39. HRMS calcd for C26H28N6O2 [M + Na]+ 479.2166, found 479.2168.
Biological Activity Assay
In vitro p97 Enzyme Inhibition Assay
The inhibitory activities of the compounds against p97 enzyme were tested as follows. The compounds to be tested were dissolved in DMSO and serially diluted 3-fold from 10 μM, each concentration point repeat 3 times. P97 hexamer enzyme (60 μg/mL, purchased from the Public Protein/Plasmid Library, China) and ATP (100 μM) were added to the 384-well plate with a total volume of 4 μL. After mixing, the mixture was incubated at 25°C for 60 min. According to the instructions, ADP Glo reagents 1 and 2 (Promega, Madison, WI) were added. CLARIO Star Plate Reader was used to detect luminescence values of each test hole and calculate the inhibition rate. The IC50 values of each compound were calculated by fitting the nonlinear curves with GraphPad Prism 8.
In vitro Antitumor Activity Assay
The tumor cell lines used in the cell proliferation inhibition experiment were all from Jiangsu Chia Tai Fenghai Pharmaceutical Co., Ltd. In 384-well plate, 18 μL medium (1000 cells) was added to each well. After 24 h incubation at 37°C, 5% CO2, the compounds to be tested were dissolved in DMSO, and then 2μL of the compounds were added to each well (the content of DMSO was less than 0.1%), the initial concentration of the compounds was 10 μM, and diluted twice, with a total of 10 concentration points. After 72 hours of continuous culture, 10 μL Cell-Titer glo reagent was added to each well, and the absorbance of each well at 450 nm was tested. The inhibition rates of each concentration point were calculated according to the absorbance, and the IC50 values were calculated by fitting the sigmoid curve with GraphPad software.
Molecular Docking
The three-dimensional (3D) crystal structure of VCP protein (PDB ID: 6MCK) was retrieved from the RCSB PDB database (URL: http://www.rcsb.org/). The protein was then pretreated by the protein preparation workflow module in the Maestro software. The molecular docking procedure was conducted utilizing the Glide module within Maestro software under extra precision (XP) mode. The docking box was centered on the native ligand CB5083 extracted from the crystal structure. Optimal docking poses were selected based on the Glide scoring function (Gscore) and docking score parameters.
In vitro Liver Microsome Stabilities
The 100 μL total reaction system used to test the stability of the liver microsomes of the compounds contained PB buffer (100 mm, pH 7.4), MgCl2-PB buffer (3 mm), compounds (1 μM), microsomal protein (0.5 mg/mL, Corning) and β-NADPH (1 mm). The control group without β-NADPH. The samples were incubated at 37°C and sampled at 0, 5, 15, 30, and 60 min. To each sample, 300 μL of ice-cold acetonitrile containing internal standards was added, mixed, and centrifuged at 5500 g for 10 minutes, and the supernatant was collected. 150 μL of supernatant was aspirated and 150 μL of water was added. After mixing, the content of the compounds to be tested was detected by LC-MS/MS and the curve was fitted.
Rat Pharmacokinetics
The male rats were obtained from Hunan SJA Laboratory Animal Co., Ltd. Twelve male sd rats (240–280 g) were randomly divided into 4 groups with 3 rats in each group. The compounds were dissolved in DMSO and castor oil, and then normal saline was added (DMSO/castor oil/NaCl=5%/5%/90%). The compounds were administered intravenously at a dose of 1mg/kg and orally at a dose of 10 mg/kg. Blood was collected from the fundus venous plexus at 2 min, 5 min, 15 min, 30 min, 1 h, 2 h, 4 h, 8 h and 24 h, and then added to a centrifuge tube containing heparin sodium. Whole blood was centrifuged at 6800 rpm for 6 min at 4°C to obtain plasma. The concentration of compounds in plasma was analyzed by LC-MS/MS, and non-ventricular pharmacokinetic parameters were analyzed by WinNonlin 6.3 program.
In vivo Antitumor Activity Study
BALB/c nude mice (female, 6–8 W, 18–22 g) were purchased from Vital River Laboratory Animal Technology Co., Ltd. All animal experiments were approved by the Laboratory Animal Ethics Committee of Nanjing Comer Pharma (protocol number: KMYY-AW-2021). Throughout the experiment, animal body weight and tumor volume were measured twice a week, and the clinical signs of the animals were observed and recorded daily. Molm-13 cells in logarithmic growth stage were collected and injected subcutaneously into Balb/c nude mice with 5 × 106 cells per mice. When the average tumor volume reached 80 ~ 120 mm3, the animals were randomly grouped (n = 5), negative control group, 50 mg/kg CB-5339 (positive control) group and 100 mg/kg V12 group. The compounds was intragastric once a day at a volume of 0.1 mL/10 g animal body weight. The tumor volume was calculated using the formula: V = length (mm) × [width (mm)]2×0.5. The male Balb/c nude mice were housed in SPF (Specific Pathogen Free) laboratory animal centers.
Statistical Analysis
All quantitative data were expressed as the mean ± SD of three independent replicates. Data analysis was performed using GraphPad Prism 8.0.2, with analysis of variance (ANOVA) used for statistical analysis. Statistical significance was defined as *p < 0.05, **p < 0.01, and ***p < 0.001.
Supporting Information
1H NMR, 13C NMR and HRMS spectra of compound V12 and V13 (Figure S1–S6).
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Statement
The in vivo antitumor activity study was entrusted to Nanjing Comer Biomedicine Co., LTD. All procedures were conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. All experimental protocols were reviewed and approved by the Nanjing Comer Biomedicine Co., LTD (protocol number: KMYY-AW-2021).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018;392(10159):2052–2090. doi:10.1016/S0140-6736(18)31694-5
2. Castaigne S, Pautas C, Terré C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, Phase 3 study. Lancet. 2012;379(9825):1508–1516. doi:10.1016/S0140-6736(12)60485-1
3. Riva M, Rizzo L, Mancini V, et al. Enasidenib, an oral therapy in mutant IDH2 relapsed/refractory acute myeloid leukemia: a real-life single center experience. Blood. 2020;136(Supplement 1):20–21. doi:10.1182/blood-2020-141987
4. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–464. doi:10.1056/NEJMoa1614359
5. Dinardo CD, Stein EM, De Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386–2398. doi:10.1056/NEJMoa1716984
6. Yu H, Wang C, Lei Y, et al. Single-institution experience of venetoclax combined with azacitidine in newly diagnosed acute myeloid leukemia patients. Int Immunopharmacol. 2024;127:111232. doi:10.1016/j.intimp.2023.111232
7. Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2018;33(2):379–389. doi:10.1038/s41375-018-0312-9
8. Panayiotidis P, Hayslip J, Mendes W, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137–2145. doi:10.1182/blood.2020004856
9. Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728–1740. doi:10.1056/NEJMoa1902688
10. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–2221. doi:10.1056/NEJMoa1516192
11. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi:10.1182/blood-2016-08-733196
12. Estey EH. Acute myeloid leukemia: 2021 update on risk‐stratification and management. Am J Hematol. 2020;95(11):1368–1398. doi:10.1002/ajh.25975
13. Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67(1):425–479. doi:10.1146/annurev.biochem.67.1.425
14. Bassermann F, Eichner R, Pagano M. The ubiquitin proteasome system-implications for cell cycle control and the targeted treatment of cancer. Biochimica Et Biophysica Acta. 2014;1843(1):150–162. doi:10.1016/j.bbamcr.2013.02.028
15. Yinon BN. Regulatory functions of ubiquitination in the immune system. Nat Immunol. 2002;3(1):20–26. doi:10.1038/ni0102-20
16. Narayanan S, Cai CY, Assaraf YG, et al. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resist Updates. 2020;48:100663. doi:10.1016/j.drup.2019.100663
17. Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway-destruction for the sake of construction. Physiol Rev. 2002;82(2):373–428. doi:10.1152/physrev.00027.2001
18. Andreeff M, Kelly KR, Yee K, et al. Results of the phase I trial of RG7112, a small-molecule MDM2 antagonist in leukemia. Clin Cancer Res. 2016;22(4):868–876. doi:10.1158/1078-0432.CCR-15-0481
19. Swords RT, Erba HP, DeAngelo DJ, et al. Pevonedistat (MLN4924), a First-in-Class NEDD8-activating enzyme inhibitor, in patients with acute myeloid leukaemia and myelodysplastic syndromes: a Phase 1 study. Br J Haematol. 2015;169(4):534–543. doi:10.1111/bjh.13323
20. Franz A, Ackermann L, Hoppe T. Create and preserve: proteostasis in development and aging is governed by Cdc48/p97/VCP. Biochim Biophys Acta. 2014;1843(1):205–215. doi:10.1016/j.bbamcr.2013.03.031
21. Stolz A, Hilt W, Buchberger A, et al. Cdc48: a power machine in protein degradation. Trends Biochem Sci. 2011;36(10):515–523. doi:10.1016/j.tibs.2011.06.001
22. Raasi S, Wolf DH. Ubiquitin receptors and ERAD: a network of pathways to the proteasome. Semin Cell Dev Biol. 2007;18(6):780–791. doi:10.1016/j.semcdb.2007.09.008
23. Wolf DH, Stolz A. The Cdc48 machine in endoplasmic reticulum associated protein degradation. Biochim Biophys Acta. 2012;1823(1):117–124. doi:10.1016/j.bbamcr.2011.09.002
24. Roux B, Vaganay C, Vargas JD, et al. Targeting acute myeloid leukemia dependency on VCP-mediated DNA repair through a selective second-generation small-molecule inhibitor. Sci, trans med. 2021;13(587):1168. doi:10.1126/scitranslmed.abg1168
25. Gopalakrishnapillai A, Szczęśniak PP, Heidelberger JB, et al. VCP inhibition induces an unfolded protein response and apoptosis in human acute myeloid leukemia cells. PLoS One. 2022;17(4):e0266478. doi:10.1371/journal.pone.0266478
26. Leinonen H, Cheng C, Pitkänen M, et al. A p97/valosin-containing protein inhibitor drug CB-5083 has a potent but reversible off-target effect on phosphodiesterase-6. J Pharmacol Exp Ther. 2021;378(1):31–41. doi:10.1124/jpet.120.000486
27. Wang XY, Wen TT, Miao H, et al. Discovery of a new class of valosine containing protein (VCP/P97) inhibitors for the treatment of colorectal cancer. Bioorg Med Chem. 2022;74:117050. doi:10.1016/j.bmc.2022.117050
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






