Back to Journals » Infection and Drug Resistance » Volume 18
Detection of Salmonella spp. Related Co-Infections Among Children with Diarrheal Diseases in Guangzhou, China
Authors Mai Q , Luo Y, Ye R, Jiang Y, Qin Y, Guo J, Lai W, Wu Y, Luo M
Received 14 January 2025
Accepted for publication 11 April 2025
Published 16 April 2025 Volume 2025:18 Pages 1895—1903
DOI https://doi.org/10.2147/IDR.S515033
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Qiongdan Mai,* Yasha Luo,* Ruoting Ye, Yongyao Jiang, Yanting Qin, Junfei Guo, Weiming Lai, Yongbing Wu, Mingyong Luo
Department of Clinical Laboratory, Guangdong Women and Children Hospital, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mingyong Luo, Department of Clinical Laboratory, Guangdong Women and Children Hospital, NO. 521 Xingnan Road, Panyu District, Guangzhou, People’s Republic of China, Tel +86-15920356428, Email [email protected]
Background: Diarrheal diseases caused by gastrointestinal pathogens contribute to the high morbidity and mortality in children worldwide. Salmonella infection is one of the leading causes of diarrhea, especially in children under 5 years of age. This study aimed to assess the prevalence of Salmonella infection and its co-infection patterns in relation to clinical symptoms.
Methods: A total of 430 stool samples of children with diarrheal diseases were collected from Guangdong Women and Children’s Hospital during January 2022 to December 2023 and used for detection. BioFire FilmArray Gastrointestinal (GI) Panel is an efficient and sensitive method used to assess infections caused by enteric pathogens simultaneously based on multiplex polymerase chain reaction (PCR) technologies.
Results: Salmonella spp. was classified as the predominant pathogen in all stool specimens, with an overall positivity rate of 36.74% (158/430), of which 35.44% (56/158) were identified as Salmonella single infection. For Salmonella-related bacterial co-infection pattern, Salmonella and enteropathogenic Escherichia coli combinations accounted for 12.66% (20/158) of Salmonella-related bacterial co-infection patterns, and Salmonella plus Clostridium difficile was found in 8.86% (14/158). For Salmonella-viral co-infection pattern, the most prevalent combination was Salmonella and adenovirus (6.33%, 10/158). Notably, the proportion of mucus stools recorded in Salmonella plus C. difficile infections was statistically higher than that in single Salmonella infections (P < 0.05).
Conclusion: This study provides a comprehensive understanding of the nature of Salmonella spp. co-infections in diarrheal diseases, and the possibility of clinical symptoms and enhanced treatments.
Keywords: enteric pathogens, gastrointestinal infection, diarrhea, FilmArray
Introduction
Diarrheal disease is the second leading cause of death in children under 5 years of age and was responsible for the deaths of 370,000 children in 2019.1–3 In China, according to some regional studies, the overall incidence of diarrhea among children under 5 years old is 2.50–3.38 episodes per year for individuals.4 Diarrhea is the passage of three or more loose or liquid stools per day, or more frequently than normal for the individual,1 and is a common symptom of gastrointestinal infection (GI) that is mainly caused by single or multiple bacterial, viral, and parasitic organisms.3,5–7 The most prevalent bacterial pathogens are Escherichia coli, Salmonella enterica, Campylobacter spp., and Shigella spp, while adenovirus, rotavirus, and norovirus were mainly responsible for viral infections. Infectious diarrhea demonstrates different seasonal distributions.4
Salmonella is a rod-shaped, gram-negative bacteria with no capsule and spore, and can be classified into typhoidal Salmonella and non-typhoidal Salmonella (NTS) serovars.8 NTS infection is a leading cause of acute gastroenteritis in pediatric patients, especially children aged under 5 years.9,10 Salmonella infection results in over 30,000 deaths annually, which was estimated by the Chinese Center for Disease Control (CDC) to be the cause of 70–80% of bacterial foodborne illnesses, ranking as one of the two diarrheal agents.11,12 Zoonotic infection caused by NTS post a significant burden for public health.13 A rising trend of Salmonellosis outbreak has been observed since the 1970s with high resistance to quinolone and beta-lactam, such as carbapenem.14,15 From the nationwide surveillance study, NTS, Shigella, C. jejuni and Y. enterocolitica displayed a “Child-pattern”, with a detection turn-point at 2–5 years.11,16 However, it remains unclear whether Salmonella-related co-infection patterns influence diarrheal symptoms among children.
The comprehensive detection of gastrointestinal pathogenic microorganisms is challenging because conventional culture methods are time-consuming and serology tests show limited sensitivity.17,18 The rapid and specific validation of gastrointestinal infectious agents is of great significance for clinical management. To overcome these difficulties, FilmArray™ technology (BioFire Diagnostics, Salt Lake City, Utah, USA) has produced rapid polymerase chain reaction (PCR) multiplexing, which is designed to simultaneously detect the most common gastrointestinal pathogens.19,20 The FilmArray GI panel (FA) not only offers high sensitivity and specificity for identifying infectious pathogens in different physiological tracts,21–23 but also provides a comprehensive way to reveal the nature of co-infection patterns among various pathogens.
The distribution of gastrointestinal pathogens, including Salmonella spp., shows significant regional characteristics. However, the distribution and clinical features of Salmonella related diarrhea in Guangzhou remain largely unknown. It is of crucial necessity to analyze the distribution and associated infection patterns of Salmonella among children, which would provide supporting information for promoting the understanding of Salmonellosis and improving bacterial gastroenteritis individualized treatment. The objectives of this study were as follows: 1) to test Salmonella in pediatric patients with diarrheal diseases using the FilmArray GI Panel to assess the overall positive rate of Salmonella; 2) to analyze the prevalence of Salmonella spp. via Film Array GI Panel and retrospective cultural results; and 3) to investigate the co-infection patterns of Salmonella with other enteric pathogens among clinical characteristics to assess the differences in symptoms.
Materials and Methods
Sample Enrollment
All stool samples in this study were obtained from patients as residual specimens of routine hospital testing procedure during January 2022 to December 2023 in Guangzhou, China. A total of 430 diarrheal stool samples from children under five years old were enrolled for FilmArray GI Panel (BioFire Diagnostics, Salt Lake City, UT, USA), and all samples were anonymized (Table S1). Second or subsequent samples from the identical patients or sample without clinical histories implicating infectious causes of diarrhea were excluded. The clinical results of fecal cultures for Salmonella spp. from 1580 patients were collected from the Laboratory Information System (LIS). These data were retrospectively categorized and analyzed to reveal the prevalence and distribution of Salmonella infection. Clinical information exported from LIS only retain gender, age, testing date, laboratory testing results and clinical syndromes. This study was approved by the Medical Ethics Committee of Guangdong Women and Children Hospital.
FilmArray GI Panel
All stool samples were tested using a commercially available multiplex PCR system, FilmArray Gastrointestinal (GI) Panel (BioFire Diagnostics, Salt Lake City, UT, USA). The FilmArray GI Panel was capable of simultaneously detecting and identifying nucleic acids from multiple bacteria, viruses, and parasites in stool samples, including Campylobacter (C. jejuni/C. coli/C. upsaliensis), Clostridium difficile (C. difficile) toxin A/B, Plesiomonas shigelloides, Salmonella, Vibrio (V. parahaemolyticus/V. vulnificus/V. cholerae), Yersinia enterocolitica, enteroaggregative Escherichia coli (EAEC), enteropathogenic Escherichia coli (EPEC), enterotoxigenic Escherichia coli (ETEC), Shiga-like toxin-producing Escherichia coli (STEC) stx1/stx2, Shigella/Enteroinvasive Escherichia coli (EIEC), Cryptosporidium, Cyclospora cayetanensis, Entamoeba histolytica, Giardia lamblia, Adenovirus F 40/41, Astrovirus, Norovirus GI/GII, Rotavirus A, Sapovirus (Genogroups I, II, IV, and V). According to FilmArray GI Panel Instruction, two process controls (RNA Process Control and PCR2 Control) were used in each essay and positive results indicated successful assay.
Salmonella Identification
Isolation and identification of Salmonella spp. was conducted according to previous research. Stool samples were plated and incubated onto XLD (Xylose Lysine Desoxycholate) agars at 36°C for 18–48h. The suspicious colonies (transparent, round, with or without black centers that reflect H2S production) were further identified by MALDI-TOF-MS (Matrix Assisted Laser Desorption Ionization-Time of Flight Mass Spectrum).
Statistical Analysis
All data on clinical characteristics were imported into Microsoft Excel. GraphPad Prism 8 was used to display the figures. Statistical analyses, including Chi-square test or Fisher’s exact test, were performed using the SPSS, version 26.0.
Results
Gender and Age Demographics of Patients
For the FilmArray GI Panel, 430 children aged <5 years with diarrheal diseases were enrolled in this study (Table 1), including 184 (42.79%) females and 246 (57.21%) males. Among them, 72 (39.13%) and 86 (34.96%) were positive for Salmonella spp. No statistically significant difference was observed between the sexes in the detection of Salmonella spp. (χ2 = 0.788, P = 0.375). The majority of stool samples were collected from children under 12 months of age (235/430), followed by 1–3 years old (161/430), and over 3 years old (34/430). The positivity rates of Salmonella spp. among the three age groups were 37.87% in children aged <12 months, 38.51% in children aged 1–3 years, and 20.59% in children aged >3 years. Moreover, 1580 patients who underwent fecal culture for Salmonella spp. were categorized and analyzed. Statistical differences were observed between children aged <12 months and those aged >3 years using both the FA (χ2 = 3.866, P = 0.049) and culture methods (χ2 = 6.481, P = 0.011). Among all stool specimens, 178 were subjected to both Salmonella detection methods.
 |
Table 1 Gender and Age Distribution of Patients with Salmonella Spp. Detection |
Prevalence of Salmonella spp. in Monthly and Age Distribution
Either FA or culture detection of Salmonella spp. was defined as a positive result for Salmonella spp. prevalence. In the monthly distribution of Salmonella spp., the highest positive rate was observed in September (42.51%, 71/167), and the lowest rate was 8.49% (9/106) in February (Figure 1A). The positive rate increased from April to July, whereas a remarkable decline was observed from September to December, followed by a peak (Figure 1A). The positive detection rate of Salmonella spp. was similar among the three age groups. In accordance with the results in Table 1, the positivity rate in children aged >3 years was likely to be lower than in the other two age groups (Figure 1B).
Prevalence of All Enteric Pathogens Detected by FilmArray
Of all the stool samples, 79.3% had at least one type of enteric pathogen (Table 2). The overall positive rate of Salmonella spp. was the highest among all the bacterial pathogens, which was detected in 36.74% (158/430), followed by EPEC (19.77%, 85/430), C. difficile (19.53%, 84/430), and Campylobacter (12.56%, 54/430). Among enteric viral pathogens, adenovirus contributed to the majority of infections (20.23%, 87/430). The proportions of bacterial single infection (Table 3), viral single infection, bacterial–bacterial infection, viral–viral infection and bacterial–viral infections were 24.19% (104/430), 10.00% (43/430), 18.60% (80/430), 3.02% (13/430), and 23.49% (101/430), respectively.
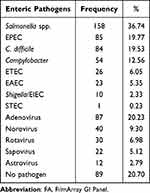 |
Table 2 Frequency of Enteric Pathogens Detected by FA |
 |
Table 3 Infection Patterns of Enteric Pathogens |
Co-Infection Patterns of Salmonella spp
Among the stool samples positive for Salmonella spp., 35.44% (56/158) were positive for Salmonella spp., whereas 37.34% (59/158) of stool samples were positive for both Salmonella spp. and other enteropathogenic bacteria. Moreover, 30 stool samples (18.99%) tested positive for Salmonella spp. and enteric viruses. A total of 13 (8.23%) stool samples were positive for Salmonella spp. and bacterial-viral co-infection. In the Salmonella mixed infection, Salmonella plus EPEC (12.66%, 20/158), Salmonella plus C. difficile (8.86%, 14/158) and Salmonella plus adenovirus (6.33%, 10/158) were the first three highest compositions. Salmonella, C. difficile and EPEC combination was detected in 4.43% (7/158) of all stool samples. Salmonella plus Norovirus, Salmonella plus EAEC were detected in 4.43% (7/158) and 2.53% (4/158) of the stool specimens, respectively (Table 4).
 |
Table 4 Infection Patterns of Salmonella Spp. Detected by FA |
Clinical Symptoms in Different Salmonella Co-Infection Patterns
The percentage of female patients with Salmonella single infection, Salmonella plus C. difficile, Salmonella plus EPEC, and Salmonella plus adenovirus were 42.86%, 35.71%, 40.00%, and 50.00%, respectively (Table 5). Similarly, most of the patients in the four groups were under 1 year of age, and 80.00% of the children in Salmonella plus EPEC group were under 12 months of age. Clinical symptoms of fever were observed in 67.86% (38/56) of Salmonella single infections, 71.43% (10/14) of Salmonella infections plus C. difficile, 60.00% (12/20) of Salmonella infections plus EPEC, and 50% of Salmonella infections plus adenovirus. Vomiting symptoms were recorded in 14.29% of patients with Salmonella single infections. All patients in the four groups had diarrhea, whereas the blood stools were recorded in 42.86% of Salmonella single infections and in 42.86% of Salmonella plus C. difficile. Notably, mucus stools were recorded in Salmonella plus C. difficile at a high rate of 92.86% (13/14), which was significantly higher than that in Salmonella infections alone (P < 0.05).
 |
Table 5 Symptom Distribution of Different Salmonella Infection Patterns |
Discussion
Salmonellosis is responsible for one of the major causes of diarrhea, and salmonellosis outbreaks have post a great threat to worldwide developing regions, especially China.14 Salmonella spp. is primarily transmitted through contaminated foods such as eggs, milk, meat, and drinking water.12,24 Although children’s consumption of nutritious foods has increased, their immature immune systems heighten susceptibility to Salmonella infections. This study found the high Salmonella positive rate among children with gastroenteritis, which was lower than the finding of Truong J et al and Spina A et al,25,26 for the Salmonella infection is likely to be associated with climate, regions and living habit. Notably, co-infection with Clostridium difficile correlated with exacerbated diarrhea severity, highlighting the necessity to enhance pediatric dietary hygiene management and reinforce preventive strategies against gastrointestinal infections. Furthermore, healthcare institutions should optimize rapid pathogen detection technologies to facilitate comprehensive enteric pathogen screening in pediatric populations.
Given that the gastrointestinal tract is home to a large number of microorganisms, co-infection is a common enteric pathogen in diarrheal infections. The high burden of coinfection with multiple enteric pathogens in children with diarrhea should be given more attention.27 The FilmArray GI Panel is an efficient and sensitive method for detecting enteric pathogens including the most prevalent bacteria, viruses, and parasites, which have a significant potential for improving the detection of important enteric microorganisms. From our data, the overall positivity rates of enteric pathogens and co-infections were higher than those reported elsewhere.28,29 A key possible factor is that children under five years of age are more vulnerable to infectious diarrhea than children over five years of age, especially in developing countries.
In this study, children co-infected with Salmonella spp. and C. difficile displayed a high frequency of fever and mucus stools compared with Salmonella single infection. It has been reported that children coinfected with rotavirus and toxin-producing C. difficile have more severe clinical characteristics and are more vulnerable to severely dehydrated.30 However, Shafiq M et al found that co-infection with other pathogens did not affect the severity of C. difficile infection or cause treatment failures.31 Moreover, although co-infection with Campylobacter and Salmonella was observed at low levels, it was estimated that co-culture with Salmonella spp. significantly promoted the survival of Campylobacter jejuni under aerobic conditions.32 Based on various gastrointestinal infection patterns and distinguished clinical symptoms, it would be reasonable to apply individualized treatments to the pediatric patients. Assessment of multiple possible infectious etiologies may contribute to better pharmacological management of acute gastroenteritis among children, whereas the strategies of the pathogenesis of concurrent infections require further investigation. Interactions between different enteropathogenic microorganisms are poorly understood. Therefore, it is reasonable to reveal and interpret the underlying mechanisms involved in pathogenic co-infections using multi-pathogen detection technology.
It was widely reported that age is a risk factor associated with Salmonella incidence, for the younger children were more vulnerable to infections. As estimated in a nationwide study, 34% of diarrheal patients infected with non-typhoid Salmonella are under five years of age.33 In this study, the overall positive rate of Salmonella spp. among children under 5 years of age was 36.74% by the FilmArray GI Panel and 25.19% by the culture method, which may be attributed to the difference in sample size and patient source. Based on the surveillance network, the most prevalent pathogen in Beijing is Shigella spp., followed by Vibrio parahaemolyticus, Salmonella spp. and EPEC.34 In another study of pathogens in children under five years of age with acute gastroenteritis in Guangzhou, China, the most prevalent pathogen was Salmonella spp.,35 which is consistent with the results of this study. Taken together, the prevalence of Salmonella spp. may vary across regions, detection methods, and patient sources.
There are some limitations to this study, including: 1) The sample size in this study was relatively small and the samples were not evenly distributed in each month, so the prevalence of Salmonella spp. detected by the FilmArray GI Panel was not representative enough. 2) Not all stool samples for the FilmArray GI Panel were simultaneously assessed using the conventional culture method, and the comparison of the two assays was limited. 3) The FilmArray GI Panel could not distinguish between Salmonella active infections and dead bacteria. 4) The FilmArray GI Panel could not determine whether the detected pathogens were pathogenic or enteric-colonized. 5) The strains and serotypes of Salmonella spp. could not be classified using the current FilmArray GI Panel.
In conclusion, this study showed that Salmonella is the predominant pathogen detected in diarrheal diseases in children under five years of age in Guangzhou, China. Furthermore, these results showed a high frequency of co-infection with Salmonella and other enteric pathogens, mainly Clostridium difficile, Enteropathogenic Escherichia coli, and adenoviruses. This study provides a comprehensive understanding of the clinical characteristics and outcomes of the potential interactions between Salmonella and other enteric microorganisms.
Data Sharing Statement
The data that support the findings are available from the corresponding author upon non-commercial request.
Ethics Approval
This study was approved by the Medical Ethics Committee of Guangdong Women and Children Hospital, China, and it was conducted according to the ethical guidelines of the Declaration of Helsinki.
Consent to Participants
The informed consent to participants was waived by the Medical Ethics Committee (NO. 202401096) of Guangdong Women and Children Hospital and the use of residual specimens was strictly anonymized in this study.
Funding
This work was supported by the Guangdong Foundation for Basic and Applied Basic Research 2022A1515012226 (to Mingyong Luo), the Guangdong Medical Science Foundation B2024025 (to Qiongdan Mai), the Guangdong Provincial Administration of Traditional Chinese Medicine 20251044 (to Weiming Lai).
Disclosure
The authors declare that they have no competing interests in publishing this paper.
References
1. WHO. Available from: https://www.who.int/health-topics/diarrhoea#tab=tab_1.
2. Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the global enteric multicenter study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. doi:10.1016/S0140-6736(13)60844-2
3. Farthing M, Salam MA, Lindberg G, et al. Acute diarrhea in adults and children: a global perspective. J Clin Gastroenterol. 2013;47(1):12–20. doi:10.1097/MCG.0b013e31826df662
4. Chen J, Wan CM, Gong ST, et al. Chinese clinical practice guidelines for acute infectious diarrhea in children. World J Pediatrics. 2018;14(5):429–436. doi:10.1007/s12519-018-0190-2
5. Bose T, Borrow R, Arkwright PD. Impact of rotavirus vaccination on diarrheal disease burden of children in South America. Expert Rev Vaccines. 2024;23(1):606–618. doi:10.1080/14760584.2024.2360212
6. Omatola CA, Mshelbwala PP, Okolo MO, et al. Noroviruses: evolutionary dynamics, epidemiology, pathogenesis, and vaccine advances-a comprehensive review. Vaccines. 2024;12(6):590. doi:10.3390/vaccines12060590
7. Ochieng JB, Powell H, Sugerman CE, et al. Epidemiology of Enteroaggregative, Enteropathogenic, and Shiga Toxin-Producing Escherichia coli among children aged <5 years in 3 countries in Africa, 2015-2018: vaccine impact on diarrhea in Africa (VIDA) study. Clin Infect Dis. 2023;76(76 Suppl1):S77–s86. doi:10.1093/cid/ciad035
8. Wang Z, Huang C, Liu Y, et al. Salmonellosis outbreak archive in China: data collection and assembly. Sci Data. 2024;11(1):244. doi:10.1038/s41597-024-03085-7
9. Chen H, Qiu H, Zhong H, Cheng F, Wu Z, Shi T. Non-typhoidal salmonella infections among children in Fuzhou, Fujian, China: a 10-year retrospective review from 2012 to 2021. Infect Drug Resist. 2023;16:2737–2749. doi:10.2147/IDR.S408152
10. Mai Q, Lai W, Deng W, et al. Prevalence, serotypes and antimicrobial resistance of salmonella isolated from children in Guangzhou, China, 2018-2023. Infect Drug Resist. 2024;17:4511–4520. doi:10.2147/IDR.S486907
11. Wang LP, Zhou SX, Wang X, et al. Etiological, epidemiological, and clinical features of acute diarrhea in China. Nat Commun. 2021;12(1):2464. doi:10.1038/s41467-021-22551-z
12. Teng L, Huang L, Zhou H, Wang B, Yue M, Li Y. Microbiological hazards in infant and toddler food in China: a comprehensive study between 2004 and 2022. Food Res Int. 2024;180:114100. doi:10.1016/j.foodres.2024.114100
13. Chen J, Huang L, An H, et al. One health approach probes zoonotic non-typhoidal salmonella infections in China: a systematic review and meta-analysis. J Global Health. 2024;14:04256. doi:10.7189/jogh.14.04256
14. Wang Z, Zhou H, Liu Y, et al. Nationwide trends and features of human salmonellosis outbreaks in China. Emerg Microbes Infect. 2024;13(1):2372364. doi:10.1080/22221751.2024.2372364
15. Ke Y, Zhu Z, Lu W, et al. Emerging bla(NDM)-positive salmonella enterica in Chinese pediatric infections. Microbiol Spectrum. 2024;12(12):e0148524. doi:10.1128/spectrum.01485-24
16. Ke Y, Teng L, Zhu Z, et al. Genomic investigation and nationwide tracking of pediatric invasive nontyphoidal salmonella in China. mLife. 2024;3(1):156–160. doi:10.1002/mlf2.12117
17. Beatty ME, Adcock PM, Smith SW, et al. Epidemic Diarrhea due to Enterotoxigenic Escherichia coli. Clin Infect Dis. 2006;42(3):329–334. doi:10.1086/499246
18. Pouzol S, Tanmoy AM, Ahmed D, et al. Clinical evaluation of a multiplex PCR for the detection of salmonella enterica serovars typhi and paratyphi A from blood specimens in a high-endemic setting. American J Trop Med Hyg. 2019;101(3):513–520. doi:10.4269/ajtmh.18-0992
19. Khare R, Espy MJ, Cebelinski E, et al. Comparative evaluation of two commercial multiplex panels for detection of gastrointestinal pathogens by use of clinical stool specimens. J Clin Microbiol. 2014;52(10):3667–3673. doi:10.1128/JCM.01637-14
20. Buss SN, Leber A, Chapin K, et al. Multicenter evaluation of the BioFire FilmArray gastrointestinal panel for etiologic diagnosis of infectious gastroenteritis. J Clin Microbiol. 2015;53(3):915–925. doi:10.1128/JCM.02674-14
21. Camprubí-Ferrer D, Cobuccio L, Van Den Broucke S, et al. Clinical evaluation of BioFire® multiplex-PCR panel for acute undifferentiated febrile illnesses in travellers: a prospective multicentre study. J Travel Med. 2023;30(3). doi:10.1093/jtm/taad041
22. Leber AL, Everhart K, Balada-Llasat JM, et al. Multicenter evaluation of biofire filmarray meningitis/encephalitis panel for detection of bacteria, viruses, and yeast in cerebrospinal fluid specimens. J Clin Microbiol. 2016;54(9):2251–2261. doi:10.1128/JCM.00730-16
23. Murphy CN, Fowler R, Balada-Llasat JM, et al. Multicenter evaluation of the biofire filmarray pneumonia/pneumonia plus panel for detection and quantification of agents of lower respiratory tract infection. J Clin Microbiol. 2020;58(7). doi:10.1128/JCM.00128-20
24. Wei Z, Xu X, Yan M, et al. Salmonella typhimurium and salmonella enteritidis infections in sporadic diarrhea in children: source tracing and resistance to third-generation cephalosporins and ciprofloxacin. Foodborne Pathogens Dis. 2019;16(4):244–255. doi:10.1089/fpd.2018.2557
25. Spina A, Kerr KG, Cormican M, et al. Spectrum of enteropathogens detected by the FilmArray GI Panel in a multicentre study of community-acquired gastroenteritis. Clin Microbiol Infection. 2015;21(8):719–728. doi:10.1016/j.cmi.2015.04.007
26. Truong J, Cointe A, Le Roux E, et al. Clinical impact of a gastrointestinal PCR panel in children with infectious diarrhoea. Arch Dis Child. 2022;107(6):601–605. doi:10.1136/archdischild-2021-322465
27. Potgieter N, Heine L, Ngandu JPK, et al. High burden of co-infection with multiple enteric pathogens in children suffering with diarrhoea from rural and peri-urban communities in South Africa. Pathogens. 2023;12(2):315.
28. Stockmann C, Pavia AT, Graham B, et al. Detection of 23 gastrointestinal pathogens among children who present with diarrhea. J Pediatric Infectious Dis Soc. 2017;6(3):231–238. doi:10.1093/jpids/piw020
29. Andersson M, Kabayiza JC, Elfving K, et al. Coinfection with enteric pathogens in east African children with acute gastroenteritis-associations and interpretations. American J Trop Med Hyg. 2018;98(6):1566–1570. doi:10.4269/ajtmh.17-0473
30. Valentini D, Vittucci AC, Grandin A, et al. Coinfection in acute gastroenteritis predicts a more severe clinical course in children. European J Clin Microbiol Infectious Dis. 2013;32(7):909–915. doi:10.1007/s10096-013-1825-9
31. Shafiq M, Alturkmani H, Zafar Y, et al. Effects of co-infection on the clinical outcomes of clostridium difficile infection. Gut Pathog. 2020;12(9). doi:10.1186/s13099-020-00348-7
32. Anis N, Bonifait L, Quesne S, et al. Survival of campylobacter jejuni co-cultured with salmonella spp. in aerobic conditions. Pathogens. 2022;11(7). doi:10.3390/pathogens11070812
33. Ran L, Wu S, Gao Y, et al. Laboratory-based surveillance of nontyphoidal salmonella infections in China. Foodborne Pathogens Dis. 2011;8(8):921–927. doi:10.1089/fpd.2010.0827
34. Friesema IH, de Boer RF, Duizer E, et al. Etiology of acute gastroenteritis in children requiring hospitalization in the Netherlands. European J Clin Microbiol Infectious Dis. 2012;31(4):405–415. doi:10.1007/s10096-011-1320-0
35. Luo X, Deng J, Luo M, Yu N, Che X. Detection and characterization of bacterial and viral acute gastroenteritis among outpatient children under 5 years old in Guangzhou, China. American J Trop Med Hyg. 2024;110(4):809–814. doi:10.4269/ajtmh.23-0725
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


