Back to Journals » Drug Design, Development and Therapy » Volume 19
Design of Experiments Assisted Formulation Optimization and Evaluation of Efavirenz Solid Dispersion Adsorbate for Improvement in Dissolution and Flow Properties
Authors Mujtaba MA , Rashid MA , Alhamhoom Y , Gangane P , Jagtap MJ, Akbar MJ, Wathore SA, Kaleem M , Elhassan GO, Khalid M
Received 11 January 2025
Accepted for publication 1 May 2025
Published 7 May 2025 Volume 2025:19 Pages 3715—3734
DOI https://doi.org/10.2147/DDDT.S517021
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Tamer Ibrahim
Md Ali Mujtaba,1,2 Md Abdur Rashid,3 Yahya Alhamhoom,3 Purushottam Gangane,4 Mohini Janardan Jagtap,4 Mohammad J Akbar,5 Sandeep Ashokrao Wathore,6 Mohammed Kaleem,7 Gamal Osman Elhassan,8 Mohammad Khalid9
1Department of Pharmaceutics, Faculty of Pharmacy, Northern Border University, Arar, Saudi Arabia; 2Center for Health Research, Northern Border University, Arar, Saudi Arabia; 3Department of Pharmaceutics, College of Pharmacy, King Khalid University, Abha, Saudi Arabia; 4Department of Pharmaceutics, Dadasaheb Balpande College of Pharmacy, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India; 5Department of Pharmaceutics, College of Pharmacy, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 6Department of Pharmaceutics, SVP College of Pharmacy, Hatta, Maharashtra, India; 7Department of Pharmacology, Dadasaheb Balpande College of Pharmacy, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra, India; 8Department of Pharmaceutics, College of Pharmacy, Qassim University, Buraidah, Saudi Arabia; 9Department of Pharmacognosy, College of Pharmacy, Prince Sattam bin Abdulaziz University, Al-Kharj, Saudi Arabia
Correspondence: Purushottam Gangane, Email [email protected] Mohammed Kaleem, Email [email protected]
Background: Efavirenz (EFZ) is an anti-HIV drug that has been administered as first-line treatment, which exhibits low solubility and poor oral bioavailability. Therefore, the current study aimed to develop a solid dispersion adsorbate (SDA) to enhance the dissolution rate and flow properties of EFZ for solid oral dosage forms.
Methods: The SDA of EFZ was prepared using the fusion method with PEG-6000 and poloxamer-188 as carriers, along with avicel PH-102 and aerosil-200 as adsorbents. 32 full factorial approach was employed to formulate the SDA and evaluate the effects of two independent factors X1: the ratio of PEG-6000 to EFZ in the solid dispersion, and X2: the ratio of aerosil-200 to the solid dispersion. The dependent factors analyzed were Y1: the time required for 85% of the drug release, and Y2: angle of repose.
Results: The optimized formulation (F9) was selected through numerical optimization, demonstrating the desired drug release and excellent flow properties of the pre-compressed SDA. Fourier transform infrared (FTIR) spectroscopy, Differential scanning calorimetry (DSC), X-ray diffraction (XRD), and Scanning electron microscopy (SEM) of SDA showed the transformation of crystalline to amorphous form of EFZ, which is responsible for improving drug dissolution. The direct compression method was used to prepare SDA-EFZ tablets (equivalent to 25 mg EFZ) along with plain EFZ. The dissolution efficiency increased from 50.68% for plain EFZ tablets to 96.18% for EFZ-SDA tablets. Furthermore, the cumulative percentage drug release (%CDR) from SDA tablets was nearly double that of plain EFZ tablets. Stability testing indicated no significant changes in drug content and %CDR of the SDA tablets.
Conclusion: The findings of this study suggest that the SDA method is an effective approach for enhancing the dissolution and flow characteristics of EFZ and may serve as an alternative strategy for preparing solid dosage forms in commercial applications.
Keywords: solid dispersion adsorbate, efavirenz, factorial design, drug delivery, in vitro dissolution
Graphical Abstract:

Introduction
The common problem with the number of active pharmaceutical ingredients (API) is the low and variable dissolution rate and oral bioavailability due to poor aqueous solubility at physiological pH. Efavirenz (EFZ), an antiviral agent, is widely used drugs for the treatment of HIV infection.1 It comes under the biopharmaceutical classification system (BCS) class II category because of its high permeability and poor water solubility. EFZ is chemically (S)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1- benzoxazin-2-one 6 (Figure 1).2 It shows low oral bioavailability (40–45%), which is practically insoluble over a pH range of 1.2–8.0 and exhibits a pKa of 10.2.3,4 In such cases, dissolution is the rate-limiting step in drug absorption, and improvement in solubility can augment the bioavailability and reduce variability in the bioavailability of a drug. Several methods have been used to augment the solubility and dissolution rate of EFZ.5 These include the preparation of microspheres, emulsions, nanosuspensions, self-microemulsions, and solid dispersions (SD). SD denotes the dispersion of one or more water-insoluble drugs in a hydrophilic inert solid base using various methods, including the solvent evaporation method, fusion method, and melting-solvent method.3,6,7 The formation of SD aids in boosting the bioavailability of drugs by increasing their solubility and dissolution rate in water. The drug molecules remained in a high-energy state in SD. This makes it difficult to compress, pulverize, flow, and return to the crystalline form with reduced solubility during storage.8 The SD adsorbate (SDA) is a novel hybrid technology that combines melt and SD adsorption to form a free-flowing powder. This method involves the adsorption of SD onto a hydrophilic, high surface area adsorbent, which tends to increase solubility, dissolution rates, and bioavailability.9,10 The adsorbent also aids in the compressibility of SD, which is used to formulate it as a tablet or capsule dosage form.11 Nanoscale pores with large surface areas and pore volumes were observed in porous adsorbents. Porous adsorbents/carriers used in pharmaceuticals include porous ceramic, porous silicon dioxide, and magnesium aluminometasilicate (Neusilin).12,13 The fusion method was used for solid dispersion formulation, because it is simple and economical.14 This method avoids the use of organic solvents and achieves intermixing of drugs with polymer at the molecular level. Thus, the absence of the risk associated with the residual solvents is one of the advantages of this method. Easy processing is an added benefit because no special treatment is needed to bring the melt mixture to a solid state.15
 |
Figure 1 Chemical structure of efavirenz. |
The Design of Experiment (DoE) is an effective statistical method for variable selection and optimization.16 It is based on the simultaneous adjustment of several elements to determine the parameter configuration that maximizes one or more outputs of interest while requiring the fewest experimental runs for testing, resulting in overcoming the time, effort, and financial barriers that have traditionally been major hurdles in formulation research.17,18 Plackett-Burman, Fractional Factorial Design, Full Factorial Design (FFD), and Response Surface Methodology are some of the most common DoE approaches used in pharmaceutical product development. FFD is the best method for investigating several intervention components because it improves model prediction ability by estimating the major effects from the average of other effects.19,20
The current study aimed to formulate and optimize the SD of EFZ to enhance the dissolution of the drug with the fusion method using PEG-4000, PEG-6000, and Poloxamer-188 as solubilizers for the SD formulation. A three-level, two-factor (32) FFD was used to optimize the formulation and identify the main effect and interaction effect between the examined components on dissolution and flow properties. The secondary goal was to adsorb the melt dispersion onto a porous adsorbent to transform it into free-flowing and compressible granules. Adsorption on porous adsorbent also enhances the surface area, which contributes to improving the dissolution rates. Avicel PH-102 and aerosil-200 were used as adsorbents, as both adsorbents are reported to have good adsorptive properties.21 The optimal formulation was chosen by numerical optimization and evaluated using Fourier transform infrared (FTIR) spectroscopy, Differential scanning calorimetry (DSC), X-ray diffraction (XRD), scanning electron microscopy (SEM), and in vitro dissolution analysis. The optimized SDA was formulated as a tablet and characterized, including the stability testing of the optimized formulation.
Materials and Methods
Materials
Efavirenz (Glenmark Pharmaceuticals Ltd., Mumbai, India), Aerosil-200 (Mylan Labs, Hyderabad, India), and Spray dried lactose (Cipla, Mumbai, India) were obtained as gift samples. PEG-4000, PEG-6000, Poloxamer-188, Avicel PH102 (microcrystalline cellulose), talc, and magnesium stearate were purchased from S.D. Fine Chemicals Ltd. India. All other compounds used in this study were of analytical grade.
Solubility Study of Efavirenz
The saturation solubility of the drug was examined in 0.1 N HCl, acetate buffer (pH 4.4, 6.4, 6.8, and 7.4), and distilled water. The maximum amount of drug was added to 10 mL of the above media separately in 25 mL amber-colored volumetric flask and kept at an orbital shaker bath. Shaking was performed for 24 h at 50 rpm and 37 ± 0.5°C.22 The samples were collected and passed through a 0.22 µm syringe filter. The filtrate was suitably diluted with the same solvent, and then the absorbance of EFZ was measured at 247nm using a validated UV-spectrophotometric procedure.23 The linearity was obtained in the range of 3‐15 µg/mL and showed a good linear relationship with R2 = 0.9827.
Screening of Carriers Based on Phase Solubility Studies
The solubility of EFZ in PEG-4000, PEG-6000, and Poloxamer-188 was evaluated as previously described methods.24 In separate volumetric flasks, 10 mL of 4%, 6%, 8%, 10%, and 12% solutions of each polymer in water was taken. EFZ was added in excess quantity to each of the above-mentioned solutions and was kept on a shaker for 24 hrs at 37 °C. The solutions were then filtered using Whatman’s filter paper. UV-spectrophotometric (Shimadzu U-1800, Japan) was used to quantify the amount of dissolved drug in the sample. Stability constants were calculated using Higuchi-Connors theory.25
The apparent stability constant was computed using the following formula.
Gibbs’ free energy of transfer (ΔG°) values (distilled water to polymer solution) were calculated using the following formula:
So – Solubility of EFZ in polymer
Ss – Solubility of EFZ in water
Formulation of Solid Dispersion (SD)
Screening of Carrier to EFZ Ratio in SD
We used PEG-6000 and Poloxamer-188 as carriers/hydrophilic solubilizers to prepare SD. These are commonly used carriers in preparing SD as they possess good surfactant properties, low melting point (~58–63°C), and adequate safety for oral consumption.26,27 These are FDA-approved excipients used to lower viscosity, improve wettability, and increase stability.28 Pilot batches of EFZ SD were prepared using the fusion method, and different carriers to EFZ ratios of 0.25:1, 0.50:1, 0.75:1, 1:1, and 2:1 were tested in SD formulation. These different ratios were tested for each carrier, resulting in 10 trial batches. The carrier was melted in a petri dish, and the EFZ was dispersed by continuous stirring on the melted carriers. The content in the petri dish was instantly cooled to room temperature to obtain SD, which was then collected, sifted, and stored in a desiccator until subsequent analysis.
Screening of Adsorbent to SD Ratio
Avicel PH-102 and Aerosil-200 were used as adsorbents to improve the flow characteristics of the SD. Aerosil-200 has an adsorption capacity of 3.2 mL/g and a surface area of 300 m2/g. It is used as a free-flowing agent to enhance the properties of the powders. It also improves the distribution of active pharmaceutical ingredients.29 The selected EFZ-SD formulation was adsorbed onto avicel PH-102 and aerosil-200 separately at different ratios to prepare EFZ-SD adsorbate (SDA) granules. The angle of repose of EFZ-SDA granules was measured using the fixed funnel method.
Preparation of EFZ-SDA Granules
EFZ-SD was prepared as described in the previous section using PEG 6000 as a carrier, which was chosen based on an earlier investigation. The PEG 6000 was melted in a porcelain dish on a water bath at 60°C. EFZ was added to the molten carrier and dispersed by stirring. During the dispersal of EFZ in PEG 6000, the mass shall be maintained at 60°C to achieve uniform distribution of EFZ in a carrier.22 Adsorbent (aerosil-200) was then added to the molten mixture and mixed. This mass was allowed to cool and dry at room temperature to produce SDA. The dried SDA was passed through a sieve. 20 to obtain SDA granules.30 SDA granules were stored in a desiccator until subsequent analysis.
Experimental Design
To measure the influence of the two independent factors on dissolution and flow properties, we used a three-level, two-factor (32) FFD with the Design-Expert program version 13 (Stat-Ease, Inc., Minneapolis, MN). The numbers −1, 0, and +1 were used to designate the low, medium, and high degrees of each variable, respectively. The independent variables chosen were the ratio between the carrier (PEG-6000) and EFZ in the SD, denoted by X1, and the ratio between the adsorbent (Aerosil-200) and SD, denoted with X2. FFD suggested nine formulations as shown in Table 1. Dissolution characteristics (time needed for 85% drug release - t85: Y1) and flow characteristics (angle of repose: Y2) were chosen as dependent variables. The responses were calculated using an interactive and polynomial statistical model as follows:
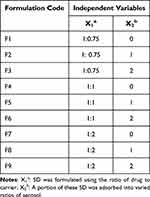 |
Table 1 32 FFD Layout of Different Batches of SDA Formulation |
In the above equation, the dependent variable is denoted by Y, and the average response across all trials is denoted by b0. The predicted coefficients for the factors X1, X2, and X1X2 are denoted as b1, b2, b11, b12, and b22. They depicted the mean result of varying each factor from the lowest to the highest value individually. X1X2 is an interaction term that demonstrates how responses change when two factors are altered simultaneously. To explore the non-linearity in a relationship, polynomial terms ( ,
,  ) were added.31 The Design-Expert software enabled the production of polynomial equations and all significant values. The control of variables influencing the outcomes was also evaluated. Polynomial equations for the influenced variables, viz., drug release and the angle of repose, were determined. Non-significant coefficients were eliminated to simplify the generated polynomial equations. To confirm the experimental data, we used analysis of variance (ANOVA) and the F-test. Graphical optimization and the overlay plots were used to select the ideal preparation.32
) were added.31 The Design-Expert software enabled the production of polynomial equations and all significant values. The control of variables influencing the outcomes was also evaluated. Polynomial equations for the influenced variables, viz., drug release and the angle of repose, were determined. Non-significant coefficients were eliminated to simplify the generated polynomial equations. To confirm the experimental data, we used analysis of variance (ANOVA) and the F-test. Graphical optimization and the overlay plots were used to select the ideal preparation.32
Characterization of SDA
Fourier Transform Infrared (FTIR) Analysis
Potassium bromide (KBr) press was used to compress the powder at 20 psi for 10 min to form the pellets of pure EFZ, PEG-6000, PEG-6000 SD, Aerosil 200, and SDA. The samples were scanned using an FTIR spectrophotometer (Alpha, Bruker, Germany) over the scan range of 4000 cm−1 to 400 cm−1.33,34
Differential Scanning Calorimetry (DSC) Analysis
The thermal behavior of the EFZ, PEG 6000, and SDA was investigated using a DSC instrument (DSC60 Shimadzu, Japan). The samples were weighed and sealed in a sample pan and then heated at a rate of 10° C/min in an inert environment flushed with dry nitrogen. Thermal behavior was investigated for a temperature range of 35–300 °C.34,35
X-Ray Diffraction (XRD) Analysis
PEG-6000, EFZ, and SDA were grounded into powder in a mortar and pestle. The XRD spectra of the powdered samples were recorded in a Philips X-ray diffractometer with scanning angle spanning between 0° and 40° of 2θ.20,35
Surface Morphology Analysis
The morphological features of EFZ and SDA were evaluated using scanning electron microscopy (SEM) (Model - XL30 ESEM with EDAX, Philips, Eindhoven, Netherlands). Using 10 mA current, the dried EFZ and SDA were gold-coated using a sputter cutter. After gold coating, the sample was viewed at different magnifications at a voltage of 15 kV.36,37
Determination of Drug Content Uniformity in SD and SDA
SD and SDA equivalent to 10 mg of EFZ were solubilized in 50 mL of methanol. Then, 1 mL solution was withdrawn and diluted 10 times with methanol. The absorbance of the sample was measured at 247 nm using UV-visible spectrophotometry to determine the amount of drug content.38
In vitro Dissolution Studies of SD and SDA
The in vitro dissolution tests were carried out for the SD and SDA with a USP Type 2 (paddle) dissolution apparatus with 900 mL of 1% w/v sodium lauryl sulfate (SLS) solution.39 SLS was used in a dissolving medium because of its ability to maintain sink conditions with poorly water-soluble drugs.40 The SD and SDA equivalent to 25 mg of EFZ were placed in dissolution media. The temperature was maintained at 37±0.5°C, and the paddle speed was 50 rpm. 5 mL of the solution was removed at each interval, and each aliquot was replaced with an equal volume of fresh dissolving medium. The aliquots were filtered and spectrophotometrically measured at 247 nm. The dissolution tests were carried out in triplicate. The percent drug release was calculated by comparing the absorbance of the diluted aliquots, and the dissolution profile was obtained by plotting the percent drug release against time.
Preparation of Tablets
The EFZ-SDA tablets were prepared using the direct compression method. Table 2 shows the compositions of SDA and plain EFZ tablets. The optimized formulation of SDA granules was blended thoroughly for 10 min with sodium starch glycolate and microcrystalline cellulose. The mixture was blended with magnesium stearate and talc for another five minutes and compressed using a rotary tablet machine. The prepared tablets were examined for different quality control tests, such as hardness, friability, and weight variation.41
 |
Table 2 Formula of SDA Tablets and Plain EFZ Tablets |
Stability Studies
The stability of the formulation was determined according to ICH guidelines Q1A (R2).42 The optimized formulation was kept in a stability chamber for six months at 40±2°C and 75±5% RH for accelerated stability studies. The samples were placed in vials that were sealed using rubber plugs and aluminum caps. After the stability period, the samples were removed, and in-vitro parameters were evaluated.43
Results and Discussion
Phase Solubility Analysis
The solubility of EFZ in 0.1 N HCl, acetate buffer (pH 4.4, 6.4, 6.8, and 7.4), and distilled water was assessed. EFZ is sparingly soluble in water and slightly soluble in HCl or acetate buffer (Figure 2A).44 The drug did not show pH-dependent solubility in GI tract pH conditions. EFZ is hydrophobic and contains functional groups such as Cl, CF3, cyclopropane, and alkyl groups as shown in Figure 1. It has an NH, which can be protonated, but NH-C=O makes it enol enol-extended conjugation. With a pKa of 10.2, the drug is weakly basic, and a log P value of 4.6 indicates the low solubility of EFZ. A phase solubility analysis was conducted to determine the solubility of EFZ in three polymeric carriers (PEG-4000, PEG-6000, and poloxamer-188) to select the best carrier for the formulation of SD (Figure 2B). For each polymer, it was observed that an increase in their concentration from 4% to 12% resulted in an increase in the solubility of EFZ, but EFZ demonstrated noticeably greater solubility in poloxamer-188 and PEG-6000 compared to PEG-4000. The regression coefficient (R2) for EFZ in PEG-4000, PEG-6000, and poloxamer-188 were 0.9726, 0.9812, and 0.9826, respectively, indicating a good correlation between the variables.
Gibbs' free energy (ΔG°) is associated with the energy that determines whether a reaction will be spontaneous or not. Negative Gibbs free energy transfer values show the spontaneous solubilization process. While ΔG° values were calculated from the solubility of EFZ in pure distilled water and the polymer solution, the stability constant was estimated using the phase solubility graph. Table 3 shows the spontaneous solubilization of EFZ in the polymer solution as indicated by the negative ΔG° value. More negative ΔG° values were recorded for poloxamer-188 and PEG-6000 than for PEG-4000. Poloxamer-188 exhibited a higher stability constant than those of PEG-4000 and PEG-6000. The stability constant was affected by the slope values. The solubilization of the drug by the polymer is directly proportional to its slope.45 Based on these results, we selected PEG-6000 and poloxamer-188 as the carrier for SD preparation.
 |
Table 3 Gibbs Free Energy for EFV in Different Carrier Solutions |
Effect of Concentration of Carriers on Drug Release
The in vitro dissolution pattern of EFZ with Poloxamer-188 or PEG-6000 SD at various ratios and per se EFZ is shown in Figure 3. As the proportion of carriers to the drug increased from 0.25:1 to 2:1, an increase in the cumulative drug release was observed. Drug release from formulations with carrier-to-drug ratios of 0.75:1, 1:1, and 2:1 did not differ significantly. However, an analysis of the dissolution profiles indicated that an increase in the proportion of carriers increased the amount of drug released during the given period.45 The release of EFZ from SD with PEG-6000 as the carrier was higher than that from SD with poloxamer-188. Thus, SD with a 2:1 ratio of PEG-6000 to the drug was selected for the optimization process in the preparation of SDA.
Selection of Adsorbent to SD Ratio
The prepared SD exhibited poor flow properties and compressibility, which made it challenging to pulverize. To improve the flow properties of SD, we used aerosil-200 and avicel PH-102 as adsorbents, owing to their good adsorptive properties. When SD was adsorbed onto Aerosil-200, the angle of repose values decreased significantly compared with the results obtained from SD adsorption on avicel PH-102 (Figure 4). The angle of repose of the SDA prepared with aerosil-200 was less than 25°, indicating good flow properties. This result was not observed when avicel PH-102 was used as an adsorbent for EFZ SD, with an angle of repose in the range of 27° to 34°. This may be ascribed to the greater specific surface area of aerosil-200 (200 ± 20 m2/g) than that of Avicel PH102 (1.21–1.30 m2/g).46 This allowed for a larger adsorption on the surface of Aerosil-200. A significant improvement in the flow property and reduction in the angle of repose were observed when the ratio of adsorbent to SD was increased from 2:(2:1) to 3:(2:1). However, no such improvement in flow was observed with a further increase in the adsorbent to SD ratio from 3:(2:1) to 4:(2:1). A further increase in the ratio was not observed as the results were approached constancy. Furthermore, given the bulky nature of aerosil-200, higher proportions of aerosil-200 were not considered to minimize the bulk volume of the formulation.
 |
Figure 4 Influence of different adsorbents on angle of repose. Carrier to drug ratio was kept constant at 2:1, adsorbent concentration was increased from 2 to 4, and angle of repose was measured. |
Evaluation of the Effect of Formulation Variables on t85 (Y1) Using Factorial Design
Figure 5 depicts the response surface plot, which reveals the correlation between independent variables and the time required for drug release to reach 85% (t85). For nine developed formulations (F1-F9), t85 was in the range of 81.58 to 90.35 min. The cumulative drug release (%) of all nine formulations was estimated, and we observed that all formulations showed a good release pattern, releasing more than 85% drug in 30 min, except formulations F1, F3, and F5 (Table 4). Table 5 describes the built-in equation that connects the response t85 (Y1) with the transitioned factor. The model was significant, as indicated by the ANOVA results. For response t85, the correlation coefficient (r2) is 0.9832, indicating a good fit. The generated polynomial equations were simplified (p>0.10) by removing insignificant coefficients. The coefficients with p values less than 0.05 were kept. Consequently, the following polynomial equation was derived as part of a reduced model for t85.
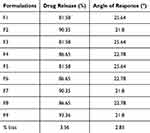 |
Table 4 Percent Drug Release and Angle of Repose of SDA Formulations |
 |
Table 5 Polynomial Equation Derived for Dependent Responses |
 |
Figure 5 (A) Response surface plot and (B) Contour plot for the influence of different adsorbents on drug release of SDA. |
In the condensed model, the value of the coefficient X1 has a negative sign. The condensed model showed that the ratio between carrier and drug (X1) was inversely proportional to the t85 value. The t85 values for all formulations were found to be less than 30 min except for trials F1, F3, and F5. The decrease in t85 may be associated with the wetting effect of the carrier and micellar solubilization.47
Evaluation of the Influence of Formulation Variables on Flow Characteristic (Y2) Using Factorial Design
The association between the independent factors and the angle of repose varied from 21.80 to 25.64 for all nine formulations (F1-F9) (Table 4), according to the response surface plot (Figure 6). Table 5 presents the fitted equation that explains the relationship between the response (Y2) and the transformed factor. A high coefficient and F value indicate that the regression model fits the data well. The correlation coefficient (r2) for the angle of repose was >0.9, indicating the goodness of fit of the model. Nonsignificant terms (p>1.0) were removed from the polynomial equation to run the model. We kept the coefficients with p values ˂ 0.05. Consequently, the following polynomial equation was derived from a reduced model for the angle of repose.
 |
Figure 6 (A) Response surface plot and (B) Counter plot for the angle of repose of SDA. |
A negative value for the coefficient X2 (−1.92) indicates that it significantly affects the angle of repose. In addition to formulations F1, F3, and F5, the angle of repose given in Table 4 was less than 25° for all formulations. Trials F1, F3, and F5 had poor flow properties and compressibility compared with the remaining trials because they were not adsorbed onto the aerosil-200, making them difficult to pulverize. According to reports, aerosil-200 is a porous calcium silicate with many surface-located interparticle (12 µm) and intraparticle (0.15 µm) pores. This increased the surface area available for the adsorption of SD and enhanced the flow characteristics.48 The simplified model demonstrated a decrease in the angle of repose with an increase in the ratio of the adsorbent to SD (X2). An angle of repose below 25° indicated excellent flow characteristics. These findings support the previously published literature.49
Checkpoint Batch
To confirm the precision of the model and the contribution of the generated polynomial equation to response prediction, a checkpoint set was established. Theoretical values were determined by varying the values in the polynomial equation. Following that, the experimental and predicted results were compared at a 95% confidence level and reported as a percentage of bias (Table 4). There was no discernible difference between them; hence, the model was verified because the percentage bias value was below 4%.
Selection of Optimization Batch
All responses with various targets were optimized using graphical optimization (overlay plot, Figure 7). Constraints on the results of the dependent and independent variables led to the creation of an ideal formulation. The constraints of the % cumulative drug release minimum (80% at 60 min) and angle of repose maximum (25°C) were common for all formulations. Design-Expert software was used to calculate the suggested levels of independent variables. The design space is visible in the white area of the overlay plot. The optimized batch for the applied constraints was at the upper spot of the design space. As an optimized formulation, F9 is recommended to maintain both independent values at an optimum value.
 |
Figure 7 Optimization of SDA granules using (A) an overlay plot and (B) a desirability plot. |
Characteristics of Optimized Formulation
FTIR Spectroscopy Analysis
FTIR spectroscopy was used to examine the possible interactions between the drug and the chosen polymer.50 FTIR peaks for the pure drug, PEG-6000, SD, and SDA revealed that there had been no alterations in the positions of the characteristic absorption peaks, which signifies no changes in the bonds of the various functional groups present in the drug (Figure 8). The FTIR spectra of EFZ showed the characteristic bands at 3318.5 cm-1 (for N–H) and 1749.47 (for C=O).51 The FTIR spectra of the SDA formulation contained all the peaks related to the functional groups of the drug and PEG-6000. The drug and PEG bond were formed, according to the FTIR peak near 1600 cm−1 in the case of FTIR spectra of PEG 6000 SD formulation.52 A peak near 1600 cm−1 on the FTIR spectrum of PEG 6000 SD denotes the PEGylation.53 There were slight variations in the drug’s peak intensities in FTIR spectra of SDA formulation at 3318 cm−1 and 1749 cm−1 (the distinct peak of EFZ). This implied that intermolecular hydrogen bonding or Van der Waals interactions between the drug and polymer might enhance the solubility of a drug.54 The formed hydrogen bond between the drug and polymer can easily break down in biological fluids, which results in higher drug release and solubility of the developed formulation.
DSC Analysis
The DSC thermograms of EFZ, PEG 6000, and SDA granules (F9) are presented in Figure 9. The EFZ showed a sharp endothermic peak at ~139°C (Figure 9A). This peak corresponded to its melting point and demonstrated its crystallinity. A similar DSC profile for EFZ was reported previously with the same melting point.55 Similar to a previous study, PEG 6000 exhibited a sharp endothermic peak at 63°C, which corresponded to its melting point (Figure 9B).56 The thermogram of SDA granules (F9 formulation) did not show any melting endotherm because of the conversion of EFZ into an amorphous form (Figure 9C). These results indicate that the crystalline nature of the drug was reduced with the fusion method to form SD using PEG-6000 as carriers.
 |
Figure 9 Differential scanning calorimetry thermogram of (A) EFZ, (B) PEG-6000, and (C) SDA granules (F9 formulation) show no thermal peak of EFZ, indicating conversion of EFZ into an amorphous form. |
XRD Analysis
The XRD pattern of the EFZ revealed several distinct peaks at angles of 6.053, 10.37, 10.92, 12.21, 13.10, and 14.10 (Figure 10A), signifying its crystalline nature.57 The XRD pattern of PEG-6000 revealed a distinctive collection of diffraction peaks. These peaks were observed at angles of 13.45, 14.43, 14.84, 17.01, and 18.87 (Figure 10B). The distinctive peaks (Figure 10C), which were present in the diffraction pattern of EFZ, were absent from the diffraction pattern of the SDA. Such a change in the XRD pattern implied the reduced crystallinity of the EFZ in SDA compared to that of pure EFZ. The improved dissolution of EFZ has been caused by a decrease in drug crystallinity. These findings are in good agreement with the DSC results.
SEM Analysis
Figure 11 shows the presence of an irregularly shaped crystalline agglomerate of EFZ-SDA under the SEM at different magnifications. Aerosil-200 possesses inter- and intra-specific pores on its surface that provide a much larger surface area. Such a large surface area facilitates the complete adsorption of the molten mass of EFZ SD on the porous surface of Aerosil-200.58 The SDA did not exhibit any particle agglomeration and possessed free-flowing properties.
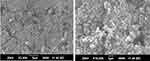 |
Figure 11 Scanning Electron microscopy of SDA granules at (A) 3500X magnification and (B) 10,000X magnification. |
Evaluation of EFZ-SDA Tablets
Tablets of EFZ SDA granules were compressed using the direct compression method with a bulk equivalent to 25 mg EFZ. The tablets were also compressed from the plain EFZ powder using the direct compression method. Physical qualities (Table 6) and dissolution patterns (Figure 12) were assessed. EFZ-SDA tablets were superior to the plain EFZ tablets in terms of all physical characteristics, including thickness, hardness, and friability (Table 6). The dissolution studies were conducted using an aqueous sodium lauryl sulfate solution as a dissolution medium. The dissolution profile of plain EFZ tablets and SDA tablets using a dissolution medium of buffer (pH 3.8) showed that EFZ was released from SDA tablets faster than the plain tablets. From SDA tablets, more than 70% drug was released within 20 min, and more than 95% drug was released within 60 min. On the other hand, only 18% of the drug was released from plain tablets after 20 min, and only 62% after 60 min. The time taken for the release of 50% of the drug from the SDA tablet was only 15 min, whereas the time required for the same amount of drug release from the plain EFZ tablet was 47 min. Within 30 min, 90% of the drug was released from the SDA tablet, whereas only 22% of the drug was released from the plain EFZ tablet. The dissolution efficiency was found to be enhanced from 50.68% in the case of plain EFZ tablets to 96.18% for EFZ-SDA tablets. The improved dissolution may be caused by hydrogen bonding between the drug and carrier and adsorption on the adsorbent, which enhances wettability and decreases crystallinity of the drug.59 The SDA technique utilized in the present study was not only able to improve the flow properties of the drug but also improve the dissolution rate of EFZ.
 |
Table 6 Evaluation of SDA and Plain EFZ Tablets |
 |
Figure 12 In-vitro dissolution study of SDA tablets and Plain EFZ tablets. |
Stability Studies
Accelerated stability studies of EFZ-SDA at 40 ± 2°C temperature and 75 ± 5% relative humidity for 6 months were performed. The results showed no significant changes in the drug content and drug release patterns from the SDA tablets (Table 7). The results of the stability study demonstrated the efficiency of aerosil in enhancing the physical stability of the drug and preventing the transition of the drug from less crystalline to crystalline form.
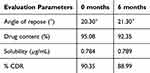 |
Table 7 Stability Study of SDA Tablets |
Conclusion
In this study, a physically stable SDA of EFZ was prepared using the hybrid technology, which is the integration of SD formulation and adsorption of SD on adsorbent carriers. The present study demonstrated the employability of the SDA hybrid technique in augmenting the dissolution rate and flow attributes of the formulation. As discussed earlier, EFZ exhibits limited oral bioavailability, which can lead to virological failure in HIV-infected patients. Thus, achieving higher bioavailability at a safe dose remains the best achievable recourse for therapeutic effectiveness. The factorial design used for the optimization of SDA suggested the ratio of 2:(2:1) of adsorbent to SD, where SD was formulated using a 2:1 ratio of PEG 6000 and EFZ, to be employed for the preparation of EFZ-SDA. The prepared SDA of EFZ at a given ratio demonstrated improved dissolution and better flow characteristics when compressed as tablets. Furthermore, the aging conditions had no impact on the physical stability and drug release of SDA. Therefore, SDA is a novel method to enhance the dissolution and flow properties of drugs. In conclusion, SDA techniques can be employed in the manufacturing of solid dosage forms of drugs with low solubility and variable bioavailability.
Acknowledgments
The authors extended their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a large group research project under grant number RGP.2/537/45. The authors are also thankful for providing the research facility at Dadasaheb Balpande College of Pharmacy, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur-440037, Maharashtra, India.
Disclosure
The authors claim they have no competing interests to disclose.
References
1. Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet. 1999;36(4):289–304. doi:10.2165/00003088-199936040-00004
2. Marquardt H. Clarke’s analysis of drugs and poisons (3rd Edition, 2004). Toxicology. 2004;203(1–3). doi:10.1016/j.tox.2004.06.010
3. Kamble RN, Mehta PP, Kumar A. Efavirenz self-nano-emulsifying drug delivery system: in vitro and in vivo evaluation. AAPS Pharm Sci Tech. 2016;17(5). doi:10.1208/s12249-015-0446-2
4. Pawar J, Tayade A, Gangurde A, Moravkar K, Amin P. Solubility and dissolution enhancement of efavirenz hot melt extruded amorphous solid dispersions using combination of polymeric blends: a QbD approach. Eur J Pharm Sci. 2016;88:37–49. doi:10.1016/j.ejps.2016.04.001
5. Obitte NC, Rohan LC, Adeyeye CM, Parniak MA, Esimone CO. The utility of self-emulsifying oil formulation to improve the poor solubility of the anti HIV drug CSIC. AIDS Res Ther. 2013;10(1):14. doi:10.1186/1742-6405-10-14
6. Sandhya P. Self-nano emulsifying drug delivery system of efavirenz: formulation, in vitro evaluation and characterization. Asian J Pharm Clin Res. 2019;12(12):217–226. doi:10.22159/ajpcr.2019.v12i12.35227
7. Madhavi B, Kusum B, Krishna Chatanya C, Madhu MN, Sri Harsha V, Banji D. Dissolution enhancement of efavirenz by solid dispersion and PEGylation techniques. Int J Pharm Investig. 2011;1(1):29–34. doi:10.4103/2230-973x.76726
8. Nikam VK, Shete SK, Khapare JP. Most promising solid dispersion technique of oral dispersible tablet. Beni-Suef Univ J Basic Appl Sci. 2020;9(1):62. doi:10.1186/s43088-020-00086-4
9. Yadav M, Sarolia J, Vyas B, Lalan M, Mangrulkar S, Shah P. Amalgamation of solid dispersion and melt adsorption technique: improved in vitro and in vivo performance of ticagrelor tablets. AAPS Pharm Sci Tech. 2021;22(8):257. doi:10.1208/s12249-021-02138-z
10. Mahajan A, Surti N, Koladiya P. Solid dispersion adsorbate technique for improved dissolution and flow properties of lurasidone hydrochloride: characterization using 3 2 factorial design. Drug Dev Ind Pharm. 2018;44(3):463–471. doi:10.1080/03639045.2017.1397687
11. Wang Y, Zhao Q, Hu Y, et al. Ordered nanoporous silica as carriers for improved delivery of water-insoluble drugs: a comparative study between three-dimensional and two dimensional macroporous silica. Int J Nanomed. 2013;8:4015–4031. doi:10.2147/IJN.S52605
12. Guntaka PR, Lankalapalli S. Solid dispersion—a novel approach for bioavailability enhancement of poorly water-soluble drugs in solid oral dosage forms. Asian J Pharm Clin Res. 2019;17–26. doi:10.22159/ajpcr.2019.v12i2.29157
13. Zhang X, Xing H, Zhao Y, Ma Z. Pharmaceutical dispersion techniques for dissolution and bioavailability enhancement of poorly water-soluble drugs. Pharmaceutics. 2018;10(3):74. doi:10.3390/pharmaceutics10030074
14. Shamsuddin, Fazil M, Ansari S, Ali J. Development and evaluation of solid dispersion of spironolactone using fusion method. Int J Pharm Investig. 2016;6(1):63–68. doi:10.4103/2230-973x.176490
15. Dugar RP, Gajera BY, Dave RH. Fusion method for solubility and dissolution rate enhancement of ibuprofen using block copolymer poloxamer 407. AAPS Pharm Sci Tech. 2016;17(6):1428–1440. doi:10.1208/s12249-016-0482-6
16. Mujtaba A, Ali M, Kohli K. Formulation of extended release cefpodoxime proxetil chitosan-alginate beads using quality by design approach. Int J Biol Macromol. 2014;69:420–429. doi:10.1016/j.ijbiomac.2014.05.066
17. Ali Mujtaba M, Kaleem M, Chaware R, et al. Development and optimization of proniosomal formulation of irbesartan using a box–behnken design to enhance oral bioavailability: physicochemical characterization and in vivo assessment. ACS Omega. 2024;9:16346–16357. doi:10.1021/acsomega.3c10506
18. Rampado R, Peer D. Design of experiments in the optimization of nanoparticle-based drug delivery systems. J Control Release. 2023;358:398–419. doi:10.1016/j.jconrel.2023.05.001
19. Emam MF, El-Ashmawy AA, Mursi NM, Emara LH. Optimization of meloxicam solid dispersion formulations for dissolution enhancement and storage stability using 33 full factorial design based on response surface methodology. AAPS Pharm Sci Tech. 2022;23(7):248. doi:10.1208/s12249-022-02394-7
20. Mujtaba MA, Gangane P, Ali A, et al. Karanjin-loaded soya lecithin-based ethosomal nanogel for the therapeutic intervention of psoriasis: formulation development, factorial design-based optimization, in vitro and in vivo assessment. Biomed Mater. 2024;19(5):055012. doi:10.1088/1748-605X/ad5e51
21. Sruti J, Niranjan Patra C, Swain S, et al. Improvement in the dissolution rate and tableting properties of cefuroxime axetil by melt-granulated dispersion and surface adsorption. Acta Pharm Sin B. 2013;3(2):113–122. doi:10.1016/j.apsb.2013.01.001
22. Pawar J, Suryawanshi D, Moravkar K, et al. Study the influence of formulation process parameters on solubility and dissolution enhancement of efavirenz solid solutions prepared by hot-melt extrusion: a QbD methodology. Drug Deliv Transl Res. 2018;8(6):1644–1657. doi:10.1007/s13346-018-0481-0
23. Slabiak Oksana I, Ivanchuk Iryna M, Klimenko LY, Tokaryk Galyna V, Kolisnyk Iuliia S. Development and validation of UV-spectrophotometric procedures for efavirenz quantitative determination. Int J Pharm Qual Assur. 2018;9(3):231–240. doi:10.25258/ijpqa.v9i3.13653
24. Mujtaba A, Fule R, Amin P, et al. Development of hot melt extruded co-formulated artesunate and amodiaquine- soluplus® solid dispersion system in fixed-dose form: amorphous state characterization and pharmacokinetic evaluation. Curr Drug Metab. 2024;25(7):505–522. doi:10.2174/0113892002330772240912055518
25. Salman ZN, Al-Ani I, Al Azzam KM, Majeed BJM, Abdallah HH, Negim ES. Enhancement of apixaban’s solubility and dissolution rate by inclusion complex (β-cyclodextrin and hydroxypropyl β-cyclodextrin) and computational calculation of their inclusion complexes. ADMET DMPK. 2023;11(4):533–550. doi:10.5599/admet.1885
26. Alruwaili NK, Zafar A, Imam SS, et al. Formulation of amorphous ternary solid dispersions of dapagliflozin using PEG 6000 and Poloxamer 188: solid-state characterization, ex vivo study, and molecular simulation assessment. Drug Dev Ind Pharm. 2020;46(9):1458–1467. doi:10.1080/03639045.2020.1802482
27. Nair AR, Lakshman YD, Anand VSK, Sree KSN, Bhat K, Dengale SJ. Overview of extensively employed polymeric carriers in solid dispersion technology. AAPS Pharm Sci Tech. 2020;21(8):1–20. doi:10.1208/s12249-020-01849-z
28. Yousaf AM, Malik UR, Shahzad Y, Mahmood T, Hussain T. Silymarin-laden PVP-PEG polymeric composite for enhanced aqueous solubility and dissolution rate: preparation and in vitro characterization. J Pharm Anal. 2019;9(1):34–39. doi:10.1016/j.jpha.2018.09.003
29. Li X, Peng H, Tian B, et al. Preparation and characterization of azithromycin - Aerosil 200 solid dispersions with enhanced physical stability. Int J Pharm. 2015;486(1–2):175–184. doi:10.1016/j.ijpharm.2015.03.029
30. Shah PJ, Patel MP, Shah J, Nair AB, Kotta S, Vyas B. Amalgamation of solid dispersion and melt adsorption techniques for augmentation of oral bioavailability of novel anticoagulant rivaroxaban. Drug Deliv Transl Res. 2022;12(12):3029–3046. doi:10.1007/s13346-022-01168-9
31. Malakar J, Nayak AK. Formulation and statistical optimization of multiple-unit ibuprofen-loaded buoyant system using 2 3-factorial design. Chem Eng Res Des. 2012;90(11):1834–1846. doi:10.1016/j.cherd.2012.02.010
32. Castro SG, Bruni SS, Lanusse CE, Allemandi DA, Palma SD. Improved albendazole dissolution rate in pluronic 188 solid dispersions. AAPS Pharm Sci Tech. 2010;11(4):1518–1525. doi:10.1208/s12249-010-9517-6
33. Mujtaba MA, Desai H, Ambekar A, et al. Development of chitosan/sodium carboxymethyl cellulose-based polyelectrolyte complex of dexamethasone for treatment of anterior uveitis. Biomed Mater. 2024;19(6):065016. doi:10.1088/1748-605X/ad7e6b
34. Kharwade R, Ali N, Gangane P, Pawar K, More S, Iqbal M. DOE-assisted formulation, optimization, and characterization of tioconazole-loaded transferosomal hydrogel for the effective treatment of atopic dermatitis: in vitro and in vivo evaluation. Gels. 2023;9(4):303. doi:10.3390/gels9040303
35. Fule R, Kaleem M, Asar TO, et al. Formulation, optimization and evaluation of cytarabine-loaded iron oxide nanoparticles: from in vitro to in vivo evaluation of anticancer activity. Nanomaterials. 2023;13(1):175. doi:10.3390/nano13010175
36. Zhao X, Liu X, Gan L, Zhou C, Mo J. Preparation and physicochemical characterizations of tanshinone IIA solid dispersion. Arch Pharm Res. 2011;34(6):949–959. doi:10.1007/s12272-011-0612-3
37. Mahajan NM, Wanaskar K, Ali N, et al. Innovative wound healing hydrogel containing chicken feather keratin and soy isoflavone genistein: in vivo studies. Gels. 2023;9(6):462. doi:10.3390/gels9060462
38. Vedha Hari BN, Lu CL, Narayanan N, Wang RR, Zheng YT. Engineered polymeric nanoparticles of efavirenz: dissolution enhancement through particle size reduction. Chem Eng Sci. 2016;155:366–375. doi:10.1016/j.ces.2016.08.019
39. Sabbatini B, Romano Perinelli D, Filippo Palmieri G, Cespi M, Bonacucina G. Sodium lauryl sulfate as lubricant in tablet formulations: is it worth it? Int J Pharm. 2023;643:123265. doi:10.1016/j.ijpharm.2023.123265
40. Sheik Abdul Kadhar Mohamed Ebrahim HR, Chungath TT, Sridhar K, et al. Development and validation of a discriminative dissolution medium for a poorly soluble nutraceutical tetrahydrocurcumin. Turkish J Pharm Sci. 2021;18(5):565–573. doi:10.4274/tjps.galenos.2021.91145
41. Mujtaba A, Kohli K. In vitro/in vivo evaluation of HPMC/alginate-based extended-release matrix tablets of cefpodoxime proxetil. Int J Biol Macromol. 2016;89:434–441. doi:10.1016/j.ijbiomac.2016.05.010
42. Bhairav BA, Jagtap LR, Saudagar RB. Solubility and dissolution enhancement of pioglitazone using solid dispersion technique. Int J Curr Pharm Res. 2017;9(5):186–193. doi:10.22159/ijcpr.2017v9i5.22326
43. Kotta S, Aldawsari HM, Badr-Eldin SM, Nair AB, Kaleem M, Dalhat MH. Thermosensitive hydrogels loaded with resveratrol nanoemulsion: formulation optimization by central composite design and evaluation in MCF-7 human breast cancer cell lines. Gels. 2022;8(7):450. doi:10.3390/gels8070450
44. Nel M, Samsodien H, Aucamp ME. Using natural excipients to enhance the solubility of the poorly water-soluble antiretroviral, efavirenz. J Drug Deliv Sci Technol. 2022;71:103332. doi:10.1016/j.jddst.2022.103332
45. Alves LDS, De La Roca Soares MF, De Albuquerque CT, et al. Solid dispersion of efavirenz in PVP K-30 by conventional solvent and kneading methods. Carbohydr Polym. 2014;104(1):166–174. doi:10.1016/j.carbpol.2014.01.027
46. Chella N, Shastri N, Tadikonda RR. Use of the liquisolid compact technique for improvement of the dissolution rate of valsartan. Acta Pharm Sin B. 2012;2(5):502–508. doi:10.1016/j.apsb.2012.07.005
47. Deepti, Dureja H, Madan AK. Solid dispersion adsorbates for enhancement of dissolution rates of drugs. PDA J Pharm Sci Technol. 2007;61(2):97–101.
48. Sharma S, Sher P, Badve S, Pawar AP. Absorption of meloxicam on porous calcium silicate: characterization and tablet formulation. AAPS Pharm Sci Tech. 2005;6(4):E618–25. doi:10.1208/pt060476
49. Weerapol Y, Limmatvapirat S, Jansakul C, Takeuchi H, Sriamornsak P. Enhanced dissolution and oral bioavailability of nifedipine by spontaneous emulsifying powders: effect of solid carriers and dietary state. Eur J Pharm Biopharm. 2015;91:25–34. doi:10.1016/j.ejpb.2015.01.011
50. More SM, Rashid MA, Kharwade RS, et al. Development of solid self-nanoemulsifying drug delivery system of rhein to improve biopharmaceutical performance: physiochemical characterization, and pharmacokinetic evaluation. Int J Nanomed. 2025;20:267–291. doi:10.2147/IJN.S499024
51. Gaur PK, Mishra S, Bajpai M, Mishra A. Enhanced oral bioavailability of Efavirenz by solid lipid nanoparticles: in vitro drug release and pharmacokinetics studies. Biomed Res Int. 2014;2014:363404. doi:10.1155/2014/363404
52. Yousefi A, Esmaeili F, Rahimian S, Atyabi F, Dinarvand R. Preparation and in vitro evaluation of a pegylated nano-liposomal formulation containing docetaxel. Sci Pharm. 2009;77(2):453–464. doi:10.3797/scipharm.0806-08
53. da Costa MA, Seiceira RC, Rodrigues CR, Hoffmeister CRD, Cabral LM, Rocha HVA. Efavirenz dissolution enhancement I: co-micronization. Pharmaceutics. 2013;5(1):1–22. doi:10.3390/pharmaceutics5010001
54. Fandaruff C, Rauber GS, Araya-Sibaja AM, et al. Polymorphism of anti-HIV drug efavirenz: investigations on thermodynamic and dissolution properties. Cryst Growth Des. 2014;14(10):4968–4975. doi:10.1021/cg500509c
55. Patel GV, Patel VB, Pathak A, Rajput SJ. Nanosuspension of efavirenz for improved oral bioavailability: formulation optimization, in vitro, in situ and in vivo evaluation. Drug Dev Ind Pharm. 2014;40(1):80–91. doi:10.3109/03639045.2012.746362
56. Adibkia K, Ghajar S, Osouli-Bostanabad K, Balaei N, Emami S, Barzegar-Jalali M. Novel gliclazide electrosprayed nano-solid dispersions: physicochemical characterization and dissolution evaluation. Adv Pharm Bull. 2019;9(2):231. doi:10.15171/apb.2019.026
57. Ataollahi N, Broseghini M, Ferreira FF, Susana A, Pizzato M, Scardi P. Effect of high-energy milling on the dissolution of anti-HIV drug efavirenz in different solvents. ACS Omega. 2021;6(19):12647–12659. doi:10.1021/acsomega.1c00712
58. Ghareeb MM, Abdulrasool AA, Hussein AA, Noordin MI. Kneading technique for preparation of binary solid dispersion of meloxicam with poloxamer 188. AAPS Pharm Sci Tech. 2009;10(4):1206–1215. doi:10.1208/s12249-009-9316-0
59. Zhang J, Guo M, Luo M, Cai T. Advances in the development of amorphous solid dispersions: the role of polymeric carriers. Asian J Pharm Sci. 2023;18(4):100834. doi:10.1016/j.ajps.2023.100834
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.










