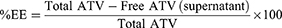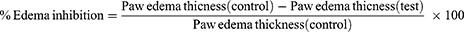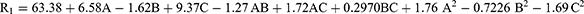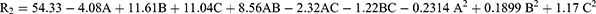Back to Journals » International Journal of Nanomedicine » Volume 19
Development and Evaluation of Nano-Vesicular Emulsion-Based Gel as a Promising Approach for Dermal Atorvastatin Delivery Against Inflammation
Authors Abdallah MH , Shawky S, Shahien MM, El-Horany HES, Ahmed EH , El-Housiny S
Received 8 May 2024
Accepted for publication 23 October 2024
Published 7 November 2024 Volume 2024:19 Pages 11415—11432
DOI https://doi.org/10.2147/IJN.S477001
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mian Wang
Marwa H Abdallah,1 Seham Shawky,2 Mona M Shahien,3 Hemat El-Sayed El-Horany,4,5 Enas Haridy Ahmed,6,7 Shaimaa El-Housiny8
1Department of Pharmaceutics, College of Pharmacy, University of Ha’il, Ha’il, 81442, Saudi Arabia; 2Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Al-Azhar University, Cairo, 11651, Egypt; 3Department of Pediatrics, College of Medicine, University of Ha’il, Ha’il, 81442, Saudi Arabia; 4Department of Biochemistry, College of Medicine, University of Ha’il, Ha’il, 81442, Saudi Arabia; 5Department of Medical Biochemistry, Faculty of Medicine, Tanta University, Tanta, 31511, Egypt; 6Department of Anatomy, College of Medicine, University of Ha’il, Ha’il, 81442, Saudi Arabia; 7Department of Anatomy and Embryology, Faculty of Medicine, Ain Shams University, Cairo, 11566, Egypt; 8Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Modern University for Technology and Information, Cairo, 4410240, Egypt
Correspondence: Marwa H Abdallah, Email [email protected]
Introduction: Atorvastatin (ATV), a medication used to reduce cholesterol levels, possesses properties that can counteract the damaging effects of free radicals and reduce inflammation. However, the administration of ATV orally is associated with low systemic bioavailability due to its limited capacity to dissolve in water and significant first-pass effect. This study aimed to assess the appropriateness of employing nano-vesicles for transdermal administration of ATV in order to enhance its anti-inflammatory effects.
Methods: ATV-loaded transethosomes (ATV-TEs) were optimized using the 33 Box-Behnken design. The ATV-TEs that were created were evaluated for their vesicle size, encapsulation efficiency (% EE), and percent release of drug. The optimum formulation was integrated into a hydroxypropyl methylcellulose (HPMC) emulsion-based gel (ATV-TEs emulgel) using jojoba oil. ATV-TEs emulgel was examined for its physical characteristics, ex vivo permeability, histological, and anti-inflammatory effect in a rat model of inflamed paw edema.
Results: The optimized transethosomes exhibited a vesicle size of 158.00 nm and an encapsulation efficiency of 80.14 ± 1.42%. Furthermore, the use of transethosomal vesicles effectively prolonged the release of ATV for a duration of 24 hours, in contrast to the pure drug suspension. In addition, the transethosomal emulgel loaded with ATV exhibited a 3.8-fold increase in the transdermal flow of ATV, in comparison to the pure drug suspension. ATV-TEs emulgel demonstrated a strong anti-inflammatory impact in the carrageenan-induced paw edema model.
Discussion: This was evident from the significant reduction in paw edema, which was equivalent to the effect of the standard anti-inflammatory medicine, Diclofenac sodium.
Conclusion: In summary, transethosomes, as a whole, might potentially serve as an effective method for delivering drugs via the skin. This could improve the ability of ATV to reduce inflammation by increasing its absorption through the skin.
Keywords: nano-vesicles, anti-inflammatory test, emulsion-based gel, histopathological study
Introduction
The transdermal drug delivery system (TDDS) is thought to be the most effective approach to deliver medication with a limited bioavailability. Drug administration via the transdermal route has the potential to resolve problems like hepatic first-pass metabolism, enzymatic drug degradation, and intravenous administration pain while also improving patient compliance with dose control when compared to oral and parenteral routes of administration. TDDS could be developed in a variety of ways to encourage skin penetration and enable appropriate, regulated medication absorption into the bloodstream.1
Over the past three decades, a lot of research has been conducted to investigate the effectiveness of transdermal delivery systems, such as liposomes,2 niosomes,3 transfersomes,4 and ethosomes,5 which are vesicle carriers. Because of their reduced transdermal delivery of the medication, classic liposomal systems typically generate a drug reservoir in the upper layers of the stratum corneum, preventing drugs from penetration of skin deeper layers. Therefore, there was a need for generating advanced nanovesicles to enhance the skin permeation and improve the therapeutic efficiency of drugs via transdermal delivery.
It has been demonstrated that ethosomes have much greater transdermal permeability and can cross the skin barrier than regular liposomes. Ethosomes, a non-invasive nano-vesicular delivery system, can enhance medication delivery via the skin. These soft flexible vesicular carriers contain glycols, phospholipids, and ethanol at high concentrations. Ethosomal formulations containing ethanol could have a synergistic effect that improves drug penetration through the skin lipids. Furthermore, the ethanol effect, which increases the permeability of membranes and the fluidity of lipid molecules, enables the soft, flexible vesicles to penetrate deeply into the skin bilayers.6 High alcohol concentrations in this class of biodegradable ethosomes have caused a negative charge formation, which has improved drug transport and bioavailability.7 Because of their unique structure and high lamellarity, ethosomes can encapsulate and disperse both lipophilic and hydrophilic drugs through the layers of the skin. Lipophilic includes simvastatin8 and valsartan,9 while hydrophilic drugs include buspirone10 and betahistine dihydrochloride.11
Moreover, the vesicular systems can be incorporated into emulsion-based gel to boost the distribution and stability of the formulation.12 Emulgel refers to jellified emulsions, which can be either oil-in-water or water-in-oil. These emulgel formations are achieved by including a gelling agent into the emulsion.13 Emulgel is a substance that has the ability to function as both an emulsion and a gel. This capability allows it to work as a dual control release mechanism.14 Gel is added to emulsion to enhance its capacity to penetrate and maintain its stability. Gels are mostly made up of synthetic or natural cellulose polymers as HPMC. These polymers have been shown to have enough viscosity and bioadhesive qualities as well as to be compatible with nano-vesicles to enhance drug solubility and delivery.
Statins are a widely recognized class of drugs used to decrease cholesterol levels. They work by blocking the enzyme HMG-CoA reductase, which is involved in the production of cholesterol.15 In relation to several medical problems, statins have demonstrated novel therapeutic efficiencies, including anti-inflammatory action as described by Bradbury et al, an anticancer activity addressed by Hassanabad, and recently identified antifungal activity.16–18
Atorvastatin calcium (ATC) is a commonly prescribed medication for reducing cholesterol levels. It works by competitively inhibiting the enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA). This medicine is used to treat conditions such as hyperlipidemia and atherosclerosis.19 Furthermore, ATC has been found to possess direct anti-inflammatory effects independently on its lipid lowering efficacy through the inhibition of pro-inflammatory cytokines released by macrophages.20 The clinical studies provided evidence that ATC has good anti-inflammatory effects by reducing the levels of the inflammatory marker C-reactive protein (CRP) in patients.21
The formulation of atorvastatin calcium (ATV), has many challenges that hinder the achievement of the ideal release pattern with high bioavailability.22 It is a white powder, very slightly soluble in water (11 μg/mL), practically insoluble in methylene chloride, and slightly soluble in ethanol (0.5 mg/mL). It is classified as a class II drug according to the biopharmaceutical categorization system (BCS). ATV has little solubility in water and is poorly absorbed when taken orally, with a bioavailability of around 14%, which is mostly attributed to the considerable first pass hepatic metabolism.23 Various formulation techniques have been employed to solve these challenges, including nanoparticles,24 nanolipospheres,25 solid dispersion,26 co-crystallization,27 and self-emulsifying system.28
To overcome these challenges, an appropriate approach is required to develop sustained transdermal drug delivery systems that can effectively treat inflammation over an extended period. The main objective of this work was to develop lipid-based nano-vesicles in a hydrogel form containing ATV, in order to improve the pharmacodynamics effectiveness and epidermal permeability, while avoiding the disadvantages associated with oral administration.
Materials and Methods
Materials
Atorvastatin calcium was supplied by Epico.co.10th of Ramadan city, Egypt. Soy lecithin, hydroxypropyl methyl cellulose, span 60, propylene glycol, ethanol, chloroform, and methanol were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Box-Behnken -Response Surface Methodology Design
The effect of different formulation parameters on the entrapment efficiency (%) and percentage of drug release after 8 hours (%) of the generated nano-vesicular formulation was systemically investigated using Box-Behnken design–response surface methodology (BBD–RSM) using Design Expert® software (Ver. 11, Stat-Ease, Minneapolis, MN, USA). Table 1 contains a list of the design’s details. Three independent variables were selected: lipid amount (A), which ranged from 70 mg to 120 mg, surfactant concentration (B), which ranged from 0% v/v to 25% v/v and ethanol concentration (C), which lay between 0% v/v and 30% v/v. Three factors were chosen as dependent variables: encapsulation efficiency % (R1) and percentage of drug release after 8 hours (Q8h, R2). Table 2 provides the values for each variable as well as the batch codes. The ideal formula and desirable attributes were determined based on performing 17 runs with five center points. The model’s significance was assessed using ANOVA, which also validated the statistical analysis results. Three-dimensional surface plots and contour plots were created to link the outcomes of the statistical study.
 |
Table 1 BBD-RSM for Optimization of Nano-Vesicular Formulations Loaded with ATV |
 |
Table 2 Different ATV-Loaded Nano-Vesicular Formulations |
Formulation of Nanovesicles
Atorvastatin loaded ethosomes were generated using the methodology described by Touitou et al with some modification.29 The specified amount of lipid was mixed with ATV and dispersed in distilled water, and then the mixture was heated in a water bath at 40°C till obtaining colloidal dispersion. In another container, the organic phase was prepared by heating a mixture of ethanol and propylene glycol 40 °C in a water bath. Once both mixtures reached 40 °C, the organic phase was then added to the aqueous phase and stirred continuously for 20 minutes using a magnetic stirrer (Magnetic Stirrer–AREC, VELP Scientifica, Milano, Italy) R. The resulting dispersion was sealed, allowed to cool, kept out of the light, and kept in storage at 4°C for further investigation.30,31 The transethosomes (TEs) loaded with Atorvastatin were generated by the same technique except adding specified amount of surfactant to the lipid and ATV mixture before heating to 40 °C. The transfersomes loaded with ATV were produced using the thin-layer hydration method.32 Briefly, the specified amounts of lipid and surfactant according to the lists described in Table 2, and ATV (100 mg) were mixed in a round bottomed flask and then dissolved in methanol/chloroform (2:1 v/v). The mixture was stirred at room temperature at 100 rpm until the formation of homogenous dispersion. After that, the solvent was extracted under low pressure in a rotary evaporator at 50 °C and 100 rpm till leaving a thin coating on the flask wall. The evaporation process was run for an additional two hours to ensure the removal of all solvents. Next, five milliliters of PBS (pH, 7.4) were added to hydrate the lipid film with agitation for one hour until formation of transfersomes. The obtained transfersomal dispersion was stirred for 20 minutes and was kept at 4°C, sealed hermetically until further studies. Atorvastatin loaded liposomes were manufactured according to same methodology without using surfactant in the lipid mixture. The composition of different nano-vesicular formulation is demonstrated in Table 2.
Characterization of ATV Loaded Nano-Vesicular Formulations
Particle Size Determination
The size of the optimized transethosomes containing ATV was assessed using a Nano ZS Zetasizer (Nano–ZS90, Malvern Instruments Ltd., Malvern, Worcestershire, UK) at 25 ± 1.0 °C while keeping the angle of scattering fixed at 90°. Deionized water was added to the transethosomal dispersion for dilution to the required degree of scattering intensity.33,34
Transmission Electron Microscopy (TEM)
The optimal nanovesicles’ morphological characteristics were inspected utilizing a transmission electron microscope (Joel JEM 2100, Tokyo, Japan). One undiluted formulation drop was placed on a film-coated 200-mesh copper grid. It was then negatively stained with 2% aqueous phosphotungstic acid (PTA) solution and let to dry before observation using a transmission electron microscope (TEM).
Percentage of encapsulation efficiency Determination
The vesicular dispersion of the generated formulations was subjected to centrifugation at 12,000 rpm using (TGL-16 Tabletop High Speed Refrigerated Centrifuge, China) for one hour at 4°C. The concentration of Atorvastatin in the supernatant was measured after suitable dilution with PBS (pH, 7.4) using a spectrophotometer by recording the absorbance at 246 nm. Each measurement is conducted three times. The drug concentration encapsulated in the manufactured nanovesicles loaded with ATV was determined by subtracting the amount of drug contained in the supernatant from the total initial amount of Atorvastatin employed during the production. The encapsulation efficiency of the ATV nanovesicles is determined using the following calculation:
Investigation of in vitro Drug Release
Drug release investigations were conducted using the dialysis method. The obtained ATV loaded nano-vesicular formulations were placed into a dialysis membrane bag with a molecular weight cutoff of 12–14 k Da (Sigma-Aldrich (St. Louis, MO, USA). The dialysis membranes were soaked in PBS, pH 7.4 overnight prior to the in vitro release experiment. The bag was securely tied at both ends. During the dialysis process, ATV-loaded nanovesicles and ATV suspension (corresponding to 5 milligrams ATV) were loaded in the dialysis bags which then sited inside a beaker filled with 150 mL of PBS and then subjected to incubation at a temperature of 37 °C in a water bath that was set to shake at a speed of 150 rpm. The release of atorvastatin from nano-vesicles was quantified by extracting 1 mL samples at specific time intervals (0.5, 1, 2, 3, 4, 6, 8, 12 and 24 h). The spectrophotometric determination of the released amounts of atorvastatin was performed at wavelengths of 246 nm.35 A calibration curve was constructed using atorvastatin as reference standards.
Stability Studies of the Optimized Transethosomes Loaded with ATV (ATV-TEs)
The stability of the optimized ATV-TEs formulation was assessed based on many characteristics, including encapsulation efficiency (EE), and in vitro drug release. The study was conducted in accordance with the guiding principles of the International Conference on Harmonization (ICH). The sample was held at two different temperatures, specifically 4 ± 1 °C and 25 ± 1 °C, for durations of 3 months.
Formulation of ATV-TEs Emulgel
The Emulgel was made by incorporating the gelling agent, hydroxypropyl methylcellulose (HPMC, 4% w/w), into distilled water and rotating the mixture until a uniform gel was formed. The oil phase was generated by dissolving Tween 80 in jojoba oil. The aqueous phase (distilled water) was slowly poured into the oily phase while constantly vortexed for 10 min till production of a white emulsion. The generated emulsion was added to the pre-formulated gel and mixed together until a homogenous jojoba oil-based emulgel was achieved. The optimized ATV-nanovesicle formulation equivalent to ten milligrams of ATV was combined with the produced emulgel using magnetic stirrer until the production of ATV-nano-vesicular emulgel formulation (1%).36 Additionally, nano vesicular gel that included ATV was manufactured by mixing the hydrogel (4% HPMC) with a specified amount of the optimized nano-vesicles loaded with ATV until the formation of uniform gel free from any clumping.
Evaluation of the Nanovesicular Emulgel Loaded with Atorvastatin (ATV-TEs)
The ATV nano-vesicular emulgel was assessed for many properties including homogeneity, drug content, pH, spreadability, viscosity, drug release, and ex vivo permeation studies. The prepared nano-vesicular emulgel formulation was inspected visually for their color, appearance, grittiness, consistency and phase separation.37 The spreadability of ATV nano-vesicular emulgel was assessed by sandwiching half a gram of the produced formulation between two transparent circular glass slides. The spreading was achieved by letting them sit for 5 minutes after putting a weight of about one kilogram on the upper side.38 The circle’s diameter was measured to determine its spreadability. The generated nano-vesicular emulgel was dispersed in twenty milliliters of distilled water, and then the pH value was determined using a digital pH meter (PCT-407, Taipei City, Taiwan).39,40 The measurement was conducted three times, and the mean value plus standard deviation were computed. The viscosity of the generated ATV-TEs emulgel was determined using a Brookfield viscometer (Model DV-II, USA) with spindle number 06 rotating at a speed of 10 rpm at 25 °C.38 The Atorvastatin content in ATV-TEs emulgel was determined by adding 0.5 g of the prepared formulation (equal to 5 mg of medication) to a clean 100 mL volumetric flask and filling it with methanol. The mixture was stirred for 2 hours and then let to stand for 24 hours.41 The solution was filtered, and the samples were examined utilizing spectrophotometry at 246 nm.
In vitro Release Investigation
The in-vitro release of ATV nano-vesicular emulgel and gel compared to ATV nanovesicles and ATV suspension was evaluated utilizing the same procedures shown in section 2.5.
Ex-Vivo Investigation
Animal
About twenty male rats weighing around 200–220 g were used. The animals’ housing conditions were alternated between light and dark cycles every 12 hours, with a constant temperature of 25 ± 2°C. The experiments were done in accordance with the rules and guidelines of the Research Ethics Committee (REC) of Ha’il University No (H-2023-386 on 23/10/2023).
Skin Permeation Study
The experiment involved testing the ATV nano-vesicular emulgel, gel, nano-vesicles and drug suspension using cell diffusion apparatus via hairless rat skin. Prior to conducting the experiment, the abdomen skin of the rat was purified of fatty tissues, and then washed with phosphate-buffered saline (pH 7.4). The skin samples were placed between the receptor and donor compartments of a diffusion cell, with the dermis faced the receptor medium and the stratum corneum exposed to the donor cell. A weighted quantity of ATV-TEs, ATV-TEs gel, and ATV-TEs emulgel (500 mg) equivalent to (5 mg) of ATV was placed in the donor compartment. The receptor compartment was filled with phosphate-buffered saline (pH 7.4) that was maintained at a temperature of 37 ± 1 °C and swirled at a speed of 100 rpm. At predetermined time intervals spanning 12 hours, 1 mL samples were extracted from the receptor media and replaced with an equivalent volume of new medium. The concentration of Atorvastatin in each sample was determined using spectrophotometry at a wavelength of 246 nm. A graph was created to show the quantity of ATV that passed through rat skin per unit area (in μg/cm2) as a function of time (in hours). The steady state flux (JSS) was determined by analyzing the slope of the linear section of the graph showing the cumulative amount of ATV penetrated per unit area (μg/cm2) over time (h).41 The enhancement ratio (ER) was also calculated by measuring the ratio of JSS from the tested sample to JSS from the control.
Ex vivo Skin Deposition Study
Skin retention study aimed to assess the ability of vesicular carriers to keep the encapsulated medication within the skin, in comparison to a drug suspension. The study was conducted using optimized transethosomes, ATV-TEs gel, ATV-TEs emulgel, and pure drug suspension to determine the ATV concentration in the skin at the end of ex vivo permeation study. After 12 hours, the skin attached to the diffusion cell was removed and the skin surface was washed three times with warm phosphate buffer. The drug retention in the skin was evaluated by cutting the cleansed skin into small parts, smashing it fully in a beaker, and then adding ten milliliters of methanol. The beaker was agitated using a water shaker bath at 37 degrees Celsius for two hours to fully extract the drug.42 The ATV content in the filtrate was measured using UV spectroscopy after appropriate dilution.
Histopathological Evaluation of the Transethosomal Formulation
Three abdominal same sized rat skin samples were analyzed. The first sample was served as negative control and was treated with 0.9% (w/v) sodium chloride solution. The second sample was treated with the conventional gel (ATV-TEs gel) and the third sample was treated with the optimized ATV-loaded transethosomal emulgel (ATV-TEs emulgel). The skin samples were subjected to washing process using phosphate buffer (pH 7.4), then immersed overnight in ten percent neutral formalin. Subsequently, the skin samples were dehydrated with ethanol after being cut vertically, and then embedded in paraffin blocks. The microtome-taken fine slices were stained with hematoxylin and eosin (H & E). The stained sections were observed using a microscope with a 25-x magnification.43
In vivo Anti-Inflammatory Efficiency
Experimental Design
The anti-inflammatory efficiency of ATV was evaluated using carrageenan-induced paw inflammation in rats. Edema was created in the rats’ left hind paw by subcutaneous injecting of 1% (w/v) carrageenan solution in normal saline. The ATV-TEs gel, ATV-TEs emulgel, and marketed (Diclofenac gel) formulations were administered 30 minutes before carrageenan injection. The paw measured was measured at 0.5, 1, 2, 3, 4, 5, 6 and 8 hours intervals using digital caliper.24 Rats were randomly divided into 5 groups, with each group containing 5 animals.
Group I was related to the control group, which was subjected to inflammation only without treatment. Group II received ATV-TEs gel topically at a dose of 10 mg/kg.22 Group III received ATV-TEs emulgel topically at a dose of 10 mg/kg. Group IV received topical marketed formulation (Diclofenac gel). The percentage of inhibition of paw edema in the drug-treated group was compared to the carrageenan control group and computed using a specific formula.
Statistical Analysis
All experiments were conducted at least three times. The results are presented as the mean ± standard deviation. A one-way ANOVA was conducted, followed by a pairwise comparison post-hoc test when necessary. A p-value less than 0.05 is considered statistically significant.
Results and Discussion
Analysis of Data by BBD
According to the data in Table S1, the ANOVA analysis revealed that, the quadratic model had significant statistical impact, as indicated by high F-values and low p-values (<0.0001), suggesting a low probability of random variation in these F-values. Furthermore, the R2 values, which quantify the extent of variance around the averages provided by the models, were near one, thereby confirming the strong correlation between the actual and projected values (Figure S1).31 Furthermore, there was a strong correlation between the predicted and adjusted R2 values. The design software suggests that the low difference between them (<0.2) indicates this. In addition, the plots of externally studentized residuals versus runs indicated that the points were randomly distributed within the control boundaries, providing evidence of low error probabilities and strong model attractiveness and fitness (Figure S2).
Impact of Independent Variables on the Evaluated Responses
Impact of the Independent Factors on EE%
The percentage of encapsulation efficiency (EE%) in all 17 ATV formulations ranges from 49.13 ± 1.52% (F7) to 81.22 ± 2.06% (F4). Equation (1) of the quadratic model in addition to surface and contour plots (Figure S3) demonstrated a positive correlation between both lipid and ethanol concentration and the percentage of encapsulation efficiency (% EE). In contrast, surfactant concentration had a negative effect on the percentage of encapsulation efficiency of ATV-loaded nano-vesicular formulations. As the lipid content increased, an increase in percentage EE was noted (Figure 1a). The percentage of EE% increased from 63.37 ± 1.83% to 81.22 ± 2.06% in formulations F2 and F4, respectively, when the content of phospholipid was increased from 70 to 120 mg at constant level of surfactant and ethanol. The increase in EE percentage (% EE) was attributed to the elevation in phospholipid concentration, which can be attributed to the lipophilic properties of ATV. Lipophilic drugs tend to have a higher affinity for the lipophilic core of the nano-vesicular formulations. There was a negative relationship observed between surfactant and the percentage of EE (Figure 1b). The percentage of encapsulation efficiency (% EE) was determined to be 52.64 ± 2.84%, 59.14 ± 2.11%, 71.85 ± 1.53%, and 73.60 ± 1.18% in F16 (liposomes), and ethosomal formulations (F9, F8, and F13), respectively, which contained 0% of surfactant. The percentage of EE was decreased to 49.48 ± 1.24%, 57.78 ± 1.98%, 69.89 ± 2.41%, and 67.16 ± 1.05% in F1 (transfersomes), and transethosomal formulations (F14, F12, and F10), respectively, as the concentration of surfactant increased to 25% w/w. This is because the surfactant employed in the formulations may be more effectively integrated into the lipid bilayer, leading to increasing the fluidity and formation of more permeable membrane and thereby decreasing the EE%.44 Adding surfactant at higher concentrations can also result in the formation of mixed micelles, which can explain the observed decrease in the EE%.
On the other hand, ethanol exhibited a direct impact on the percentage of encapsulation efficiency of ATV-loaded nano-vesicular formulations. Figure 1c demonstrates that ethanol positively influenced the encapsulation of ATV in the nano-vesicular formulations. An elevation in the EE% was noticed by raising the ethanol content. Transfersomes (F7) containing 0% ethanol had an EE% of 49.13 ± 1.52%, while transethosomes (F2) with 30% ethanol had an EE% of 63.37 ± 1.83%. Formulation (F1, transfersomes) with 0% ethanol had an EE% of 49.48 ± 1.24%, while formulation (F12) with 30% ethanol had an EE% of 69.89 ± 2.41%. Formulations F16 (liposomes) and F8 (ethosomes) showed comparable results, as seen in Table 2. The obtained results could be attributed to the enhanced solubility of ATV in the polar surface of transethosomes by increasing ethanol concentration, which may be due to its co-solvent action.45 Consequently, a greater quantity of ATV could have been retained in the aqueous core of the nano-vesicles.
Impact of the Independent Factors on in vitro Drug Release
The drug release pattern is a crucial indicator for predicting drug performance in vivo. The percentage of drug release after 8 hours (Q8h) in all 17 ATV formulations ranges from 29.58 ± 2.66% to 77.06 ± 2.93% as shown in Table 2 and was influenced by numerous manufacturing factors. Equation (2) of the quadratic model demonstrates a positive correlation between the two independent factors (B, C) while showing a negative correlation between lipid concentration (A) and the percentage of drug release after 8 hours (Q8h). Figure 1d displayed an inverse correlation between the lipid quantity and Q8h. The average Q8h of transethosomes generated with 70 mg of lipid (F2; 72.31 ± 3.29%) was considerably greater than transethosomes prepared with 120 mg of lipid (F4; 59.54 ± 2.59%) at the same level of surfactant and ethanol. The decreased Q8h values with increasing lipid concentration could be due to the elevated rigidity of the created nano-vesicles and the development of thicker frameworks resulting from the increase in phospholipid quantities.46
There was a positive correlation observed between surfactant concentration and the percentage of drug release after eight hours (Q8h) (Figure 1e). The percentage of ATV released from F9 (ethosomes), containing 70 mg phospholipid, 15% (v/v) ethanol and 0% (v/v) surfactant (54.89 ± 1.85%), was lower than that released from F14 (transethosomes), composed 70 mg phospholipid, 15% (v/v) ethanol and 25% (v/v) surfactant (61.87 ± 2.50%). The enhanced drug release observed at higher surfactant concentration may be due to the less structured and more permeable lipid bilayers of vesicles, which facilitated drug release.47,48 Another explanation for our findings is that at a high concentration of surfactant, the drug encapsulation efficiency decreases, causing disruption of the vesicles’ lipid membranes. This leads to the membranes becoming less ordered and more leaky, resulting in the leakage of the encapsulated drug.49 Additionally, the higher concentration of ethanol resulted in a faster percentage of drug release (Figure 1f). As indicated by Table 2, F6 that had 120 mg lipid, 0% (v/v) ethanol and 12.5% (v/v) surfactant showed drug release (42.86 ± 1.44%) after eight hours, whereas F4, which contained 70 mg phospholipid, 30% (v/v) ethanol and 12.5% (v/v) surfactant showed higher drug release (59.54 ± 2.59). The increased concentration of ethanol may be the cause of the increased flexibility and fluidity observed in the lipid bilayers of nano-vesicular formulations. Moreover, ethanol could disturb the lipid bilayers of the vesicles, allowing the drug to diffuse across membranes more easily.50,51
Optimization of Atorvastatin Loaded Nano-Vesicular Formulations
The experimental design was used to select the optimized ATV-nanovesicles based on the desirability criterion. The optimization goals are to maximize EE% and Q8h. Figure 2a and b shows contour plots illustrating the desirability and graphical optimization of the formulation design space. The optimized nano-vesicular formulation (transethosomes) loaded with ATV was composed of 120 mg lipid, 23.7% (v/v) surfactant and 30% (v/v) ethanol, with a desirability value of 0.952. The experimental trial yielded predicted responses of 78.23%, 77.06%, for EE % and Q8h, respectively (Figure 3), while the actual values were 80.14 ± 1.42%, and 76.03 ± 1.94%.
 |
Figure 2 Design optimization model concerning independent variables and dependent responses; (a) desirability and, (b) graphical optimization contour plot. |
 |
Figure 3 The components of the optimum formulation and its predicted responses according to Box Behnken Design. |
Characterization of the Optimized ATV Loaded Transethosomes (ATV-TEs)
Particle Size and Surface Morphology
The optimum ATV transethosomal formulation was assessed for particle size, and polydispersity index (PDI). The optimized formulation had a vesicular size of 158.00 nm. Polydispersity index (PDI) indicates the consistent size distribution of the transethosomal vesicles. A lower PDI score indicates a greater degree of homogeneity between the particles. The optimum ATV transethosomal formulation had a PDI value of 0.363 (Figure 4a), which is below 0.5, suggesting a uniform size distribution.52 Transmission electron microscopy (TEM) was utilized to analyze the structure of the produced system. The TEM micrograph of the optimized ATV-TEs revealed non-aggregated and spherical vesicles with a smooth surface and narrow size distribution, as shown in Figure 4b. The obtained figure indicates that the vesicles’ diameter noticed in the micrograph was within the nano-scale range and aligned with the measurement obtained from the vesicle size analyzer.
 |
Figure 4 Particle size (a) and TEM image (b) of the optimized ATV-TEs. |
Stability Study of ATV-TEs
One of the most important factors that determines the effectiveness of the colloidal dispersions is their stability. The stability of the optimized ATV-TEs was assessed after 1 and 3 months of storage at temperatures of 4 ± 1 °C and 25 ± 1 °C. These findings are demonstrated in Figure S4. Our results indicated that there were no statistically significant differences (p < 0.05) between the fresh formulation and the formulation after storage. This provides strong evidence that the formulation is stable and confirms the effectiveness of transethosomes as a nano-carrier.
Characterization of the ATV Transethosomal Emulgel
The created ATV-TEs emulgel was uniform in consistency and had a good homogeneous texture. The appearance of the transethosomal gel was observed to be translucent, whitish in color, none gritty without coarse particles and phase separation. The pH of the prepared ATV-TEs emulgel was 6.23 ± 0.25, falling within the normal pH range for topical formulations, suggesting that it will be safe when applied to the skin without any risk of skin irritation.53 As a result, the pH value reported from the transethosomal emulgel was determined to be appropriate, as the formulation is intended for dermal administration. The spreadability value of the transethosomal emulgel was 57.33 ± 2.31 mm, which demonstrates that the product can be easily spread on the skin surface with minimal effort. The viscosity of the ATV-TEs emulgel was measured to be 90533.33± 1350 cP. The therapeutic efficacy of any pharmaceutical dosage form is directly impacted by the homogeneity of the drug content, which can be measured by the percentage assay of the drug. 17 The drug content of the ATV-TEs gel was measured to be 97.93 ± 2.64%, indicating good content uniformity, and they were within the range of 90 to 110% as specified by the US Pharmacopeia. Therefore, the drug loss during the formulation development was found to be insignificant based on this result. For semisolid formulations, viscosity is an important factor since it makes the formulation easier to apply topically and mainly affects the drug’s release and diffusion from its carrier.54
In vitro Release Investigation
Figure 5 displays the in vitro release patterns of Atorvastatin from different generated formulations using phosphate buffer. Approximately 96.78% ± 3.41 of ATV was liberated from the free drug suspension within nearly 8 hours. The percentage of ATV released after 8 hours was 76.03 ± 1.94%, 51.47± 3.23%, and 42.82 ± 1.69 for transfersomes, transethosomal gel and for transethosomal emulgel, respectively. The generated nano-vesicular formulations yielded significantly lower amounts of ATV compared to the free drug. One possible explanation for the greater ATV release rate from the drug suspension compared to different transethosomal formulations is that the hydrophobic drug, Atorvastatin binds more strongly to the hydrophobic portion of transfersomes, which slows down the drug release. The addition of a gelling agent, which enhances the thickness of the formulations, can lead to a decrease in the release of Atorvastatin from transethosomal gel and emulgel formulations compared to transethosomes due to increased viscosity. The Atorvastatin release percentage from ATV-TEs gel was significantly greater than the release percentage from ATV-TEs emulgel. The gel formulation has a high-water content, facilitating the drug’s diffusion into the release medium.39 The lower release of Atorvastatin from ATV-TEs emulgel compared to the ATV-TEs gel formulation was due to the increased viscosity of the emulgel, hindering the effective diffusion of the medication. Furthermore, the decrease in water content and the addition of jojoba oil may account for the decreased drug release.55 These findings were consistent with Manian et al, who concluded that, the release rate of Diclofenac sodium gel was found to be significantly higher than that of Diclofenac sodium emulgel.56
 |
Figure 5 In-vitro release of Atorvastatin from free drug suspension, transethosomes (ATV-TEs), transethosomal (ATV-TEs) gel, and transethosomal (ATV-TEs) emulgel. |
Ex vivo Permeation Study
Both ATV-TEs gel and ATV-TEs emulgel exhibited considerably lower amount of drug permeated at the end of 24 hours through skin (300.75 ± 14.89 µg/cm2 and 497.03 ± 33.71 µg/cm2), respectively, compared to ATV-TEs dispersion (618.43 ± 27.69) (p < 0.05). The relatively lower permeation of ATV from both ATV-TEs gel and ATV-TEs emulgel compared to TEs dispersion could be related to the elevated viscosity of ATV-TEs gel and ATV-TEs emulgel, which interfered with the drug’s diffusion into the release media.54 Additionally, transethosomal dispersion, ATV-TEs gel and ATV-TEs emulgel exhibited significantly greater ATV penetration in terms of both rate and extent compared to the free drug suspension, as shown in Figure 6. The flux (J) values were determined and the findings indicated a significant difference (p < 0.05) between TEs dispersion (51.12 ± 1.64 µg/cm2 h), ATV-TEs emulgel formula (41.78 ± 2.56 µg/cm2 h) and ATV-TEs gel formula (24.46 ± 1.82 µg/cm2 h) compared to free drug suspension (11.02 ± 1.45 µg/cm2 h). The values of enhancement ratio for TEs dispersion, ATV-TEs emulgel and ATV-TEs gel were 4.7, 3.8 and 2.2, respectively, indicating a notable increase in ATV penetration through the skin. The relatively higher ATV permeation from transethosomal formulations could be due to the synergistic impact of nano-sized vesicles and vesicle elasticity influenced by the presence of surfactant and ethanol.57 Moreover, the incorporation of jojoba oil during formulation of ATV-TEs emulgel could enhance the skin permeability as a result of penetration enhancer effect of jojoba oil.58
 |
Figure 6 Ex vivo permeation study of different ATV formulation from abdominal rat skin. |
Ex vivo Skin Deposition Study
Figure 7 displays the amount of ATV depositing in the skin layers following the ex vivo permeation study. Transethosomal formulations demonstrated a significant increase in ATV deposition into the skin. The quantities of ATV deposited from different transethosomal formulations into skin layers were determined to be 1476.15 ± 29.66 μg, 877.75 ± 35.42 μg, and 1118.88 ± 45.23 μg for transethosomes, ATV-TEs gel, and ATV-TEs emulgel, respectively, compared to 664.09 ± 40.94 μg, for pure ATV suspension. Our findings may be related to the permeability enhancing properties of the transethosomal components, particularly the surfactants and ethanol.59,60 Additionally, the significantly higher deposition of ATV into skin layers is attributed to the great deformability of ATV transethosomal formulations, allowing them to pass across the para-cellular gap of skin tissues. Greater ATV penetration into the skin layers could indicate a potential increase in transdermal flux. The increased deposition of the transethosomes in the skin allowed them to operate as a reservoir, resulting in a prolonged release of ATV in the deep layers of the skin between administrations. Our study demonstrated that inclusion of surfactant, ethanol, and phospholipid in the ATV-TEs significantly enhanced the penetration of ATV and its deposition in the skin by fold for transethosomes, ATV-TEs gel, and ATV-TEs emulgel, respectively, compared to drug suspension.
 |
Figure 7 Amount of ATV retained in the skin following 24 hours of ex vivo permeation experiment. Values are expressed as mean ± (SD). |
Histopathological Study
Histopathological investigations were done to evaluate the safety of the drug-loaded transethosomal formulations following topical application. Skin samples treated with both ATV-TEs emulgel and ATV-TEs gel were compared to skin sample treated with normal saline solution (Figure 8). The skin samples treated with normal saline displayed a clearly defined structure consisting of the epidermis, dermis, and subcutaneous layers. Additionally, it exhibited normal skin appendages and vascularity, as depicted in Figure 8A. Additionally, no indications of inflammation or irritation were observed in the stained skin samples treated with either optimized ATV-TEs emulgel (Figure 8B) or the ATV-TEs gel (Figure 8C). Moreover, there were no changes in the skin’s structure when either the ATV-TEs emulgel or ATV-TEs gel was applied topically. The results strongly confirm the safety and tolerability of the studied formulations.61
 |
Figure 8 Histopathological investigation of (A) negative control; (B) skin section subjected to ATV-TEs gel; and (C) skin section subjected to ATV-TEs emulgel. |
Anti-Inflammatory Efficiency of ATV- TEs Emulgel
The carrageenan-induced rat hind paw method was utilized to evaluate the anti-inflammatory efficiencies of ATV-TEs gel and ATV-TEs emulgel in comparison to the marketed gel formulation (Diclofenac Gel). The reduction in rat hind paw swelling was employed as a measure of anti-inflammatory efficacy. Figure 9 illustrates the variations in the size of rat hind paw edema caused by carrageenan injection in comparison to non-treated control group. It was observed that carrageenan injection into rat hind paws of control group caused noticeable swelling, reaching its highest point after 4 hours and then slowly declining. Rats pretreated with ATV-TEs gel, ATV-TEs emulgel and marketed formulation showed significant reduction in paw edema compared to control animals (p < 0.05). The maximum edema inhibition percentage following topical application of ATV-TEs gel was 39.48 ± 5.78% after four hours, 60.29 ± 7.36% after six hours following topical application of ATV-TEs Emulgel, and 66.46 ± 6.41% after four hours following topical application of marketed formulation. Animals treated with ATV-TEs emulgel showed significant reduction in edema size compared to group treated with ATV-TEs gel (p < 0.05). The ATV-TEs emulgel showed the greatest potential for anti-inflammatory effects. The study offers valuable insights on how the combination of jojoba oil and ATV in transethosomal emulgel could boost the effectiveness and permeability of topical formulations, indicating a synergistic interaction between the two components. The improved transdermal permeability of ATV TEs and compared to plain ATV contribute to the superior and persistent anti-inflammatory action of ATV-TEs emulgel (p > 0.05). Remarkably, the anti-inflammatory efficacy of ATV-TEs emulgel was equivalent to that of the conventional anti-inflammatory medication (Diclofenac sodium) (p > 0.05).
 |
Figure 9 Influence of different ATV preparations on rats mean hind paw edema. |
Conclusions
Our study examined the effectiveness of transethosomes in delivering Atorvastatin, via the skin. The study utilized a response surface approach, specifically a 33 Box-Behnken design, to develop and improve transethosomes loaded with Atorvastatin (ATV-TEs). The optimized transethosomal system exhibited nano-sized vesicles with a smooth spherical form, an acceptable vesicle size, and prolonged drug release for 24 hours. In addition, the incorporation of the optimized vesicles into emulsion-based gel led to the preparation of ATV-TEs emulgel, which exhibited satisfactory physical characteristics in terms of pH, viscosity, and spreadability. Additionally, they significantly enhanced the permeability of ATV across the skin. Furthermore, ATV-TEs emulgel demonstrated an intense anti-inflammatory effect using the carrageenan-induced paw edema model. This was evident from the significant reduction in rat paw volume, which was equivalent to the effect of the standard anti-inflammatory drug, Diclofenac sodium. These findings suggest that ATV-TEs emulgel has the potential to reduce swelling. In conclusion, our data indicate that transethosomes might be considered an effective technique for delivering ATV through the skin, enhancing the effectiveness of the medication for inflammation treatment.
Data Sharing Statement
The data presented in this study are contained within the article.
Institutional Review Board Statement
Our study has been approved by the Research Ethics Committee (REC) at the University of Ha’il, No (H-2023-386 on 23/10/2023).
Informed Consent Statement
Not applicable.
Acknowledgments
The authors thank the Scientific Research Deanship at the University of Ha’il, Saudi Arabia, for funding this research through project number RG-23 240.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the Scientific Research Deanship at University of Ha’il, Saudi Arabia, through project number RG-23 240.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Yu YQ, Yang X, Wu XF, Fan YB. Enhancing permeation of drug molecules across the skin via delivery in nanocarriers: novel strategies for effective transdermal applications. Front Bioeng Biotechnol. 2021;9:646554. doi:10.3389/fbioe.2021.646554
2. Souto EB, Macedo AS, Dias-Ferreira J, Cano A, Zielińska A, Matos CM. Elastic and ultradeformable liposomes for transdermal delivery of active pharmaceutical ingredients (APIs). Int J Mol Sci. 2021;22(18):9743. doi:10.3390/ijms22189743
3. Zaid Alkilani A, Abu-Zour H, Alshishani A, Abu-Huwaij R, Basheer HA, Abo-Zour H. Formulation and evaluation of niosomal alendronate sodium encapsulated in polymeric microneedles: in vitro studies, stability study and cytotoxicity study. Nanomaterials. 2022;12(20):3570. doi:10.3390/nano12203570
4. Yuan M, Niu J, Xiao Q, et al. Hyaluronan-modified transfersomes based hydrogel for enhanced transdermal delivery of indomethacin. Drug Deliv. 2022;29(1):1232–1242. doi:10.1080/10717544.2022.2053761
5. Paliwal S, Tilak A, Sharma J, et al. Flurbiprofen loaded ethosomes - transdermal delivery of anti-inflammatory effect in rat model. Lipids Health Dis. 2019;18(1):133. doi:10.1186/s12944-019-1064-x
6. Elsayed MMA, Abdallah OY, Naggar VF, Khalafallah NM. Lipid vesicles for skin delivery of drugs: reviewing three decades of research. Int J Pharm. 2007;332(1):1–16. doi:10.1016/j.ijpharm.2006.12.005
7. Opatha SAT, Titapiwatanakun V, Chutoprapat R. Transfersomes: a promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics. 2020;12(9):855. doi:10.3390/pharmaceutics12090855
8. An K, Sun Y, Wu Y, Yuan H, Cui Z, Xu L. Preparation and in vitro percutaneous penetration of simvastatin ethosome gel. Artif. Cells Nanomed. Biotechnol. 2013;41(5):315–318. doi:10.3109/10731199.2012.743903
9. Nasr AM, Moftah F, Abourehab MAS, Gad S. Design, formulation, and characterization of valsartan nanoethosomes for improving their bioavailability. Pharmaceutics. 2022;14(11):2268. doi:10.3390/pharmaceutics14112268
10. Shumilov M, Touitou E. Buspirone transdermal administration for menopausal syndromes, in vitro and in animal model studies. Int J Pharm. 2010;387(1–2):26–33. doi:10.1016/j.ijpharm.2009.11.029
11. El-Menshawe SF, Ali AA, Halawa AA, Srag El-Din AS. A novel transdermal nanoethosomal gel of betahistine dihydrochloride for weight gain control: in-vitro and in-vivo characterization. Drug Des Devel Ther. 2017;Volume 11:3377–3388. doi:10.2147/DDDT.S144652
12. Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000;50(1):27–46. doi:10.1016/S0939-6411(00)00090-4
13. Ajazuddin AA, Tripathi DK, Swarna TV, Maurya J, Patel S. Mechanism responsible for mucoadhesion of mucoadhesive drug delivery system: a review. International Journal of Applied Biology; 2011.
14. Ali Khan B, Ullah S, Khan MK, Alshahrani SM, Braga VA. Formulation and evaluation of Ocimum basilicum-based emulgel for wound healing using animal model. Saudi Pharm J. 2020;28(12):1842–1850. doi:10.1016/j.jsps.2020.11.011
15. Matusewicz L, Meissner J, Toporkiewicz M, Sikorski AF. The effect of statins on cancer cells. Tumor Biol. 2015;36(7):4889–4904. doi:10.1007/s13277-015-3551-7
16. Bradbury P, Traini D, Ammit AJ, Young PM, Ong HX. Repurposing of statins via inhalation to treat lung inflammatory conditions. Adv. Drug Delivery Rev. 2018;133:93–106. doi:10.1016/j.addr.2018.06.005
17. Hassanabad AF. Current perspectives on statins as potential anti-cancer therapeutics: clinical outcomes and underlying molecular mechanisms. Transl Lung Cancer Res. 2019;8(5):692. doi:10.21037/tlcr.2019.09.08
18. Eita AS, Makky AMA, Anter A, Khalil IA. Atorvastatin-loaded emulsomes foam as a topical antifungal formulation. Int J Pharm X. 2022;4:100140. doi:10.1016/j.ijpx.2022.100140
19. Xie W, Huang H, Xiao S, Yang X, Zhang Z. Effect of statin use on cardiovascular events and all-cause mortality in immune-mediated inflammatory diseases: a systematic review and meta-analysis involving 148,722 participants. Pharmacol Res. 2020;160:105057. doi:10.1016/j.phrs.2020.105057
20. Peng S, Pu J, Shao Q. Atorvastatin inhibits inflammatory response, attenuates lipid deposition, and improves the stability of vulnerable atherosclerotic plaques by modulating autophagy. Front Pharmacol. 2018;9:347926. doi:10.3389/fphar.2018.00438
21. Milajerdi A, Larijani B, Esmaillzadeh A. Statins influence biomarkers of low grade inflammation in apparently healthy people or patients with chronic diseases: a systematic review and meta-analysis of randomized clinical trials. Cytokine. 2019;123:154752. doi:10.1016/j.cyto.2019.154752
22. Shaker MA, Elbadawy HM, Al Thagfan SS, Shaker MA. Enhancement of atorvastatin oral bioavailability via encapsulation in polymeric nanoparticles. Int J Pharm. 2021;592:120077. doi:10.1016/j.ijpharm.2020.120077
23. Prabhu P, Patravale V. Dissolution enhancement of atorvastatin calcium by co-grinding technique. Drug Delivery Transl Res. 2016;6(4):380–391. doi:10.1007/s13346-015-0271-x
24. Abdelkader DH, Abosalha AK, Khattab MA, Aldosari BN, Almurshedi AS. A novel sustained anti-inflammatory effect of atorvastatin-calcium PLGA nanoparticles: in vitro optimization and in vivo evaluation. Pharmaceutics. 2021;13(10):1658. doi:10.3390/pharmaceutics13101658
25. Khafagy E-S, Motawee AO, Ghorab MM, Gardouh AR. Atorvastatin-loaded pro-nanolipospheres with ameliorated oral bioavailability and antidyslipidemic activity. Colloids Surf. B. 2023;227:113361. doi:10.1016/j.colsurfb.2023.113361
26. Iqbal A, Hossain S, Shamim A, Islam M, Siddique AT. Formulation, in vitro evaluation and characterization of atorvastatin solid dispersion. Trop J Pharm Res. 2020;19(6):1131–1138. doi:10.4314/tjpr.v19i6.2
27. Nada A. Atorvastatin Cocrystals: tablet Formulation and Stability. Asian J Pharmaceutics (AJP). 2020;14(4).
28. Gardouh AR, Nasef AM, Mostafa Y, Gad S. Design and evaluation of combined atorvastatin and ezetimibe optimized self-nano emulsifying drug delivery system. J Drug Delivery Sci Technol. 2020;60:102093. doi:10.1016/j.jddst.2020.102093
29. Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. Ethosomes—novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 2000;65(3):403–418. doi:10.1016/S0168-3659(99)00222-9
30. Bragagni M, Mennini N, Maestrelli F, Cirri M, Mura P. Comparative study of liposomes, transfersomes and ethosomes as carriers for improving topical delivery of celecoxib. Drug Delivery. 2012;19(7):354–361. doi:10.3109/10717544.2012.724472
31. Ghanbarzadeh S, Arami S. Enhanced transdermal delivery of diclofenac sodium via conventional liposomes, ethosomes, and transfersomes. Biomed Res. Int. 2013;2013:616810. doi:10.1155/2013/616810
32. Moqejwa T, Marimuthu T, Kondiah PPD, Choonara YE. Development of stable nano-sized transfersomes as a rectal colloid for enhanced delivery of cannabidiol. Pharmaceutics. 2022;14(4):703. doi:10.3390/pharmaceutics14040703
33. Elsheikh MA, El-Feky YA, Al-Sawahli MM, Ali ME, Fayez AM, Abbas H. A brain-targeted approach to ameliorate memory disorders in a sporadic alzheimer’s disease mouse model via intranasal luteolin-loaded nanobilosomes. Pharmaceutics. 2022;14(3):576. doi:10.3390/pharmaceutics14030576
34. Abdallah MH, Abu Lila AS, Shawky SM, et al. Experimental design and optimization of nano-transfersomal gel to enhance the hypoglycemic activity of silymarin. Polymers. 2022;14(3):508. doi:10.3390/polym14030508
35. Rohilla R, Garg T, Bariwal J, Goyal AK, Rath G. Development, optimization and characterization of glycyrrhetinic acid–chitosan nanoparticles of atorvastatin for liver targeting. Drug Delivery. 2016;23(7):2290–2297. doi:10.3109/10717544.2014.977460
36. Abdallah MH, Elghamry HA, Khalifa NE, et al. Ginger extract-loaded sesame oil-based niosomal emulgel: quality by design to ameliorate anti-inflammatory activity. Gels. 2022;8(11):737. doi:10.3390/gels8110737
37. Akram MA, Khan BA, Khan MK, Alqahtani A, Alshahrani SM, Hosny KM. Fabrication and characterization of polymeric pharmaceutical emulgel co-loaded with eugenol and linalool for the treatment of trichophyton rubrum infections. Polymers. 2021;13(22):3904. doi:10.3390/polym13223904
38. Mwangi AN, Njogu PM, Maru SM, et al. Meloxicam emulgels for topical management of rheumatism: formulation development, in vitro and in vivo characterization. Saudi Pharm J. 2021;29(4):351–360.
39. Abdallah MH, Elghamry HA, Khalifa NE, et al. Development and optimization of erythromycin loaded transethosomes cinnamon oil based emulgel for antimicrobial efficiency. Gels. 2023;9(2):137. doi:10.3390/gels9020137
40. Abdallah MH, Lila ASA, Anwer MK, Khafagy E-S, Mohammad MS, Soliman M. Formulation, development and evaluation of ibuprofen loaded nano-transferosomal gel for the treatment of psoriasis. J Pharm Res Int. 2019;31(6):1–8. doi:10.9734/jpri/2019/v31i630356
41. Qushawy M, Nasr A, Abd-Alhaseeb M, Swidan S. Design, optimization and characterization of a transfersomal gel using miconazole nitrate for the treatment of candida skin infections. Pharmaceutics. 2018;10(1):26. doi:10.3390/pharmaceutics10010026
42. Elshall AA, Ghoneim AM, Abdel-Mageed HM, Osman R, Shaker DS. Ex vivo permeation parameters and skin deposition of melatonin-loaded microemulsion for treatment of alopecia. Future J Pharm Sci. 2022;8(1):28. doi:10.1186/s43094-022-00418-4
43. Nemr AA, El-Mahrouk GM, Badie HA. Hyaluronic acid-enriched bilosomes: an approach to enhance ocular delivery of agomelatine via D-optimal design: formulation, in vitro characterization, and in vivo pharmacodynamic evaluation in rabbits. Drug Delivery. 2022;29(1):2343–2356. doi:10.1080/10717544.2022.2100513
44. Zaki RM, Seshadri VD, Mutayran AS, et al. Wound healing efficacy of rosuvastatin transethosomal gel, i optimal optimization, histological and in vivo evaluation. Pharmaceutics. 2022;14(11):2521. doi:10.3390/pharmaceutics14112521
45. Bin Jardan YA, Ahad A, Raish M, Al-Jenoobi FI. Preparation and characterization of transethosome formulation for the enhanced delivery of sinapic acid. Pharmaceutics. 2023;15(10):2391. doi:10.3390/pharmaceutics15102391
46. Ibrahim TM, Abdallah MH, El-Megrab NA, El-Nahas HM. Transdermal ethosomal gel nanocarriers; a promising strategy for enhancement of anti-hypertensive effect of carvedilol. J Liposome Res. 2019;29(3):215–228. doi:10.1080/08982104.2018.1529793
47. Rao M, Sukre G, Aghav S, Kumar M. Optimization of metronidazole emulgel. J Pharm. 2013;2013:1–9. doi:10.1155/2013/501082
48. Abdallah MH. Transfersomes as a transdermal drug delivery system for enhancement the antifungal activity of nystatin. Int J Pharm Pharm Sci. 2013;5:560–567.
49. Mahmood S, Taher M, Mandal UK. Experimental design and optimization of raloxifene hydrochloride loaded nanotransfersomes for transdermal application. Int j Nanomed. 2014;9:4331. doi:10.2147/IJN.S65408
50. Albash R, Abdelbary AA, Refai H, El-Nabarawi MA. Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: in vitro, ex vivo, and in vivo evaluation. Int J Nanomed. 2019;14:1953–1968. doi:10.2147/IJN.S196771
51. Alam P, Imran M, Jahan S, Akhtar A, Hasan Z. Formulation and characterization of hesperidin-loaded transethosomal gel for dermal delivery to enhance antibacterial activity: comprehension of in vitro, ex vivo, and dermatokinetic analysis. Gels. 2023;9(10):791. doi:10.3390/gels9100791
52. Mazyed EA, Abdelaziz AE. Fabrication of transgelosomes for enhancing the ocular delivery of acetazolamide: statistical optimization, in vitro characterization, and in vivo study. Pharmaceutics. 2020;12(5):465. doi:10.3390/pharmaceutics12050465
53. Zaid Alkilani A, Hamed R, Abdo H, et al. Formulation and evaluation of azithromycin-loaded niosomal gel: optimization, in vitro studies, rheological characterization, and cytotoxicity study. ACS Omega. 2022;7(44):39782–39793. doi:10.1021/acsomega.2c03762
54. Teaima MH, Alsofany JM, El-Nabarawi MA. Clove oil endorsed transdermal flux of dronedarone hydrochloride loaded bilosomal nanogel: factorial design, in vitro evaluation and ex vivo permeation. AAPS Pharm Sci Tech. 2022;23(6):182. doi:10.1208/s12249-022-02337-2
55. Khalifa NE, Abdallah MH, Elghamry HA, et al. Development of tea tree oil based nanoemulgel loaded with azithromycin for enhancing the antibacterial activity. Processes. 2023;11(6):1836. doi:10.3390/pr11061836
56. Manian M, Jain P, Vora D, Banga AK. Formulation and evaluation of the in vitro performance of topical dermatological products containing diclofenac sodium. Pharmaceutics. 2022;14(9):1892. doi:10.3390/pharmaceutics14091892
57. Abdulbaqi IM, Darwis Y, Assi RA, Khan NAK. Transethosomal gels as carriers for the transdermal delivery of colchicine: statistical optimization, characterization, and ex vivo evaluation. Drug Des Devel Ther. 2018;12:795–813. doi:10.2147/DDDT.S158018
58. Rajan R, Vasudevan DT. Effect of permeation enhancers on the penetration mechanism of transfersomal gel of ketoconazole. J Adv Pharmaceut Technol Res. 2012;3(2):112–116. doi:10.4103/2231-4040.97286
59. Hassan AS, Hofni A, Abourehab MA, Abdel-Rahman IA. Ginger extract–loaded transethosomes for effective transdermal permeation and anti-inflammation in rat model. Int j Nanomed. 2023;Volume 18:1259–1280. doi:10.2147/IJN.S400604
60. Verma D, Fahr A. Synergistic penetration enhancement effect of ethanol and phospholipids on the topical delivery of cyclosporin A. J Control Release. 2004;97(1):55–66. doi:10.1016/j.jconrel.2004.02.028
61. Abdelnabi DM, Abdallah MH, Elghamry HA. Buspirone hydrochloride loaded in situ nanovesicular gel as an anxiolytic nasal drug delivery system: in vitro and animal studies. AAPS Pharm Sci Tech. 2019;20(3):1–14. doi:10.1208/s12249-018-1211-0
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.