Back to Journals » Drug Design, Development and Therapy » Volume 19
Design, Synthesis, and Evaluation of Nitroxide Radical Derivatives Based on Rhein as Potential Anti-Aging Agents Targeting the Keap1-Nrf2 Pathway
Authors Wang J, Peng X, Zhang X, Lin J, Zhang Q, Li J, Cui L, Zhao L
Received 8 January 2025
Accepted for publication 29 May 2025
Published 19 June 2025 Volume 2025:19 Pages 5153—5167
DOI https://doi.org/10.2147/DDDT.S516209
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Muzammal Hussain
Jie Wang,1,* Xuejing Peng,1,2,* Xinyue Zhang,1 Jia Lin,3 Qili Zhang,1,2 Jiaojiao Li,1 Longchen Cui,1 Lei Zhao1,2,4– 6
1College of Pharmacy, Gansu University of Chinese Medicine, Lanzhou, People’s Republic of China; 2Northwest Collaborative Innovation Center for Traditional Chinese Medicine Co-Constructed by Gansu Province & MOE of PRC, Gansu University of Chinese Medicine, Lanzhou, People’s Republic of China; 3College of Pharmacy, Gansu Health Vocational College, Lanzhou, People’s Republic of China; 4Key Laboratory of Chemistry and Quality of TCM of the College of Gansu Province, Gansu University of Chinese Medicine, Lanzhou, People’s Republic of China; 5Gansu Province Engineering Laboratory for TCM Standardization Technology and Popularization, Gansu University of Chinese Medicine, Lanzhou, People’s Republic of China; 6Gansu Pharmaceutical Industry Innovation Research Institute, Lanzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Zhao, College of Pharmacy, Gansu University of Chinese Medicine, 35 Dingxi East Road, Chengguan District, Lanzhou, 730000, People’s Republic of China, Tel +86 13893395136, Email [email protected] Longchen Cui, College of Pharmacy, Gansu University of Chinese Medicine, 35 Dingxi East Road, Chengguan District, Lanzhou, 730000, People’s Republic of China, Tel +86 13919908921, Email [email protected]
Purpose: Targeting the crucial Keap1-Nrf2-ARE antioxidant pathway, we selected Rhein - a natural anthraquinone from traditional Chinese medicine with established Keap1-Nrf2 inhibitory activity as our lead compound. Through rational structural modification by incorporating nitroxide radicals at the 3-carboxyl position, we aimed to develop enhanced Keap1-Nrf2 modulators with anti-aging potential.
Patients and Methods: A series of rhein nitroxide derivatives were synthesized, and their free radical scavenging activity was assessed in vitro using DPPH and ABTS methods. Compound 4b, demonstrating significant activity, was selected for further evaluation. Its effects on the survival of L02 hepatocytes under oxidative stress and the lifespan and stress tolerance of Caenorhabditis elegans (C. elegans) were investigated. Additionally, the impacts of 4b on antioxidant enzyme activity, malondialdehyde (MDA) levels, and reactive oxygen species (ROS) accumulation under oxidative stress were assessed. Molecular docking was conducted to analyze interactions between 4b and the Kelch domain of Keap1.
Results: Compound 4b exhibited potent free radical scavenging activity, with IC50 values of 0.51 ± 0.09 mM against DPPH radicals and 0.12 ± 0.03 mM against ABTS radicals. It significantly improved the survival rate of L02 hepatocytes under oxidative stress, maintaining 95.42% viability (p < 0.01). In the C. elegans model, 4b extended the average lifespan and enhanced stress resistance, increasing GSH activity, reducing MDA content, and decreasing ROS accumulation. Molecular docking showed that 4b penetrates deeply into the Kelch domain of Keap1, forming stable interactions with key residues.
Conclusion: Compound 4b demonstrates superior antioxidant and anti-aging effects compared to the parent compound rhein, representing a highly promising anti-aging candidate and Keap1-Nrf2 signaling pathway modulator with potential as a novel therapeutic agent for age-related diseases.
Keywords: rhein, nitroxide radical, antioxidant, anti-aging, keap1-Nrf2 pathway
Graphical Abstract:

Introduction
Accumulated damage from oxidative stress is a significant factor in the development of various diseases and is closely associated with most age-related conditions and the aging process itself.1,2 Increasing evidence indicates that the accumulation of reactive oxygen species (ROS), mitochondrial dysfunction, and DNA damage play pivotal roles in the aging process.3–6 Keap1-Nrf2-ARE signaling pathway is a crucial mechanism by which cells respond to oxidative stress damage. Under normal conditions, Nrf2 is anchored in the cytoplasm by Keap1 and targeted for degradation, maintaining low levels of Nrf2. During oxidative stress, the cysteine residues of Keap1 are oxidized, leading to the release and activation of Nrf2, which then translocates into the nucleus to activate antioxidant genes, thereby mitigating oxidative damage and playing a vital role in delaying aging.7–9 Notably, studies have found that species longevity is not correlated with the protein levels of Nrf2 itself but is significantly negatively correlated with Keap1, indicating that in long-lived species, Nrf2 activity is primarily regulated by Keap1 rather than changes in Nrf2 protein levels.10 Thus, reducing Keap1 expression or inhibiting its function may be an effective strategy for discovering anti-aging drugs.9 Currently, research on Keap1–Nrf2–ARE activators follows two main strategies. One strategy targets the modification of different cysteine residues in Keap1, such as Cys151 and Cys288, with typical drug representatives like dimethyl fumarate approved by the FDA for the treatment of multiple sclerosis and psoriasis.11,12 The other involves Keap1-Nrf2 PPI inhibitors, which block the interaction between the Kelch domain of Keap1 and Nrf2 to activate Nrf2. This strategy is unaffected by cysteine mutations in Keap1 (eg, Cys→Ser); it can activate Nrf2 under low oxidative stress conditions and offers higher specificity and applicability.13–15 Additionally, binding to the Kelch domain of Keap1 typically does not interfere with other functions of Keap1, potentially offering improved safety profiles.16,17 Despite progress in the development of drugs targeting the Keap1-Nrf2 pathway, the clinical translation of Nrf2 inhibitors still faces significant challenges. Targeting transcription factors is inherently difficult, and existing Nrf2 inhibitors mostly act through nonspecific mechanisms, lacking sufficient targeting precision.18
Natural products are a valuable resource for drug discovery. Hydroxyanthraquinones, widely distributed in plants of the Liaoke, Rubiaceae, and Liliaceae families, serve as key bioactive constituents in traditional Chinese medicines such as rhubarb, cassia, and aloe. Their biological effects are believed to be partly mediated through activation of the Nrf2 signaling pathway.19 Using a virtual screening approach on a hydroxyanthraquinone structure library, we identified rhein as a promising candidate for inhibiting the Keap1-Nrf2 PPI. After thousands of years of use and recent research, rhein has been proven to have extensive protective effects. Previous studies have shown that rhein can activate the Nrf2 signaling pathway by inhibiting inflammatory responses and oxidative stress, thereby alleviating LPS-induced intestinal barrier injury.20,21 In addition, rhein can significantly reduce vancomycin-induced nephrotoxicity by regulating the expression of renal transporters and activating the Nrf2 signaling pathway.22 In rodent models, oral administration of rhein increases nuclear Nrf2 levels in various tissues, including the intestine, brain, and lungs.21,23,24 Furthermore, rhein and its derivative, rhein-lysine (D-gal), effectively reduces ROS levels, exhibiting notable antioxidant and anti-aging activities.25,26 These findings highlight rhein as a promising lead compound for antioxidant and anti-aging drug development.
Nitroxides, as synthetic antioxidants, are a class of stable radical donors characterized by an odd number of electrons on the nitro N-O• group, which is significantly stabilized by bulky methyl or ethyl groups, thereby greatly limiting the reactivity of the nitro group and enhancing its stability.27 Nitroxides can engage in single-electron reactions with other radicals, oxidize transition metal ions, and stimulate peroxidase-like activity of hemoglobin. They have been proven effective in scavenging alkyl, aromatic oxygen, phenoxy, alkoxy, and peroxyl radicals.28,29 The stable and potent antioxidant activity of nitroxides has made this structural motif valuable in drug optimization.28,30–33 Here, we designed and synthesized a series of rhein nitroxide radical derivatives and evaluated their antioxidant and anti-aging effects in vitro and in vivo models, aiming to obtain enhanced compounds by pharmacodynamic combination.
Materials and Methods
Chemistry Section
General Chemistry Method
High-purity solvents and reagents, such as rhein, Boc-glycine, 4-oxo-tempo, N,N’-dicyclohexylcarbodiimide, tert-butyl alcohol, and ethyl acetate, were obtained from commercial suppliers. The required free radicals were synthesized in-house (see Schemes S1 and S2 in Supplementary Material for synthetic details). All solvents were reagent grade and, when necessary, were purified and dried by standard methods. All reactions were analyzed by TLC (Silica Gel HSGF254, Yantai Institute of Chemical Industry, China), the compounds were visualized by UV light, and the compounds were purified by silica gel column (200–300 Mesh). Bruker MicroTOF-QII mass spectrometer was used for high-resolution mass spectrometry analysis. The melting point was measured by a digital melting point meter. The spectra were analyzed by using an ALPHA-T infrared spectrometer. Spin single-electron measurements were performed using JES-FA300 Electron paramagnetic resonance spectrometer.
General Procedure for the Synthesis of Rhein Derivatives
A mixture of amino acids (1.00 mmol) and nitroxide radicals (0.50 mmol) in anhydrous dichloromethane (DCM, 15 mL) was treated with N,N’-dicyclohexylcarbodiimide (DCC, 2.40 mmol) and 4-dimethylaminopyridine (DMAP, 0.55 mmol) in a 50 mL flame-dried flask under nitrogen. The reaction was stirred at 25°C and monitored by TLC (silica gel GF254, DCM: MeOH= 20:1). Upon completion, the solvent was removed under reduced pressure (35°C, 100 rpm), and the crude product was purified by column chromatography (silica gel 200–300 mesh, DCM: MeOH=150:1 ~ 50:1) to afford compound A. Compound A (0.67 mmol) was dissolved in DCM (4 mL) and cooled to 0°C. Trifluoroacetic acid (2 mL) was added dropwise over 10 min. After quenching with saturated NaHCO₃ (15 mL), the organic layer was separated, dried over anhydrous Na2SO₄, and concentrated to give compound B as a white solid. Rhein (0.36 mmol) was placed in a round-bottom flask equipped with a magnetic stir bar and dissolved in anhydrous tetrahydrofuran (THF, 10 mL). To this solution were added sequentially:1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI·HCl, 0.70 mmol), 4-Dimethylaminopyridine (DMAP, 0.64 mmol), Compound B (0.48 mmol), Triethylamine (100 μL, 0.72 mmol). The reaction mixture was stirred at room temperature for 24 h under nitrogen atmosphere, with progress monitored by TLC (silica gel GF254, DCM: MeOH=50:1). Upon completion, the mixture was filtered through filter paper to remove insoluble materials, concentrated under reduced pressure (35°C, 100 rpm), and purified by column chromatography (silica gel 200–300 mesh, DCM: MeOH = 200:1~100:1) to obtain the target compound (Scheme1).
 |
Scheme 1 Synthesis of a series of rhein derivatives (1a~1h, 2a~2h, 3a~3f, 3h, 4a~4f, and 4h). Reagents and conditions: (a) DCC, DMAP, DCM, rt; (b) TFA, DCM, 0°C; (c) EDCl, DMAP, Et3N, THF, rt. |
In vitro Antioxidant Activity Evaluation
Antioxidant potential was evaluated as follows:
DPPH Radical Scavenging Assay:34 A proper amount of DPPH was obtained, and anhydrous ethanol was prepared into a solution of 0.2 mm, which was stored in the dark. DMSO was used as the sample solvent to dissolve the nitroxyl radical derivatives of rhein. A 50 μL derivative solution of different concentrations was mixed with 150 μL of DPPH working solution and labeled as A1; 50 μL of derivative solution of different concentrations was mixed with 150 μL of DMSO and labeled as A2; and 50 μL of DMSO and 150 μL of DPPH were mixed and labeled as A0. The absorbance was measured at 517 nm for 30 min at room temperature. Each sample was set up with three complex holes, and the average value was obtained after three trials. The scavenging rate of DPPH was calculated by Equation 1, and IC50 of different compounds on DPPH radical scavenging was calculated.
ABTS Radical Cation Decolorization Assay:35 A solution of 7.4 and 2.6 mm was prepared from distilled water. The ABTS solution and potassium persulfate solution were mixed at 1:1 (v:v), placed in the dark room for 12–16 h, and diluted several times before use. The OD value was about 0.70±0.05 at the wavelength of 734 nm. A 50 μL derivative solution of different concentrations was mixed with 150 μL of ABTS working solution and labeled as A1; 50 μL of derivative solution of different concentrations was mixed with 150 μL of DMSO and labeled as A2; and 50 μL of DMSO and 150 μL of ABTS were mixed and labeled as A0. The absorbance was measured at 734 nm for 5 min at room temperature. Each sample was set up with three complex holes, and the average value was obtained after three trials. The scavenging rate of ABTS was calculated by Equation 1, and the scavenging IC50 of different compounds was calculated to compare their antioxidant activity.
L02 Cell Protection Assay: Human L02 hepatocytes (Procell, China) were cultured in RPMI 1640 medium (Cytiva, USA) supplemented with 10% fetal bovine serum (FBS; TIANHANG, China) and 1% penicillin-streptomycin (Solarbio, China) at 37°C in a humidified 5% CO2 incubator. Cells in the logarithmic growth phase were seeded in 96-well plates at a density of 3 × 104 to 5 × 104 cells/mL and then subjected to drug intervention. When the concentration of the compound was 5 μM, the survival rate of human normal hepatocytes (L02) was more than 80%. L02 cells were protected for 24 h with medium containing 5 μM different rhein derivatives, induced with 0.4 mM H2O2, incubated for 2h, added MTT solution, and incubated for 4h. After DMSO was dissolved, the absorbance was measured at 570 nm, and cell viability was calculated according to Equation 2.
Note: “Cell viability (%)” refers to the percentage of viable cells relative to the untreated control group, which is normalized to 100%.
In vivo Antioxidant and Anti-Aging Activity of Compound 4b
Lifespan Extension
Frozen wild-type C. elegans were thawed and centrifuged (2,000 ×g, 2 min). The pellet was treated with alkaline hypochlorite solution to isolate eggs, which were hatched overnight in M9 buffer to obtain synchronized L1 larvae. Larvae were then transferred to NGM plates seeded with OP50 and cultured at 20°C until reaching the desired developmental stage.
Compound 4, 4b, and rhein were first diluted to the desired concentrations (50, 150, and 300 µM) using OP50, and then applied to NGM agar plates. Synchronized C. elegans were then randomly assigned to groups treated with these different concentrations of the compounds. The number of survival nematodes was recorded every 24 h, and different groups of survival nematodes were transferred to the same concentration culture dish until all nematodes died. Criteria for nematode death and elimination were as follows: no movement, no response 10s after touching with platinum wire, and missing or fatal nematode due to crawling out of the Petri dish.36
Stress Resistance Assays
According to the method of life test, the plate was cultured in a constant-temperature incubator at 37°C, and the heat stress model was established.37 Survival rate was calculated hourly until all nematodes died. The oxidative stress model was established by preparing NGM medium with 400 μM juglone.38 Survival rate was calculated every hour until all nematodes died.
Determination of Oxidative Stress State Indexes
C. elegans was purchased from the Caenorhabditis Genetics Center. The culture medium (NGM) of Escherichia coli OP 50 containing the compound 4b was used in a 20 °C thermostatic incubator. The N2 nematodes, which grew to L4 stage after synchronization, were transferred to the prepared medium and cultured in the incubator at a constant temperature for 96 h. The nematodes were subjected to oxidative stress and then rinsed with M9 buffer three times, followed by collection in a 1.5 mL EP tube. Subsequently, 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) was added into a 1.5 mL EP tube to make the final concentration of 50 μM DCFH-DA. The mixture was evenly mixed, stored in the dark, observed, and photographed under a fluorescence microscope. Image J software was used to analyze the fluorescence intensity of the photos and determine the ROS level in the nematodes. Following the kit instructions, we added the extract and performed rapid homogenization on ice to detect the levels of GSH and MDA in nematodes.
Molecular Docking Study
The 3D molecular structure of rhein and its derivatives was drawn by Chemoffice software and then imported into the Schrödinger 2020–4 program. The LigPrep module was used to optimize the structure by the OPLS _ 2005 force field. Epik 28 distributed the ionization state under the condition of pH (7.0 ± 2.0). Keap1 crystal structure (PDB: 6TYM) was selected as the receptor for molecular docking. The PrepWiz module was used to optimize the protein and generate the grid file. Molecular docking was performed using Glide’s standard precision methodology.
Statistical Analysis
All experiments were performed in triplicate and repeated three times independently (n = 3). Results are expressed as the mean ± standard deviation (SD). Scavenging rates or inhibition rates were calculated, and IC50 values were determined by plotting these rates against sample concentrations (six different concentration gradients) using non-linear regression analysis. Prior to statistical analysis, the normality of data distribution was assessed using QQ plots. For normally distributed data, comparisons between two groups were performed using the independent samples t-test. For non-normally distributed data, the Mann–Whitney U-test was applied as a non-parametric alternative. Statistical significance was defined as P < 0.05. All statistical analyses were conducted using SPSS software (version 25.0), and graphs were generated using GraphPad Prism (version 10.4.0).
Results
Characterization of Compounds
1a: Boc-glycine (1.00 mmol, 0.1752 g) and compound 1 (0.53 mmol, 0.0987 g) were used as the amino acid and nitroxide radical components, respectively. The subsequent steps were performed according to Scheme 1. Yellow solid powder, 0.1868 g, yield 96.63%, m.p. 100.20~101.25°C. HR-ESI-MS: 532.1804 [M+Na]+; IR (KBr): 3416.83, 2961.04, 2921.26, 2852.50, 1629.63, 1261.15, 1093.84, 871.74, 799.78, 540.78; EPR (DCM): g factor, 2.0008.
1b: Following the synthetic procedure for compound 1a, Boc-β-alanine (1.00 mmol, 189.3 mg) was used in place of Boc-glycine to afford the target derivative. Yellow solid powder, 0.1154 g, yield 51.70%, m.p. 163.78~165.53°C. HR-ESI-MS: 546.1962 [M+Na]+; IR (KBr): 3417.83, 2922.04, 2852.10, 2358.41, 1732.54, 1628.75, 1458.11, 1379.88, 1264.67, 1091.68, 747.68, 542.21; EPR (DCM): g factor, 2.00095.
1c: Following the synthetic procedure for compound 1a, Boc-L-alanine (1.00 mmol, 189.0 mg) was used in place of Boc-glycine to afford the target derivative. Yellow solid powder, 0.0927 g, yield 50.32%, m.p. 143.39~145.03°C. HR-ESI-MS: 546.1986 [M+Na]+; IR (KBr): 3416.75, 2923.73, 2853.14, 2340.05, 1628.26, 1454.99, 1382.71, 1265.93, 1100.77, 906.17, 753.47, 539.52; EPR (DCM): g factor, 2.00096.
1d: Following the synthetic procedure for compound 1a, Boc-L-valine (1.00 mmol, 217.2 mg) was used in place of Boc-glycine to afford the target derivative. Yellow solid powder, 0.0960 g, yield 49.45%, m.p. 113.43~114.96°C. HR-ESI-MS: 574.2308 [M+Na]+; IR (KBr): 3416.28, 2971.31, 2930.79, 1711.21, 1629.52, 1506.65, 1459.59, 1366.82, 1268.71, 1157.05, 1088.17, 906.62, 755.10, 546.32; EPR (DCM): g factor, 2.00084.
1e: Following the synthetic procedure for compound 1a, Boc-L-leucine (1.00 mmol, 231.2 mg) was used in place of Boc-glycine to afford the target derivative. Yellow solid powder, 0.0812 g, yield 40.80%, m.p. 156.46~158.11°C. HR-ESI-MS: 588.2451 [M+Na]+; IR (KBr): 3416.75, 2923.73, 2853.14, 2340.05, 1628.26, 1454.99, 1382.71, 1265.93, 1100.77, 906.17, 753.47, 539.52; EPR (DCM): g factor, 2.00083.
1f: Following the synthetic procedure for compound 1a, Boc-L-methionine (1.00 mmol, 249.3 mg) was used in place of Boc-glycine to afford the target derivative. Yellow solid powder, 0.0925 g, yield 45.05%, m.p. 120.31~120.90°C. HR-ESI-MS: 606.2012 [M+Na]+; IR (KBr): 3293.10, 2975.42, 2927.04, 1704.42, 1672.54, 1630.07, 1538.07, 1453.20, 1376.49, 1272.77, 1202.94, 1165.29, 1080.43, 1012.00, 905.66, 751.65; EPR (DCM): g factor, 2.00095.
1g: Following the synthetic procedure for compound 1a, Boc-L-phenylalanine (1.00 mmol, 265.3 mg) was used in place of Boc-glycine to afford the target derivative. Yellow solid powder, 0.0893 g, yield 42.34%, m.p. 173.72~174.90°C. HR-ESI-MS: 622.2282 [M+Na]+; IR: 3422.45, 2920.66, 2851.94, 2358.42, 1630.87, 1380.76, 1263.53, 1104.50, 905.59, 745.10, 538.44; EPR (DCM): g factor, 2.00096.
1h: Following the synthetic procedure for compound 1a, N-Boc-2-methylalanine (1.00 mmol, 203.1 mg) was used in place of Boc-glycine to afford the target derivative. Yellow solid powder, 0.0856 g, yield 45.26%, m.p. 120.37~122.22°C. HR-ESI-MS: 560.2137 [M+Na]+; IR (KBr): 3414.76, 2924.19, 2853.27, 2359.10, 1633.50, 1454.11, 1382.09, 1099.23, 746.83, 539.68; EPR (DCM): g factor, 2.00096.
2a: Following the synthetic procedure for compound 1a, compound 2 (0.53 mmol, 97.6 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.1318 g, yield 73.83%, m.p. 120.08~120.96°C. HR-ESI-MS: 530.1649 [M+Na]+; IR (KBr): 3420.90, 2979.03, 2930.02, 1707.11, 1630.30, 1452.02, 1357.17, 1268.47, 1189.04, 755.29; EPR (DCM): g factor, 2.00176.
2b: Following the synthetic procedure for compound 1b, compound 2 (0.53 mmol, 97.6 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.1510 g, yield 82.33%, m.p. 141.66~147.71°C. HR-ESI-MS: 544.1834 [M+Na]+; IR (KBr): 3293.06, 2921.85, 2852.62, 1739.33, 1631.13, 1540.25, 1463.11, 1360.87, 1263.76, 1204.94, 1162.91, 1084.23, 1030.00, 801.42, 751.69; EPR (DCM): g factor, 2.00174.
2c: Following the synthetic procedure for compound 1c, compound 2 (0.53 mmol, 97.6 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.1161 g, yield 63.30%, m.p. 136.93~137.22°C. HR-ESI-MS: 544.1827 [M+Na]+; IR (KBr): 3290.34, 2931.00, 2857.42, 1709.03, 1631.44, 1567.22, 1537.55, 1427.99, 1379.40, 1321.08, 1267.47, 1202.77, 1156.48, 1086.08, 1053.16, 752.67; EPR (DCM): g factor, 2.00155.
2d: Following the synthetic procedure for compound 1d, compound 2 (0.53 mmol, 97.6 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.0856 g, yield 44.28%, m.p. 153.80~154.66°C. HR-ESI-MS: 572.2152 [M+Na]+; IR (KBr): 3426.99, 2971.75, 2926.82, 1739.46, 1631.52, 1534.14, 1461.66, 1377.21, 1268.42, 1198.32, 1152.87, 755.10; EPR (DCM): g factor, 2.00195.
2e: Following the synthetic procedure for compound 1e, compound 2 (0.53 mmol, 97.6 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.0810 g, yield 40.84%, m.p. 127.52~129.03°C. HR-ESI-MS: 586.2306 [M+Na]+; IR (KBr): 3302.17, 2963.78, 2932.00, 1743.40, 1673.38, 1631.09, 1539.02, 1453.59, 1377.17, 1273.38, 1200.97, 1156.14, 751.35; EPR (DCM): g factor, 2.00173.
2f: Following the synthetic procedure for compound 1f, compound 2 (0.53 mmol, 97.6 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.1408 g, yield 68.81%, m.p. 123.88~124.72°C. HR-ESI-MS: 604.1849 [M+Na]+; IR (KBr): 3317.34, 2929.13, 2859.80, 1742.05, 1706.85, 1630.25, 1535.20, 1428.36, 1380.05, 1272.14, 1201.45, 1165.26, 754.07; EPR (DCM): g factor, 2.00176.
2g: Following the synthetic procedure for compound 1g, compound 2 (0.53 mmol, 97.6 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.1065 g, yield 50.66%, m.p. 103.13~104.62°C. HR-ESI-MS: 620.2129 [M+Na]+; IR (KBr): 3339.87, 2977.46, 2932.03, 1742.33, 1708.52, 1630.59, 1531.81, 1450.50, 1376.37, 1271.72, 1199.58, 752.12, 701.46; EPR (DCM): g factor, 2.00172.
2h: Following the synthetic procedure for compound 1h, compound 2 (0.53 mmol, 97.6 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.0618 g, yield 32.80%, m.p. 155.78~156.52°C. HR-ESI-MS: 558.1968 [M+Na]+; IR (KBr): 3401.11, 2978.90, 2930.88, 1632.09, 1269.37, 1154.16, 755.15; EPR (DCM): g factor, 2.00174.
3a: Following the synthetic procedure for compound 1a, compound 3 (0.53 mmol, 90.2 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.0763 g, yield 43.95%, m.p. 178.41~180.25°C. HR-ESI-MS: 516.1518 [M+Na]+; IR (KBr): 3420.93, 2975.89, 2927.22, 1629.34, 1548.51, 1457.28, 1373.69, 1268.90, 1200.34, 1088.77, 1027.46; EPR (DCM): g factor, 2.00137.
3b: Following the synthetic procedure for compound 1b, compound 3 (0.53 mmol, 90.2 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.1186 g, yield 66.44%, m.p. 120.39~120.60°C. HR-ESI-MS: 530.1670 [M+Na]+; IR (KBr): 316.44, 2973.02, 2921.21, 2852.24, 1742.27, 1633.94, 1542.51, 1464.10, 1361.53, 1267.29, 1157.10, 1099.25, 899.75, 801.98, 755.21, 538.58; EPR (DCM): g factor, 2.00132.
3c: Following the synthetic procedure for compound 1c, compound 3 (0.53 mmol, 90.2 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.0898 g, yield 50.30%, m.p. 139.07~141.59°C. HR-ESI-MS: 530.1679 [M+Na]+; IR (KBr): 3285.89, 2930.05, 2857.77, 1749.57, 1707.97, 1632.26, 1537.76, 1428.71, 1380.02, 1322.13, 1268.33, 1202.39, 1158.08, 1086.30, 755.13; EPR (DCM): g factor, 2.00135.
3d: Following the synthetic procedure for compound 1d, compound 3 (0.53 mmol, 90.2 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.1100 g, yield 58.38%, m.p. 109.49~111.26°C. HR-ESI-MS: 558.1988 [M+Na]+; IR (KBr): 3422.09, 2921.86, 2853.34, 1623.08, 1476.31, 1378.78, 1299.89, 1264.24, 1217.63, 1162.17; EPR (DCM): g factor, 2.00138.
3e: Following the synthetic procedure for compound 1e, compound 3 (0.53 mmol, 90.2 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.0852 g, yield 44.07%, m.p. 164.35~165.91°C. HR-ESI-MS: 572.2127 [M+Na]+; IR (KBr): 3275.30, 2969.99, 2932.54, 1748.12, 1673.27, 1633.59, 1537.52, 1458.82, 1377.09, 1273.18, 1200.23, 1157.12, 754.20; EPR (DCM): g factor, 2.00146.
3f: Following the synthetic procedure for compound 1f, compound 3 (0.53 mmol, 90.2 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.0730 g, yield 36.55%, m.p. 123.44~125.42°C. HR-ESI-MS: 590.1716 [M+Na]+; IR (KBr): 3286.64, 2975.44, 2926.58, 1746.22, 1631.13, 1534.55, 1476.91, 1375.68, 1272.17, 1203.02, 1160.77, 1079.32, 1006.61, 750.56, 701.06; EPR (DCM): g factor, 2.00096.
3h: Following the synthetic procedure for compound 1h, compound 3 (0.53 mmol, 90.2 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.0735 g, yield 40.07%, m.p. 187.18~188.72°C. HR-ESI-MS: 544.1819 [M+Na]+; IR (KBr): 3417.62, 2981.95, 2930.54, 2358.29, 1705.56, 1631.19, 1455.76, 1383.06, 1268.54, 1146.41, 755.37, 538.39; EPR (DCM): g factor, 2.00135.
4a: Following the synthetic procedure for compound 1a, compound 4 (0.53 mmol, 91.3 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.0998 g, yield 57.25%, m.p. 152.36~154.53°C. HR-ESI-MS: 518.1679 [M+Na]+; IR (KBr): 3421.91, 2972.53, 2928.58, 1748.46, 1628.78, 1543.50, 1457.70, 1373.65, 1268.85, 1199.03, 1083.61, 1029.00, 754.83; EPR (DCM): g factor, 1.99936.
4b: Following the synthetic procedure for compound 1b, compound 4 (0.53 mmol, 91.3 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.1270 g, yield 70.87%, m.p. 143.17~144.48°C. HR-ESI-MS: 532.1819 [M+Na]+; IR (KBr): 3428.23, 2969.23, 2925.47, 1736.86, 1629.21, 1461.42, 1373.54, 1267.80, 1163.96, 1081.27, 754.95; EPR (DCM): g factor, 1.99949.
4c: Following the synthetic procedure for compound 1c, compound 4 (0.53 mmol, 91.3 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.1108 g, yield 61.83%, m.p. 184.63~185.96°C. HR-ESI-MS: 532.1829 [M+Na]+; IR (KBr): 3417.82, 3324.51, 2926.46, 2852.06, 1740.76, 1628.48, 1570.76, 1538.09, 1488.31, 1454.01, 1374.37, 1264.90, 1154.29, 1112.02, 893.81, 748.87, 540.55; EPR (DCM): g factor, 1.99945.
4d: Following the synthetic procedure for compound 1d, compound 4 (0.53 mmol, 91.3 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.0783 g, yield 41.40%, m.p. 103.27~104.56°C. HR-ESI-MS: 560.2126 [M+Na]+; IR (KBr): 3335.44, 2969.52, 2930.51, 1739.00, 1671.72, 1630.42, 1534.08, 1459.20, 1372.81, 1272.93, 1201.27, 1156.84, 1083.24, 1012.54, 750.38; EPR (DCM): g factor, 1.99958.
4e: Following the synthetic procedure for compound 1e, compound 4 (0.53 mmol, 91.3 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.0707 g, yield 66.44%, m.p. 133.21~134.36°C. HR-ESI-MS: 574.2293 [M+Na]+; IR (KBr): 3415.42, 3270.12, 2965.17, 2932.06, 2871.62, 1742.31, 1672.64, 1636.22, 1542.22, 1457.75, 1374.46, 1276.59, 1200.35, 1155.56, 1080.02, 1017.55, 901.48, 746.09; EPR (DCM): g factor, 1.99991.
4f: Following the synthetic procedure for compound 1f, compound 4 (0.53 mmol, 91.3 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.1119 g, yield 55.83%, m.p. 173.92~174.53°C. HR-ESI-MS: 592.1858 [M+Na]+; IR (KBr): 3316.24, 2973.82, 2926.14, 1740.90, 1673.10, 1630.54, 1538.70, 1453.58, 1373.56, 1270.96, 1202.66, 1162.55, 1079.24, 1011.14, 903.97, 754.44; EPR (DCM): g factor, 1.99991.
4h: Following the synthetic procedure for compound 1h, compound 4 (0.53 mmol, 91.3 mg) was used in place of compound 1 to afford the corresponding derivative. Yellow solid powder, 0.0778 g, yield 42.26%, m.p. 165.09~166.46°C. HR-ESI-MS: 546.1984 [M+Na]+; IR (KBr): 33,421.78, 2972.72, 2928.21, 1629.16, 1458.56, 1371.29, 1268.83, 1153.61, 755.29; EPR (DCM): g factor, 1.99852
The corresponding characterization spectra are provided in the Supplementary Material (Figures S10–S69).
In vitro Antioxidant Activity Evaluation
DPPH and ABTS
Compared with rhein, four series of rhein acid-tetramethylpiperidine nitroxyl radical showed better radical scavenging ability than the control (Table 1). Notably, alanine (1b, 1c, 2b, 3b, 3c, 4b, and 4c) exhibited superior scavenging compared with glycine and valine; in particular, the synthetic compounds 1b, 2b, and 4b were 30–50 times more efficient than rhein in DPPH scavenging and 30–80 times more efficient than rhein in ABTS scavenging.
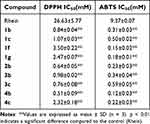 |
Table 1 IC50 of Scavenging DPPH Free Radical and ABTS Free Radical of Rhein Nitroxide Free Radical Derivatives |
Oxidative Damage Protection Assay
The protective effect against H2O2-induced oxidative damage in L02 cells was evaluated. The cell survival rate in the oxidative damage model group was 50.95%, while that in the rhein group increased to 56.76%. Among the nine previously screened compounds, all showed higher cell survival rates than the rhein group. Compound 4b exhibited the strongest protective effect, significantly increasing the cell survival rate to 95.42% (p < 0.01) (Figure 1).
In vivo Antioxidant and Anti-Aging Activity of Compound 4b
Lifespan Extension
The results of the acute toxicity experiments indicated that compound 4b did not exhibit significant toxicity to C. elegans at concentrations ≤300 µM, demonstrating that this concentration range is safe. Therefore, these concentrations were used in subsequent lifespan experiments to ensure that any observed effects were due to anti-aging properties rather than toxicity. Thus, three concentrations (50, 150, and 300 µM) were selected to assess the effects of compound 4b, 3-hydroxymethyl-2,2,5,5-tetramethylpyrroline nitroxide radical (compound 4), and rhein on the lifespan of C. elegans. Across all concentrations, treatment with 4, 4b, and rhein significantly extended the maximum and average lifespans of the worms compared with the blank group, demonstrating a dose-dependent effect. At 300 µM, compound 4b extended the maximum lifespan to 27.0 ± 0.82 days and the average lifespan to 15.41 ± 0.29 days, which was a 41.09% increase compared with the control group (p < 0.001) (Figure 2).
Stress Resistance Assays
In stress resistance experiments, C. elegans exposed to thermal stress (37°C) and oxidative stress (400 µM juglone) were treated with compound 4b. Under heat stress, the control group exhibited an average survival time of 6.33 ± 0.19 h and a maximum survival time of 11.33 ± 0.47 h. Treatment with compound 4b significantly extended both metrics, achieving a maximum survival time of 14.33 ± 0.47 h and an average survival time of 9.38 ± 0.26 h, representing a 48.09% increase (Figure 3A). Under oxidative stress conditions, compound 4b-treated worms showed an average lifespan extension of 31.91%, reaching 18.13 ± 0.52 h compared with the control group (Figure 3B).
 |
Figure 3 Effects of compounds 4, 4b, and rhein on heat stress and oxidative stress resistance of wild-type C. elegans (300 µM): (A) heat stress; (B) oxidative stress. |
Assessment of Oxidative Stress Markers
To evaluate the impact of compound 4b on oxidative stress, we used the DCFH-DA fluorescent probe method to measure ROS levels in C. elegans. As depicted in Figure 4A, treatment with compound 4b and rhein resulted in only weak fluorescence signals, whereas the control group showed strong fluorescence. Quantitative analysis using Image J software revealed that ROS levels in the 4-, 4b-, and rhein-treated groups decreased by 18.35%, 53.39%, and 32.72%, respectively, compared with the control group (p < 0.001) (Figure 4B). Additionally, measurements of malondialdehyde (MDA) levels and glutathione (GSH) activity indicated that compound 4b significantly reduced MDA levels by 34.92% (p < 0.001) (Figure 4C) and increased GSH activity by 28.73% (p < 0.01) (Figure 4D). These results demonstrated that compound 4b effectively reduced ROS accumulation and lipid peroxidation while enhancing antioxidant defenses.
Molecular Docking
Keap1 binds Nrf2 through its C-terminal Kelch domain. The Keap1/Nrf2 binding site within the Kelch domain can be divided into five pockets (P1–P5). Compared with rhein, 4b is more deeply inserted into the central cavity of the Keap1 Kelch domain (Figure 5A). Molecular docking of rhein with Keap1 protein showed that rhein could form hydrophobic interactions with P1 (Ile461, Phe478, and Ser508), P2 (Ser363), P3 (Ala556), P4 (Tyr525), and P5 (Tyr334) (Figure 5B). The carbonyl oxygen atom and hydroxyl group in the structure of rhein formed hydrogen bond interactions with the residues Ser 508 and Arg 483 in the P1 pocket of the Kelch domain, respectively. The benzene ring and the residue Arg 415 in the P1 pocket formed π-π stacking and salt bridge. Compared with rhein, compound 4b occupied more binding pockets and formed hydrophobic interactions with P1 (Ile461, Gly462, Arg483, and Ser508), P3 (Gly509, Ser555, Ala556, and Gly603), and P4 (Tyr525, Gln530, and Tyr572). In addition, the carbonyl oxygen atoms in the 4b structure formed hydrogen bond interactions with the residues Gln 530 and Arg 415, respectively, and hydrogen bonds formed between the hydroxyl groups on the tetrahydropyrrole ring and the residue Leu365 (Figure 5C). Compound 4b was inserted deep into the central cavity of the Kelch domain, exhibiting enhanced stability in binding to the Kelch domain and superior competition with Nrf2 for binding sites.
 |
Figure 5 Static structure analysis of the binding mode of rhein, 4b and the Kelch domain (PDB 6TYM); (A) The target binding site of compound; (B) rhein; (C) 4b. |
Discussion
This study used rhein, a natural compound with known Keap1 inhibitory activity, as the parent structure and introduced a nitroxide radical at the 3-position carboxyl group for structural modification, designing and synthesizing a series of novel derivatives. These derivatives exhibited significantly enhanced free radical scavenging capacity compared to the parent compound, with derivative 4b demonstrating excellent DPPH and ABTS radical scavenging activities in vitro. The liver, as the primary metabolic and detoxification organ, contains mitochondria and the cytochrome P450 enzyme system, making it a major site of endogenous ROS production. L02 human normal hepatocytes retain the metabolic characteristics of primary hepatocytes, accurately reflecting oxidative stress responses under physiological conditions. Moreover, they are highly sensitive to oxidative inducers such as H2O2, enabling the establishment of a stable and reliable oxidative damage model.39,40 Experimental results showed that compound 4b exhibited significant protective effects in an H2O2-induced oxidative damage model using L02 cells.
To further validate the in vivo activity of the compounds, this study employed C. elegans as a model organism. This choice was based on the following key considerations: First, C. elegans shares highly conserved antioxidant defense mechanisms with mammals; second, its short lifespan makes it an ideal model for rapid evaluation of anti-aging effects;41,42 and third, the C. elegans model effectively complements cellular experiments, verifying the compound’s efficacy at the whole-organism level. The study found that compound 4b demonstrated a stronger ability to extend the lifespan of C. elegans compared to the parent compound rhein, while also significantly enhancing the worms’ resistance to heat stress (37°C) and oxidative stress (juglone). These results corroborated the L02 cell experimental data, collectively indicating that compound 4b possesses significant antioxidant and anti-aging activity.
Previous molecular docking studies revealed that rhein binds to key amino acids in the Keap1 structure, and compared to known Nrf2 activators such as bardoxolone, rhein exhibited a higher binding score with Keap1.22 In this study, the introduction of a 3-hydroxymethyl-2,2,5,5-tetramethylpyrrolidine-nitroxide radical at the 3-position of rhein resulted in compound 4b, which could penetrate deeper into the central cavity of the Keap1 Kelch domain, occupying more binding sites and forming more stable interactions. This structural optimization not only enhanced the compound’s binding affinity to Keap1 but may also more effectively promote the release and activation of Nrf2 through allosteric effects.
These findings not only validate the effectiveness of targeting the Keap1-Nrf2 pathway as an anti-aging strategy but also highlight the critical role of nitroxide radical modification in enhancing antioxidant activity. This study provides new insights for the design of novel anti-aging drugs.
Conclusion
In conclusion, this study successfully designed and synthesized nitroxide radical derivatives of rhein, significantly enhancing their antioxidant and anti-aging properties. Compound 4b emerged as the most potent derivative, exhibiting superior free radical scavenging capabilities, effective protection against oxidative damage in L02 hepatocytes, and substantial lifespan extension and stress resistance improvements in C. elegans. Molecular docking studies confirmed that compound 4b formed stable and extensive interactions within the Keap1 Kelch domain, supporting its role as a potent Keap1–Nrf2 PPI inhibitor. These findings establish compound 4b as a promising anti-aging candidate. Future research will focus on exploring pharmacokinetic properties, safety evaluation, and potential synergistic applications in chronic disease management.
Funding
This work was supported by the National Natural Science Foundation of China (no. 82160457, no. 81660577), the Gansu Provincial Department of Education 2024 University scientific research innovation platform major training project (no. 2024CXPT-18), the Young Doctor Support Project of Gansu Provincial Department of Education (no. 2025QB-067), the Open Fund of Gansu Province Engineering Laboratory for TCM Standardization Technology and Popularization (no. ddyc-2022-03) and the College industry support plan project of Gansu Province (no. 2025CYZC-052).
Disclosure
Jie Wang reports a patent CN202411110059.6 pending. The authors report no other conflicts of interest in this work.
References
1. Romano AD, Serviddio G, Matthaeis A, et al. Oxidative stress and aging. J Nephrol. 2010;23(15):S29–36.
2. Kregel KCZH. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R18–36. doi:10.1152/ajpregu.00327.2006
3. Cui HKY, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 2012;646354. doi:10.1155/2012/646354
4. Golden TR, Hinerfeld DA, Melov S. Oxidative stress and aging: beyond correlation. Aging Cell. 2002;1(2):117–123. doi:10.1046/j.1474-9728.2002.00015.x
5. Mikhed YDA, Steven S. Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age-related vascular dysfunction. Int J Mol Sci. 2015;16(7):15918–15953. doi:10.3390/ijms160715918
6. Zia A, Farkhondeh T, Pourbagher-Shahri AM, et al. The roles of mitochondrial dysfunction and reactive oxygen species in aging and senescence. Curr Mol Med. 2022;22(1):37–49. doi:10.2174/1566524021666210218112616
7. Tu W, Wang H, Li S, et al. The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 2019;10(3):637–651. doi:10.14336/AD.2018.0513
8. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi:10.1146/annurev.pharmtox.46.120604.141046
9. Yu C, Xiao JH. The Keap1-Nrf2 system: a mediator between oxidative stress and aging. Oxid Med Cell Longev. 2021;2021:6635460. doi:10.1155/2021/6635460
10. Lewis KN, Wason E, Edrey YH, et al. Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proc Natl Acad Sci. 2015;112(12):3722–3727. doi:10.1073/pnas.1417566112
11. Blair HA. Dimethyl fumarate: a review in relapsing-remitting MS. Drugs. 2019;79(18):1965–1976. doi:10.1007/s40265-019-01229-3
12. Brück J, Dringen R, Amasuno A, et al. A review of the mechanisms of action of dimethylfumarate in the treatment of psoriasis. Exp Dermatol. 2018;27(6):611–624. doi:10.1111/exd.13548
13. Tran KT, Pallesen JS, Solbak SMØ, et al. A comparative assessment study of known small-molecule Keap1−Nrf2 protein–protein interaction inhibitors: chemical synthesis, binding properties, and cellular activity. J Med Chem. 2019;62(17):8028–8052. doi:10.1021/acs.jmedchem.9b00723
14. Jiang Z-Y, Xu LL, Lu M-C, et al. Structure–activity and structure–property relationship and exploratory in vivo evaluation of the nanomolar Keap1–Nrf2 protein–protein interaction inhibitor. J Med Chem. 2015;58(16):6410–6421. doi:10.1021/acs.jmedchem.5b00185
15. Shi Z, Zhang Y, Wang X, et al. Discovery of propionic acid derivatives with a 5-THIQ core as potent and orally bioavailable Keap1–Nrf2 protein–protein interaction inhibitors for acute kidney injury. J Med Chem. 2024;67(21):19247–19266. doi:10.1021/acs.jmedchem.4c01687
16. Zhang Y, Shi Z, Zhou Y, et al. Emerging substrate proteins of kelch-like ECH associated protein 1 (Keap1) and potential challenges for the development of small-molecule inhibitors of the Keap1-Nuclear factor erythroid 2-related factor 2 (Nrf2) protein–protein interaction. J Med Chem. 2020;63(15):7986–8002. doi:10.1021/acs.jmedchem.9b01865
17. Crisman E, Duarte P, Dauden E, et al. KEAP1‐NRF2 protein–protein interaction inhibitors: design, pharmacological properties and therapeutic potential. Med Res Rev. 2022;43(1):237–287. doi:10.1002/med.21925
18. Zhang DD. Thirty years of NRF2: advances and therapeutic challenges. Nat Rev Drug Discov. 2025. doi:10.1038/s41573-025-01145-0
19. Ren Q, Bakker W, de Haan L, et al. Induction of Nrf2-EpRE-mediated gene expression by hydroxyanthraquinones present in extracts from traditional Chinese medicine and herbs. Food and Chemical Toxicology. 2023:176. doi:10.1016/j.fct.2023.113802
20. Zhuang S, Yu R, Zhong J, et al. Rhein from rheum rhabarbarum inhibits hydrogen-peroxide-induced oxidative stress in intestinal epithelial cells partly through PI3K/Akt-mediated Nrf2/HO-1 pathways. J Agric Food Chem. 2019;67(9):2519–2529. doi:10.1021/acs.jafc.9b00037
21. Zhuang S, Zhong J, Bian Y, et al. Rhein ameliorates lipopolysaccharide-induced intestinal barrier injury via modulation of Nrf2 and MAPKs. Life Sci. 2019;216:168–175. doi:10.1016/j.lfs.2018.11.048
22. Zhu Y, Jin H, Huo X, et al. Protective effect of rhein against vancomycin-induced nephrotoxicity through regulating renal transporters and Nrf2 pathway. Phytother Res. 2022;36(11):4244–4262. doi:10.1002/ptr.7559
23. Antonisamy P, Agastian P, Kang C-W, et al. Anti-inflammatory activity of rhein isolated from the flowers of Cassia fistula L. and possible underlying mechanisms. Saudi J Biol Sci. 2019;26(1):96–104. doi:10.1016/j.sjbs.2017.04.011
24. Zhuang S, Zhong J, Zhou Q, et al. Rhein protects against barrier disruption and inhibits inflammation in intestinal epithelial cells. Int Immunopharmacol. 2019;71:321–327. doi:10.1016/j.intimp.2019.03.030
25. Tu Y, Liu W, Liu S, et al. Rhein, a component of rhubarb, protects against oxidative stress-induced renal aging and injury by inhibition of TNF-a-mediated autophagy and necroptosis. Res Square. 2024. doi:10.21203/rs.3.rs-5367778/v1
26. Zhen Y-Z, Lin Y-J, Li K-J, et al. Effects of rhein lysinate on D-galactose-induced aging mice. Exp Ther Med. 2016;11(1):303–308. doi:10.3892/etm.2015.2858
27. Sadowska-Bartosz I BG. The cellular and organismal effects of nitroxides and nitroxide-containing nanoparticles. Int J Mol Sci. 2024;25(3):1446. doi:10.3390/ijms25031446
28. Carloni P, Greci L, Stipa P, et al. Electron-transfer reactions. oxidation of Grignard reagents in the presence of an aminoxyl as a radical-trapping agent. J Org Chem. 1991;56(15):4733–4737. doi:10.1021/jo00015a029
29. Carloni P, Greci L, Stipa P. Antioxidants and light stabilizers. part 1. reactions of an indolinone nitroxide and phenoxy radicals. X-ray crystallographic analysis of 1-[O-(3,5-di-tert-butyl-4-hydroxy)-benzyl]-1,2-dihydro-2-methyl-2-phenyl-3-oxo-3H-indole and 3,5,3ق│5ق│ -tetra-tert-butylstilbene-4,4ق│-quinone. Polym Degrad Stab. 1993;39(1):73–83. doi:10.1016/0141-3910(93)90127-
30. Larin ACR, Pfrunder MC, Mullen KM, et al. Synergistic or antagonistic antioxidant combinations - a case study exploring flavonoid-nitroxide hybrids. Org Biomol Chem. 2023;21(8):1780–1792. doi:10.1039/d2ob02101c
31. Zhang JQ, Zhang ZW, Hui L, et al. Design, synthesis and biological evaluation of novel spin-labeled derivatives of podophyllotoxin. Nat Prod Commun. 2010;5(2):241–244. doi:10.1177/1934578x1000500214
32. Zhang ZW, Zhang JQ, Hui L, et al. First synthesis and biological evaluation of novel spin-labeled derivatives of deoxypodophyllotoxin. Eur J Med Chem. 2010;45(4):1673–1677. doi:10.1016/j.ejmech.2009.12.032
33. Csekö J, Hankovszky H, Hideg K. Synthesis of novel, highly reactive 1-oxyl-2,2,6,6-tetramethyl-1,2,5,6-tetrahydropyridine derivatives. Can J Chem. 1985;63(4):940–943. doi:10.1139/v85-156
34. Liu T, Liu X, Olajide TM, et al. Two novel lipophilic antioxidants derivatized from curcumin. Antioxidants. 2022;11(4). doi:10.3390/antiox11040796
35. Fan M-X, Chen G-L, Guo M-Q. Potential antioxidative components in Azadirachta indica revealed by bio-affinity ultrafiltration with SOD and XOD. Antioxidants. 2022;11(4). doi:10.3390/antiox11040658
36. Murillo K, Samigullin A, Humpert PM, et al. Protective effects of transient glucose exposure in adult C. elegans. Antioxidants. 2022;11(1). doi:10.3390/antiox11010160
37. Shi H, Zheng Y, Zhao J, et al. Zexie decoction reduce glucose-dependent lipid accumulation and oxidative stress in Caenorhabditis elegans. Phytomedicine. 2023:120. doi:10.1016/j.phymed.2023.155036
38. Zhu A, Zheng F, Zhang W, et al. Oxidation and antioxidation of natural products in the model organism Caenorhabditis elegans. Antioxidants. 2022;11(4). doi:10.3390/antiox11040705
39. Hu Z, Xu D, Meng H, et al. 4-octyl itaconate protects against oxidative stress-induced liver injury by activating the Nrf2/Sirt3 pathway through AKT and ERK1/2 phosphorylation. Biochem Pharmacol. 2024;220:115992. doi:10.1016/j.bcp.2023.115992
40. Li J, Ge H, Xu Y, et al. Geniposide alleviates oxidative damage in hepatocytes through regulating miR-27b-3p/Nrf2 Axis. J Agric Food Chem. 2022;70(37):11544–11553. doi:10.1021/acs.jafc.2c03856
41. Bai X, Wang M, Xu T, et al. Antioxidant and anti-aging activities of Acanthopanax senticosus polysaccharide CQ-1 in nematode Caenorhabditis elegans. Int J Biol Macromol. 2025;297:139925. doi:10.1016/j.ijbiomac.2025.139925
42. Zhang Y, Li Y, Ren T, et al. Promising tools into oxidative stress: a review of non-rodent model organisms. Redox Biol. 2024;77:103402. doi:10.1016/j.redox.2024.103402
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






