Back to Journals » Infection and Drug Resistance » Volume 18
Development and Validation of a Predictive Nomogram for Myelosuppression Risk in Chronic Hepatitis B Patients Treated with Peginterferon
Authors Fu J, Deng T, Zheng T, Shi P, Zhu W, Tao M, Wen Z, Wu X
Received 25 November 2024
Accepted for publication 28 March 2025
Published 9 April 2025 Volume 2025:18 Pages 1793—1805
DOI https://doi.org/10.2147/IDR.S508538
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Oliver Planz
Jiwei Fu,1,* Ting Deng,2,* Ting Zheng,1 Pei Shi,1 Wentao Zhu,1 Mengyu Tao,1 Zhilong Wen,1 Xiaoping Wu1
1Department of Infectious Diseases, The First Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi Province, 330006, People’s Republic of China; 2Second Department of Cardiovascular Medicine, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi Province, 330006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoping Wu, Department of Infectious Diseases, the First Affiliated Hospital, Jiangxi Medical College, Nanchang University, No. 17 Yongwai Street, Nanchang, Jiangxi, People’s Republic of China, Email [email protected]
Purpose: Peginterferon (Peg-IFN) is a common treatment for chronic hepatitis B (CHB); however, some patients developing myelosuppression as a side-effect. In this study, we identified risk factors associated with increased myelosuppression, and incorporated them into a predictive nomogram.
Patients and Methods: This study is designed as a case-control study. A total of 312 CHB patients treated with Peg-IFN from two medical centers were retrospectively enrolled between December 2019 and December 2022. Patients from the First Affiliated Hospital of Nanchang University were randomly divided into a training cohort (n=153) and a test cohort (n=55) at a 3:1 ratio. Patients from the Jiangxi Provincial People’s Hospital composed the validation cohort (n= 104). In the training cohort, based on the blood routine results of patients 1 week after Peg-IFN treatment, patients were further divided into Normal (myelosuppression grades 0-I) and Myelosuppression (grades II–IV) groups. Then uni- and multivariate logistic regression analyses were carried out to identify myelosuppression risk factors, which were subsequently incorporated into a predictive nomogram. The capability of the predictive nomogram was validated using an area under the curve (AUC) of the receiver operating characteristic (ROC) curve. The Hosmer-Lemeshow test, calibration curves, and decision curve analysis (DCA) were used to evaluate the nomogram. Finally, the developed predictive nomogram was validated both internally and externally using separate test and validation cohorts.
Results: Body mass index (BMI; odds ratio [OR]=0.841, 95% confidence interval [CI] 0.738– 0.959, P=0.010), white blood cell counts (WBC; OR=0.657, 95% CI 0.497– 0.868, P=0.003), globulin (GLB; OR=0.796, 95% CI 0.713– 0.889, P< 0.001) and serum creatinine levels (SCR; OR=1.029, 95% CI 1.002– 1.058, P=0.038) are independent risk factors for myelosuppression in Peg-IFN-treated CHB patients. A predictive nomogram was constructed by incorporating the above independent risk factors, and its performance was assessed across the training, test, and validation cohorts. The model demonstrated AUC values of 0.824 (95% CI 0.757– 0.891), 0.812 (95% CI 0.701– 0.923), and 0.870 (95% CI 0.802– 0.940), respectively, highlighting its good predictive accuracy. As for Hosmer-Lemeshow, it was P=0.351, (χ 2= 8.898) for training, P=0.514 (χ 2=6.226) for the test, and P=0.442 (χ 2=7.918) for the validation cohort. The results of the calibration curves and DCA demonstrated good concordance between predicted probabilities and observed outcomes, with the model showing higher clinical net benefit.
Conclusion: Lower BMI, WBC counts, GLB, and higher SCR levels are independent risk factors for myelosuppression among Peg-IFN-treated CHB patients. The predictive nomogram, based on those factors, is able to identify high-risk individuals for myelosuppression, thereby aiding in early alleviation of this side-effect.
Keywords: chronic hepatitis B, peginterferon, myelosuppression, predictive nomogram
Introduction
Hepatitis B virus (HBV) infection is widespread globally, and with an estimated HBV prevalence of 6.1% in China, it is a major public health challenge.1,2 Long-term HBV infection can progress to chronic hepatitis B (CHB), which may further lead to the development of liver cirrhosis and hepatocellular carcinoma, posing a significant threat to public health.3,4 Therefore, effective antiviral therapy is crucial for CHB patients to prevent disease progression and related complications.5 Currently, Subcutaneous peginterferon (Peg-IFN) is one of the first-line treatment regimens for CHB.6 Individualized Peg-IFN treatment regimens can enhance the patient’s immune system’s ability to fight against CHB, allowing certain favorable populations to achieve clinical cure of hepatitis B, which is its greatest advantage.7 However, compared to long-term oral nucleoside/nucleotide analogue (NAS) therapy, the higher incidence of adverse reactions with Peg-IFN, especially myelosuppression, requires more attention.8
Myelosuppression is clinically manifested by peripheral blood cytopenia, such as anemia, leukopenia, and thrombocytopenia.9 This Peg-IFN-associated myelosuppression effect may arise through multiple mechanisms. Some studies suggest that interferon exerts its myelosuppressive effects by directly inhibiting the activity of hematopoietic stem cells in the bone marrow, impairing the differentiation of bone marrow cells into hematopoietic cells, suppressing the release of myeloid cells, and directly depleting peripheral blood cells. These mechanisms collectively contribute to the development of myelosuppression.10–12 Furthermore, current treatments for Peg-IFN-related myelosuppression, such as administrating leukocyte-raising drugs, reducing Peg-IFN dosages, as well as suspending/interrupting Peg-IFN therapy, could effectively prevent further aggravation of myelosuppression, but they have a delayed effect and may come at the cost of potentially lowering Peg-IFN effectiveness. Additionally, the high cost of Peg-IFN could increase the economic burden for patients with severe myelosuppression, who would have their treatment interrupted.13
The process of Peg-IFN treatment is long, uncomfortable, and expensive, requiring patient adherence throughout the therapy. The early onset of myelosuppression not only poses a health risk to the patient but also easily affects their confidence in continuing interferon therapy, ultimately leading to dose reduction or discontinuation, which can impact treatment efficacy. Therefore, it is necessary to identify patients at risk of myelosuppression in advance and intervene accordingly. But no effective method currently exists to predict its occurrence in CHB patients being treated with Peg-IFN. In this study, we aimed to rectify this omission by retrospectively analyzing myelosuppression risk factors among Peg-IFN-treated CHB patients and subsequently establishing a predictive nomogram for myelosuppression risk. This model is able to identify patients at risk for myelosuppression in advance, helping clinicians make more cost-effective decisions for these patients, thereby making Peg-IFN treatment safer and more effective.
Materials and Methods
Patients and Study Design
A total of 312 CHB patients, treated with Peg-IFN in the First Affiliated Hospital of Nanchang University and Jiangxi Provincial People’s Hospital, between December 2019-December 2022 were retrospectively collected through the electronic medical record system. Among them, 208 patients from the First Affiliated Hospital of Nanchang University were randomly divided into a training cohort (n=153) and a test cohort (n=55) at a ratio of 3:1.14,15 The training cohort was a subset of participants used for developing and optimizing the predictive model, while the test cohort consisted of participants from the same hospital, used for internal validation of the model. Additionally, 104 patients from Jiangxi Provincial People’s Hospital were used as a validation cohort to externally validate the model. Afterward, the patients were divided into a Normal group and a Myelosuppression group according to whether they had myelosuppression. The study flow is detailed in Figure 1. Inclusion criteria were as follows: 1) Meeting the “Guidelines for the Prevention and Treatment of CHB (2022 edition)” criteria, in which serum HBsAg-positivity for more than 6 months was present, 2) Age 16–65 years, 3) No previous antiviral therapy. Exclusion criteria were as follows: 1) Pregnant/lactating female, 2) CHB with hepatic cirrhosis at the decompensated stage, malignant tumors, autoimmune liver disease, drug-induced hepatitis, alcoholic hepatitis, or infection with other hepatotropic viruses (ex. Hepatitis A, C, E), 3) Other severe diseases, such as respiratory/heart failure, 4) Previous history of mental illness, abnormal thyroid function, autoimmune diseases, or other contraindications against Peg-IFN administration, 5) Peg-IFN allergy, or 6) Missing data.
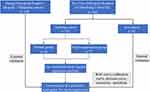 |
Figure 1 Flowchart of the study. |
The grade of myelosuppression was assessed according to the WHO myelosuppressive response grading index16 (Table 1), based on the most severe level of myelosuppression among four indicators: white blood cells, neutrophils, hemoglobin, and platelets. According to the blood routine results of patients 1 week after Peg-IFN treatment, patients with myelosuppression grades II–IV were defined as the Myelosuppression group, while 0-I was considered as the Normal group. In addition, we retrospectively collected the demographic characteristics and clinical data of these patients through the hospital’s electronic medical record system,17 including age, gender, height, and weight; history of diabetes and hypertension; family history of hepatitis B, cirrhosis, and liver cancer; history of smoking and alcohol consumption; as well as baseline laboratory parameters prior to treatment, including blood routine, liver function, kidney function, blood glucose, blood lipids, electrolytes, thyroid function, hepatitis B e antigen, and hepatitis B virus DNA. These parameters were considered as potential factors for predicting the risk of myelosuppression during Peg-IFN treatment.
 |
Table 1 The WHO Myelosuppressive Response Grading Index |
Ethics Approval and Informed Consent
The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University (REB#2019024) and in compliance with the Helsinki Declaration. As this study was retrospective, the Ethics Committee of the First Affiliated Hospital of Nanchang University waived the informed consent for this study. Of note, all patient data were anonymized and handled with strict confidentiality to ensure privacy.
Statistical Analyses
Statistical analysis was performed with R (version 4.13), SPSS (version 24.0), and GraphPad Prism (version 8.0). Categorical data were described as rates and percentages, and comparisons between groups were carried out using the χ2 test. For continuous data with normal distribution, they were described as mean ± standard deviation (SD), two-independent samples t-test was used for comparisons between 2 groups, and one-way ANOVA was used for comparisons between multiple groups. Continuous data that did not conform to a normal distribution were described as median (25–75% quartiles), the Mann–Whitney U-test was used for comparisons between 2 groups, and the Kruskal‒Wallis H rank sum test was used for comparisons between multiple groups.
Putative myelosuppression risk factors were subjected to uni-, followed by multi-variate logistic regression analysis, and a predictive nomogram was established. The predictive power of that model was analyzed using receiver operating characteristic (ROC) curve analysis, in which an area under the curve (AUC)>0.70 was considered highly accurate in predicting myelosuppression. The Hosmer-Lemeshow test was then applied to determine goodness of fit, in which P>0.05 indicated that the predictive model was well-calibrated. The calibration curve and C-index were used to evaluate the fitting of the nomogram. Finally, decision curves were used to evaluate the clinical usefulness of the predictive nomogram. For all other statistical analyses, P<0.05 was considered statistically significant.
Results
Clinical Characteristics
Among the 312 patients with CHB, 146 had myelosuppression, with a prevalence of 46.8%. 208 patients at the First Affiliated Hospital of Nanchang University included 140 males and 68 females, with a mean age of 35.01 ± 8.70 years, and 108 patients with myelosuppression. The training cohort included 105 males and 48 females, with a mean age of 34.41 ± 8.59 years, and 77 patients with myelosuppression. The test cohort included 35 males and 20 females with a mean age of 36.71 ± 8.86 years and 31 patients with myelosuppression. 104 patients from Jiangxi Provincial People’s Hospital served as the validation cohort, including 71 males and 33 females, with a mean age of 36.41 ± 8.47 years, and 38 patients with myelosuppression. A comparison of the clinical characteristics of the 3 cohorts of patients revealed no statistically significant differences (Table 2).
 |
Table 2 CHB Patient Characteristics in the Training, Test, and Validation Cohorts |
Examining Myelosuppression Grades and Patient Characteristics in the Training Cohort
In the training cohort, 27 patients had grade 0, 49 had grade I, 57 had grade II, 19 had grade III, and 1 had grade IV in terms of myelosuppression (Figure 2).
 |
Figure 2 Distribution of the different grades of myelosuppression among peginterferon (Peg-IFN)-treated chronic hepatitis B (CHB) patients in the training cohort. |
We then compared patient characteristics between normal and myelosuppression patients in the training cohort and found that significant differences between those 2 groups were present for body mass index, white blood cell, monocyte, neutrophil, eosinophil, and basophil counts, as well as direct bilirubin, globulin, albumin/globulin ratio, serum creatinine, and glycated serum protein levels. For all other patient characteristics, no significant differences were present (Table 2).
The finding of significantly lower white blood cell, monocyte, neutrophil, eosinophil, and basophil counts in the Myelosuppression group, compared to normal, was further supported by an analysis of the number of patients with decreases in different blood cell types, in which myelosuppressed patients most often had lowered neutrophil counts, followed by white blood cells. By contrast, most patients had normal levels of platelets and red blood cells, the latter represented in the form of hemoglobin (Supplementary Table 1).
Independent Risk Factors for Myelosuppression in CHB Patients Treated With Peg-IFN
To identify the risk factors associated with myelosuppression in the training cohort, univariate logistic regression analysis was first carried out on the 11 variables with statistically significant differences, in which 8 variables were significantly associated with increased myelosuppression risk (Table 3). Multivariate logistic regression analysis was subsequently conducted, in which only BMI (odds ratio [OR]=0.841, 95% confidence interval [CI] 0.738–0.959), WBC counts (OR=0.657, 95% CI 0.497–0.868), GLB (OR=0.796, 95% CI 0.713–0.889) and SCR levels (OR=1.029, 95% CI 1.002–1.058) were significantly associated with increased myelosuppression risk (Table 3).
 |
Table 3 Uni- and Multivariate Logistic Regression Analyses of Patient Characteristics Associated With Increased Myelosuppression Risk |
Establishment and Validation of the Predictive Nomogram for Myelosuppression Risk
Based on the findings of multi-variate logistic regression analyses, the risk factors of body mass index, white blood cell counts, globulin, and serum creatinine levels were incorporated into a predictive nomogram for identifying increased myelosuppression risk in Peg-IFN-treated CHB patients (Figure 3).
To confirm the validity of this nomogram, an ROC curve analysis was conducted, in which for the training cohort, the AUC was 0.824 (cutoff value 0.557, sensitivity 71%, specificity 84%), which was above the 0.70 cut-off, indicating that the formula was highly predictive for myelosuppression risk (Figure 4A). This was further confirmed by ROC curve analysis of the test cohort and validation cohort, in which the AUC were 0.812 (cutoff value 0.491, sensitivity 81%, specificity 71%) and 0.870 (cutoff value 0.333, sensitivity 87%, specificity 82%) (Figure 4B and C).
 |
Figure 4 Receiver operating characteristic (ROC) curves for (A) training, (B) test and (C) validation cohorts. Notes: AUC, area under the curve. |
The goodness of fit for the training, test and validation cohorts, and thus the calibration of the predictive nomogram, was then investigated using the Hosmer-Lemeshow test, in which for the training cohort, P=0.351, (χ2= 8.898), for the test cohort, P=0.514 (χ2=6.226), and for the validation cohort, P=0.442 (χ2=7.918), both of which were above the P>0.05 cut-off. Therefore, the nomogram did not deviate from the perfect fitting.
The calibration curves between the training cohort, test cohort, and validation cohort show good calibration with 1000 bootstrap sampling. For these 3 cohorts, there was a close correspondence between the probability of myelosuppression predicted by the nomogram and the actual probability (Figure 5A, C and E). In addition, the DCA curves for the 3 cohorts showed that the nomogram had high clinical utility compared with the assumption that all patients developed myelosuppression after 1 week of Peg-IFN therapy or the assumption that all patients did not develop myelosuppression (Figure 5B, D and F). Therefore, the nomogram was highly accurate in identifying increased myelosuppression risk among Peg-IFN-treated CHB patients.
Discussion
Myelosuppression is a common side effect after Peg-IFN treatment and has a greater impact on the ability to safely and successfully complete the full course of Peg-IFN therapy.18 Therefore, early identification of myelosuppression-prone patients is clinically significant, as it enables interventions to lower myelosuppression occurrence; this could be facilitated by the establishment of a myelosuppression risk prediction model. Here, we identified, by uni and multi-variate logistic regression analyses, that low body mass index, white blood cell counts, globulin, and high serum creatinine levels are independent risk factors for myelosuppression. These factors were incorporated into a predictive nomogram, which was found to be highly accurate when applied to the training, test, and validation patient cohorts, as determined by ROC curve, calibration curve, and DCA curve analyses.
In our study, we found that more than half of the 153 patients in the training cohort exhibited mild-to-moderate myelosuppression in the early stages. Notably, 13.1% of the patients had severe myelosuppression. This suggests that peg-IFN-related myelosuppression is quite common, and a portion of patients may progress to severe myelosuppression, which warrants attention. This may be due to our study only looking at patients at 1-week post-treatment; as the grade of myelosuppression is dose-dependent, prolonged Peg-IFN treatment may eventually lead to increased myelosuppression. Mitsunaga et al found that 21% of patients experienced severe adverse events in their study on Peg-IFN treatment for lymphomas, primarily related to a decrease in blood cell count.19 This finding supports our research results. As for the effects of Peg-IFN on specific blood cell counts, we found that myelosuppression was most prevalent in neutrophils among patients, followed by white blood cells, platelets, and red blood cells, consistent with previous reports.20 This suggests that we need to focus on patients’ neutrophil levels when treating CHB patients with peg-IFN.
Low body mass index as an independent myelosuppression risk factor was in line with a study by Miao et al,21 who found an inverse correlation between body mass index and myelosuppression incidence among azathioprine-treated autoimmune hepatitis patients (P=0.003), as well as Robins et al,22 who determined that temozolomide-treated patients with body mass index<30 kg/m2 were significantly more likely to have grade III–IV myelosuppression, compared to those ≥30 kg/m2. This inverse correlation may be due to increased body mass indices being associated with corresponding decreases in both initial and maximum peak Peg-IFN concentrations, resulting in lowered Peg-IFN effects, and subsequently lowered myelosuppression. Additionally, body mass index also serves as an indicator of the nutritional status of the human body,23 in which higher indices, within a certain range, are linked to better nutritional statuses, and subsequently, stronger immune function and resistance to drug-induced myelosuppression. The two studies mentioned above both support the notion of low body mass index as an independent myelosuppression risk factor. However, the key difference lies in the types of drugs responsible for myelosuppression in their respective studies, namely immunosuppressants and chemotherapeutic agents.
With respect to low white blood cell counts, it is an independent risk factor for myelosuppression among Peg-IFN-treated CHB patients in accordance with a previous study, where it was also a risk factor among thiopurine-treated inflammatory bowel disease patients,24 as well as another showing that low baseline WBC counts were an important predictor of post-chemotherapy myelosuppression.25 It is worth noting that although the two studies mentioned above support the findings, the drugs causing myelosuppression in their research were not Peg-IFN. Peg-IFN-induced myelosuppression leads primarily to a decrease in WBC, so the lower the baseline WBC count the greater the likelihood that myelosuppression will occur, and the greater the grade of myelosuppression that may occur. Additionally, low WBC counts may be related to the patient’s own limited bone marrow hematopoiesis or release of blood cells, resulting in a greater susceptibility to myelosuppression with the use of Peg-IFN. White blood cells are an important component of the immune system, and lowering their levels below a certain threshold could potentially result in reduced immune function and increased infections. However, a study showed that IFN-induced leukopenia in CHB patients was not associated with increased infection.26
We identified that low globulin levels were another independent myelosuppression risk factor for Peg-IFN-treated CHB patients. The study by Mavroudi et al supports the above viewpoint, as their research found that serum IgG levels were significantly lower in patients with chronic idiopathic neutropenia compared to healthy controls.27 However, the study population in their research did not include individuals who developed myelosuppression after interferon treatment. These globulins are mostly in the form of immunoglobulins, which are mainly produced by immune cells, such as plasma cells. These macromolecules are essential for the body to mount specific immune responses, particularly via promoting phagocytosis.28 One possible mechanism behind IFN-induced myelosuppression is via the activation of specific immune responses, such as myelosuppressive T cells. However, certain specific globulins may suppress the activity of these T cells. Studies have shown that immunoglobulins can inhibit the proliferation, survival, and function of certain T cell subsets.29 On the other hand, as shown in our study, lowered globulin levels are indicative of reduced immunoglobulins, which could lead to the removal of inhibitions against myelosuppressive T cell activity, and thus increase the risk of Peg-IFN-associated myelosuppression.
Finally, we also found that high serum creatinine levels were an independent risk factor for the development of myelosuppression in CHB patients treated with Peg-IFN. A study found that patients with blood creatinine higher than 75.6 μ mol/L treated with methotrexate chemotherapy are more likely to develop myelosuppression.30 These findings support our results, but it is important to note that the drug involved in the study was methotrexate, not Peg-IFN. Interferon is primarily metabolized by the liver and excreted by the kidneys. Increased serum creatinine results in decreased clearance of interferon and higher concentrations of the drug in the body. This may increase the toxic effects on the bone marrow, leading to an increased risk of myelosuppression.
There are a number of limitations to this study, one of which is its retrospective nature, meaning that there may be biases in the data collection process. Additionally, this study focused exclusively on patients from Jiangxi Province in southern China. Given the regional differences between northern and southern populations, the findings may not be fully generalizable to patients from other regions. Future studies will utilize larger patient sample sizes, from multiple medical institutions, to further examine the predictive power of our model to identify Peg-IFN-treated CHB patients at high risk for myelosuppression.
Conclusion
Low body mass index, white blood cell counts, globulin, and high serum creatinine levels were independent risk factors for myelosuppression in Peg-IFN-treated CHB patients. Based on these risk factors, a predictive nomogram was developed to identify Peg-IFN-treated CHB patients at high risk for myelosuppression, thereby enabling early intervention. This nomogram aims to provide an effective tool for preventing myelosuppression following interferon treatment, enhancing treatment adherence, improving therapeutic efficacy, and ultimately improving prognosis.
Abbreviations
Peg-IFN, Peginterferon; CHB, Chronic hepatitis B; AUC, Area under the curve; ROC, Receiver operating characteristic; DCA, Decision curve analysis; BMI, Body mass index; CI, Confidence interval; WBC, White blood cell; GLB, Globulin; SCR, Serum creatinine levels; HBV, Hepatitis B virus; NAS, Nucleoside/nucleotide analogue; PHA, Phytohemagglutinin.
Data Sharing Statement
Data in this study can be made available upon request to the corresponding author.
Acknowledgments
The authors thank all study patients and staff participating in the study. We also thank Alina Yao for her assistance in manuscript preparation and editing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was funded by the Natural Science Foundation (No. 20212ACB206010) of Jiangxi Province, China.
Disclosure
The authors report no conflict of interest in the work.
References
1. Ou TY, Huy LD, Mayne J. et al. Global mortality of chronic liver diseases attributable to Hepatitis B virus and Hepatitis C virus infections from 1990 to 2019 and projections to 2030. J Infect Public Health. 2024;17(7):102443. doi:10.1016/j.jiph.2024.04.027
2. Jiao L, Shen T, Han Y, et al. The spatial-temporal distribution of hepatitis B virus infection in China, 2006–2018. BMC Infect Dis. 2024;24(1):811. doi:10.1186/s12879-024-09716-z
3. Adali G, Aykut H, Bilgic NM, Yilmaz Y. Chronic hepatitis B and metabolic dysfunction-associated steatotic liver disease: metabolic risk factors are key drivers of hepatocellular carcinoma. Heliyon. 2024;10(18):e37990. doi:10.1016/j.heliyon.2024.e37990
4. Xiao J, Wang F, Yuan Y, et al. Epidemiology of liver diseases: global disease burden and forecasted research trends. Sci China Life Sci. 2024;68(2):541–557. doi:10.1007/s11427-024-2722-2
5. Huang D, Yuan Z, Wu D, et al. HBV Antigen-Guided Switching Strategy From Nucleos(t)ide Analogue to Interferon: avoid Virologic Breakthrough and Improve Functional Cure. J Med Virol. 2024;96(11):e70021. doi:10.1002/jmv.70021
6. You H, Wang F, Li T, et al. Guidelines for the Prevention and Treatment of Chronic Hepatitis B (version 2022). J Clin Transl Hepatol. 2023;11(6):1425–1442. doi:10.14218/jcth.2023.00320
7. Tang LSY, Covert E, Wilson E, Kottilil S. Chronic Hepatitis B Infection: a Review. JAMA. 2018;319(17):1802–1813. doi:10.1001/jama.2018.3795
8. Tseng TC, Kao JH, Chen DS. Peginterferon α in the treatment of chronic hepatitis B. Expert Opin Biol Ther. 2014;14(7):995–1006. doi:10.1517/14712598.2014.907784
9. Brigle K, Pierre A, Finley-Oliver E, Faiman B, Tariman JD, Myelosuppression MT. Bone Disease, and Acute Renal Failure: evidence-Based Recommendations for Oncologic Emergencies. Clin J Oncol Nurs. 2017;21(5 Suppl):60–76. doi:10.1188/17.Cjon.S5.60-76
10. Mucci A, Antonarelli G, Caserta C, et al. Myeloid cell-based delivery of IFN-γ reprograms the leukemia microenvironment and induces anti-tumoral immune responses. EMBO Mol Med. 2021;13(10):e13598. doi:10.15252/emmm.202013598
11. Matatall KA, Jeong M, Chen S, et al. Chronic Infection Depletes Hematopoietic Stem Cells through Stress-Induced Terminal Differentiation. Cell Rep. 2016;17(10):2584–2595. doi:10.1016/j.celrep.2016.11.031
12. Yan H, Walker FC, Ali A, et al. The bacterial microbiota regulates normal hematopoiesis via metabolite-induced type 1 interferon signaling. Blood Adv. 2022;6(6):1754–1765. doi:10.1182/bloodadvances.2021006816
13. Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ. 2019;97(3):230–238. doi:10.2471/blt.18.219469
14. Wang Y, Chen Y, Chen R, et al. Development and validation of a nomogram model for prediction of stroke-associated pneumonia associated with intracerebral hemorrhage. BMC Geriatr. 2023;23(1):633. doi:10.1186/s12877-023-04310-5
15. He B, Qiu Z. Development and validation of an interpretable machine learning for mortality prediction in patients with sepsis. Front Artif Intell. 2024;7:1348907. doi:10.3389/frai.2024.1348907
16. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. doi:10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6
17. Wu B, Niu Z, Hu F. Study on Risk Factors of Peripheral Neuropathy in Type 2 Diabetes Mellitus and Establishment of Prediction Model. Diabetes Metab J. 2021;45(4):526–538. doi:10.4093/dmj.2020.0100
18. Derungs T, Poddubnyy D, Schneider T. Pancytopenia following adjuvant therapy with interferon-gamma in a patient with disseminated nocardiosis. Int J Infect Dis. 2024;142:106997. doi:10.1016/j.ijid.2024.106997
19. Mitsunaga K, Bagot M, Ram-Wolff C, et al. Real-world study of pegylated interferon α-2a to treat mycosis fungoides/Sézary syndrome using time to next treatment as a measure of clinical benefit: an EORTC CLTG study. Br J Dermatol. 2024;191(3):419–427. doi:10.1093/bjd/ljae152
20. Hindi NN, Saleh MI. Patient characteristics associated with peglyated interferon alfa-2a induced neutropenia in chronic hepatitis C patients. Clin Exp Pharmacol Physiol. 2018;45(7):636–642. doi:10.1111/1440-1681.12934
21. Miao Q, Yan L, Zhou Y, et al. Association of genetic variants in TPMT, ITPA, and NUDT15 with azathioprine-induced myelosuppression in southwest China patients with autoimmune hepatitis. Sci Rep. 2021;11(1):7984. doi:10.1038/s41598-021-87095-0
22. Robins HI, Eickhoff J, Gilbert MR, et al. The association between BMI and BSA-temozolomide-induced myelosuppression toxicities: a correlative analysis of NRG oncology RTOG 0525. Neurooncol Pract. 2019;6(6):473–478. doi:10.1093/nop/npz006
23. Bray GA. Beyond BMI. Nutrients. 2023;15(10):2254. doi:10.3390/nu15102254
24. Broekman M, Coenen MJH, Wanten GJ, et al. Risk factors for thiopurine-induced myelosuppression and infections in inflammatory bowel disease patients with a normal TPMT genotype. Aliment Pharmacol Ther. 2017;46(10):953–963. doi:10.1111/apt.14323
25. Lyman GH, Abella E, Pettengell R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol. 2014;90(3):190–199. doi:10.1016/j.critrevonc.2013.12.006
26. Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8(7):559–568. doi:10.1038/nri2314
27. Mavroudi I, Eliopoulos AG, Pontikoglou C, et al. Immunoglobulin and B-cell disturbances in patients with chronic idiopathic neutropenia. Clin Immunol. 2017;183:75–81. doi:10.1016/j.clim.2017.07.009
28. Sun Y, Huang T, Hammarström L, Zhao Y. The Immunoglobulins: new Insights, Implications, and Applications. Annu Rev Anim Biosci. 2020;8(1):145–169. doi:10.1146/annurev-animal-021419-083720
29. Barahona Afonso AF, João CM. The Production Processes and Biological Effects of Intravenous Immunoglobulin. Biomolecules. 2016;6(1):15. doi:10.3390/biom6010015
30. Tu X, Chen R, Huang G, et al. Factors Predicting Severe Myelosuppression and Its Influence on Fertility in Patients with Low-Risk Gestational Trophoblastic Neoplasia Receiving Single-Agent Methotrexate Chemotherapy. Cancer Manag Res. 2020;12:4107–4116. doi:10.2147/cmar.S252664
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



