Back to Journals » Risk Management and Healthcare Policy » Volume 18
Development and Validation of a Questionnaire to Measure Feeding Challenges and Nutritional Problems Associated With Long-Term Enteral Nutrition Among Children With Disabilities
Authors Zaher S, Ajabnoor SM
Received 22 October 2024
Accepted for publication 14 February 2025
Published 3 March 2025 Volume 2025:18 Pages 747—757
DOI https://doi.org/10.2147/RMHP.S502223
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Sara Zaher,1 Sarah M Ajabnoor2,3
1Clinical Nutrition Department, College of Applied Medical Sciences, Taibah University, Madinah, Kingdom of Saudi Arabia; 2Department of Clinical Nutrition, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, Saudi Arabia; 3Food, Nutrition and Lifestyle Unit, King Fahd Medical Research Centre, King Abdulaziz University, Jeddah, Saudi Arabia
Correspondence: Sara Zaher, Clinical Nutrition Department, College of Applied Medical Sciences, Taibah University, P.O. Box 344, Madinah, 42353, Kingdom of Saudi Arabia, Tel +966565592420, Email [email protected]; [email protected]
Background: Children with disabilities receiving long-term enteral nutrition (EN) frequently struggle with feeding issues. This study aims to develop and validate a questionnaire to assess tube feeding challenges and nutritional problems among this group of children.
Methods: In this survey-based study, data was collected via an online survey of mothers or caregivers of children with disabilities receiving long-term EN. The questionnaire was prepared following a literature analysis of nasogastric, gastrostomy and jejunostomy-related complications in children. The validation of the tool was conducted with three experts in the field, followed by its translation into Arabic. It was then pilot-tested on six mothers of children with disabilities who receive long-term EN. The reliability of the questionnaire was assessed using Cronbach’s-alpha coefficient and measurement of test–retest inter-rater reliability.
Results: Twenty-four children were included in this study; their median age was 4.7 years. The value of Cronbach’s-alpha (α = 0.742) suggested good reliability of the tool items among the study sample. The test-retest reliability assessed by correlation coefficients showed a strong correlation for most items; the r-value of the overall tool was 0.922, P < 0.001.
Conclusion: Strong test–retest reliability was demonstrated by the developed EN feeding problem questionnaire. Suggesting that the questionnaire is reliable and valid for utilisation in children with disabilities.
Keywords: feeding problems, enteral nutrition, disability, children
Introduction
Feeding problems are common in children with disabilities. According to a recent systematic review, children who have unilateral cerebral palsy and stroke frequently experience dysphagia, which ranges between 24.2% and 88.6%.1 Up to 77% of children with disabilities report having gastroesophageal reflux disease (GERD) as one of their gastrointestinal symptoms.2 Children with neurological disabilities also experience such problems far more frequently than those who develop normally.3 The inability to safely and successfully consume food is a hallmark of feeding difficulties in children with disabilities. This can be caused by a number of factors, such as swallowing dysfunction, gastroesophageal reflux, and poor oral-motor coordination. Such feeding difficulties can lead to various negative health outcomes, most notably poor growth and weight loss.
Enteral nutrition (EN) via tube feeding is considered a life-sustaining and therapeutic intervention for children with disabilities who cannot meet their nutritional requirements via oral intake. The benefits of tube feeding include improved growth and nutritional status, malnutrition prevention, fluid intake maintenance, facilitation of intake when diet is unpalatable, improved medication compliance, reduced risk of aspiration and complications related to GERD, and overall enhanced health-related quality of life for children and their carers.4 EN can be delivered through a nasogastric, naso-jejunal, percutaneous endoscopic gastrostomy (PEG), or jejunostomy tube.5 Gastric feeding is considered the preferable method for EN access because of the high feasibility of tube insertion and provision of bolus feeds.2 However, long-term EN introduces various tube feeding challenges and nutritional problems that can profoundly impact children and their caregivers.6 These challenges include mechanical issues with feeding devices, gastrointestinal complications, and psychosocial stressors, all of which can compromise the efficacy of EN and affect the child’s quality of life. Reflux, stomach ulcers, overfeeding, and site infections are among the frequent adverse effects linked to gastrostomy feeding in children with neurological impairment.7 Nevertheless, longer-term and more robust case series studies are required to comprehend the advantages and risks of tube feeding initiation in children with disabilities.
Appropriate management of feeding problems in children receiving long-term EN involves recognition and control of contributing factors, which include feeding schedule, estimation of nutrients requirements, caring of tube feeding, and identifying EN-related complications (ie, mechanical and gastrointestinal complications).8 Regular assessment and evaluation of tube feeding problems should be conducted in children with disabilities receiving long-term EN, with collaboration between the child’s family and a multidisciplinary healthcare team. Unfortunately, there is no available standardised tool to systematically assess the tube feeding challenges and nutritional problems associated with long-term EN in children with disabilities. For instance, Bell et al (2019) developed and validated a tool to screen for problems specifically related to oral feeding, swallowing, and undernutrition in paediatric patients with cerebral palsy, but excluded patients with feeding tubes.9 They included questions related to oromotor function, feeding-related difficulties (such as longer mealtimes, coughing during meals, or difficulty with particular textures), and indicators of undernutrition (such as low weight gain or growth faltering).9 Similarly, other studies have focused on developing and validating tools for assessing quality of life in adult patients receiving home EN and parenteral nutrition.10,11 Although the NutriQoL questionnaire is thorough, its applicability to paediatric patients may be limited because it was validated largely in adult populations.10 Overall, the screening tools developed by these studies did not address the tube feeding related problems in children with disabilities. Future studies should focus on creating and validating assessment tools that are specific to tube feeding practices, age-appropriate, and inclusive of a wider spectrum of disabilities. A well-designed questionnaire could therefore provide a standardised method for assessing the multifaceted aspects of enteral feeding in children with disabilities, including mechanical, gastrointestinal, and nutritional adequacy. It could also facilitate the identification of specific problems that require intervention, guide clinical decision-making, and support research efforts aimed at improving care practices.
The present study aims to develop and validate a comprehensive questionnaire specifically designed to assess tube feeding challenges and nutritional problems associated with long-term EN in children with disabilities. The development of a robust assessment tool will help to enhance clinical practice, inform targeted interventions, and ultimately improve the quality of life for children receiving long-term EN and their caregivers.
Materials and Methods
Study Design and Sampling
In this survey-based study, all mothers or caregivers of children with disabilities receiving long-term EN (more than 4 weeks) and living in Saudi Arabia were eligible to participate. The study complies with the Declaration of Helsinki and it was approved by the ethics committee at Taibah University, certificate number (2024/177/203 CLN). The consent for participation was obtained through a mandatory question confirming agreement to participate in the study.
Convenient sampling method was used in this study, where data was collected via an online survey, distributed through various social media platforms including WhatsApp and X between December 2023 and March 2024. The study collected information on feeding challenges and nutritional problems associated with long-term EN among children with disabilities. The participants were given the option to provide their contact information if they were willing to participate in the test–retest reliability of the questionnaire.
Questionnaire Development and Validation
The tool was developed to collect data on the feeding challenges and nutritional problems associated with long-term EN among children with disabilities. The development of the tool followed a step-by-step approach (Figure 1). The English-language questionnaire was prepared following a literature analysis of nasogastric-, gastrostomy- and jejunostomy-related complications in children.12–17 The most reported tube feeding and nutritional problems in the literature were tube leakage, tube occlusion or obstruction, infection, vomiting, gastroesophageal reflux, diarrhoea and constipation. In addition, weight gain and weight loss were also reported among children receiving long-term EN.
 |
Figure 1 Flow diagram of validation process. |
Expert validation of the tool was conducted with 3 experts in the field who provided insight into the indication of EN among children with disabilities, feeding and nutritional problems associated with its long-term application, as well as the challenges faced by caregivers. The questionnaire was modified according to their feedback.
The English questionnaire was then translated into Arabic-language using the forward translation method. Two translators completed the translation and ensured the cultural appropriateness of the questionnaire. The Arabic-language version of the questionnaire was then developed and pilot-tested on 6 mothers of children with disabilities on EN feeding. Based on the received feedback after pilot testing, clarifications for some items were included.
The final version of the questionnaire included 15 sociodemographic questions and 12 Likert scale questions that are related to tube feeding (10 questions to assess feeding and nutritional problems associated with EN among disabled children and 2 questions to assess feeding difficulties experienced by the caregivers). The participants were asked to rate the frequency of each item on a scale of 1 (Never), 2 (Rarely), 3 (Sometimes), 4 (Often) and 5 (Always). Additional 15 questions about health-related conditions and the nutritional status of the child were included in the questionnaire but were not part of the rating scale questionnaire. These items were not selected for analysis in the current study as they were not directly relevant to the validation study objectives. Finally, the participants were given the option to provide their contact information if they were willing to participate in the test–retest reliability of the questionnaire.
Reliability of the Questionnaire
The reliability of the questionnaire was assessed by Cronbach’s alpha coefficient, and the test–retest inter-rater reliability through the intraclass correlation coefficient. The participants were given a one-week gap before they answered the questionnaire for the second time.
Statistical Analysis
Data was analysed using the Statistical Package for Social Sciences software program version 22 (SPSS 22, SPSS Inc., Chicago, IL, USA). The normality of continuous variables was assessed using the Shapiro–Wilk test. Data are presented as frequencies and percentages. Continuous variables are presented as mean ± standard deviation (SD) and median (interquartile range [IQR]). A total Likert rating score of the items was calculated for each participant to be used in the statistical analysis. A Spearman Correlation was performed to assess inter-rater reliability between the first and the second responses of participants to the questionnaire. An r-value of 0.8–1 was considered a strong correlation, 0.5–0.8 a moderate correlation and 0.3–0.5 a weak correlation. p-values of <0.05 were considered statistically significant.
Results
A total of 24 children were included in the final analysis, 54.1% of them girls. The median (IQR) age of the children was 4.7 (1.9–6.9) years, with approximately 54% diagnosed with cerebral palsy. The general characteristics of the study participants are presented in Table 1.
 |
Table 1 Demographic Characteristics of Children Included in the Study (n = 24) |
Around 45.8% of the participants indicated that their children sometimes experience constipation and 37.5% vomiting. Nearly 20.8% of the participants indicated that their children sometimes experience tube obstruction, GERD and weight loss, 17% that their children sometimes experience tube leakage, and 12.5% that their children sometimes suffer from infection of the stoma site. Constipation was the most frequently reported nutritional problem associated with EN among our study sample, with an average Likert score of 3 (2–3.75), followed by vomiting with 2.5 (1–3) and weight loss 2.5 (1–3) (Table 2).
 |
Table 2 Description of the Items Included in the Newly Developed Tool to Assess Feeding Challenges and Nutritional Problems Associated With Long-Term EN in Children With Disabilities |
The overall Cronbach’s alpha of the developed questionnaire was α = 0.742 suggesting a good reliability of the tool items among the study sample. The Cronbach’s alpha coefficient did not markedly increase after deleting any item, which indicates a similar contribution of all items to the developed tool (Table 3).
 |
Table 3 Internal Reliability of the Tool Items |
The test–retest reliability assessment showed a good correlation for most items. A strong correlation was shown for items related to tube obstruction (r = 0.877), infection (r = 0.826), vomiting (r = 0.923) GERD (r = 0.822), and weight loss (r = 0.856). The items related to diarrhoea (r = 0.760) and constipation (r = 0.634) showed a moderate correlation. Items related to weight gain (r = 0.496) dumping syndrome (r = 0.480), and tube leakage (r = 0.301) showed a weak correlation. The overall r value of all items was 0.922, P < 0.001 (Table 4).
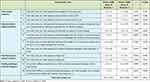 |
Table 4 Test–Retest Reliability Correlation Matrix |
Discussion
This study examined the relative validity and reliability of a newly developed questionnaire aimed at assessing feeding and nutritional problems associated with EN therapy in children with disabilities. The questionnaire included 10 items to assess feeding and nutritional problems associated with EN among disabled children and 2 items for difficulties experienced by their caregivers. Together, all questionnaire items showed good reliability among the study sample in determining the likelihood of tube feeding issues. Comparing the two different time points at which the questionnaire was administered, all items were found to be significantly correlated, except for that related to tube leakage. The strength of correlation was moderate for items related to dumping syndrome and weight gain but was strong for all others.
The measurement of tube feeding and nutritional problems in disabled children receiving EN have not been well documented. Most of the available published research with similar topics employ questionnaires for assessing the health-related quality of life in adult patients receiving home EN.18 A single study did evaluate a screening instrument in paediatric patients with cerebral palsy; however, this study did not include patients receiving EN.9 Using the new instrument, the authors were able to identify all children with severe malnutrition as well as those with eating and drinking disabilities.9 This screening instrument was intended for use in an outpatient setting for patients with cerebral palsy, who have a high prevalence of feeding/swallowing issues, which can lead to prolonged undernutrition. Another study evaluated the validity of an Arabic version of the Feeding Handicap Index (FHI) questionnaire, which aimed at assessing the physical, functional, and emotional effects of feeding and swallowing issues in children with disabilities.19 Unfortunately, due to the limited number of studies investigating the applicability of EN-related feeding problem questionnaires in children with disabilities, it is difficult to compare the results of the current study against other research findings.
In the present study, the first three items of the questionnaire were related to problems associated with all types of EN access devices. Good reliability was shown for the two items related to tube obstruction and infection of the tube insertion site. In general, difficulties associated with enteral feeding such as tube leakage, obstruction, and infection at the stoma site may have an impact on the quality of life of the patients.20 Identifying such EN-related problems early is paramount for children with disabilities, especially for avoiding undernutrition.
Problems related to gastrointestinal symptoms were covered in items 4 to 8 of the questionnaire. The test–retest reliability correlation was significant for all items related to gastrointestinal symptoms indicating good reliability. However, the item related to dumping syndrome showed only a week yet significant correlation. Compared to oral feeding, tube feeding is known to speed up stomach emptying, causing dumping syndrome.21 Significant gastrointestinal tract dysfunction, manifesting as impaired oral-motor function, GERD, aspiration, altered gastric emptying, and constipation, can arise from damage to the developing central nervous system. All of these factors have the potential to exacerbate eating difficulties in children with disabilities and provide more complex long-term management problems.22 Recognizing and treating gastrointestinal issues promptly is important as they have the potential to exacerbate problems with nutritional status and feeding.23
Regular monitoring of nutritional status for children with neurological impairments, along with timely and suitable treatment, is crucial for improving their health and enhancing their families’ quality of life.24 For these children, recognizing the importance of anthropometric nutritional red flags like weight status have been emphasized in the guidelines of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN).2 The questionnaire used in this study included two items related to the weight status of the child, and both showed good reliability. Given that children with neurological impairments often face challenges with routine assessment of body weight due to mobility restrictions, a validated caregiver-reported tool provides a practical alternative for tracking weight trends.
Nevertheless, the final two items of the questionnaire provide a short and simple means to identify the degree of difficulties faced by caregivers in determining the child’s nutritional requirements and handling the tube feeding administration. Evidence has shown that once a gastrostomy feeding tube is inserted in a disabled child, the carers’ quality of life is significantly improved.25 The perception of mothers toward tube feeding administration is very important as it may influence adherence to feeding guidelines.26,27 Therefore, families caring for children with a disability must receive ongoing support regarding feeding and nutritional problems.
The current study has some limitations. The small sample size and the short duration between the two responses might limit the applicability of the validity and reliability findings to a larger population. However, previous nutritional studies assessing the reliability of new questionnaires have used a similar methodological approach.28,29 The small sample is considered sufficient for tool pretesting due to the exploratory nature of the current study. A strength of the current study is that it is the first study to develop and validate a tube feeding problems questionnaire specifically designed for disabled children with long-term EN. In addition, the newly developed questionnaire was designed and validated based on the children’s mother’s opinions, allowing the identification of the most common feeding and nutritional issues that can be closely observed in the home setting. Finally, the sample recruited for the study was diverse and not restricted to only one type of disability, as in previous research.9 Therefore, the findings of this study may be generalisable in children with different types of disabilities.
Conclusion
This study is the first to evaluate the relative validity and reproducibility of an independent questionnaire to be used by parents/caregivers to check for feeding and nutritional problems in disabled children receiving long-term EN. The questionnaire was found to be reliable and valid in the tested sample; thus, it could be implemented as an assessment tool in future studies. The tool may also help in raising awareness of undernutrition and feeding issues, and in the prompt identification of children who may benefit from early intervention and management programs, ultimately leading to better long-term outcomes for these patients.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Ethical approval was authorized by Taibah University (2024/177/203 CLN). Informed consent for publication was obtained from the participants before filling out the questionnaire.
Acknowledgment
The authors would like to thank the parents and caregivers of the children for their cooperation in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
No conflict of interest to declare.
References
1. Sherman V, Greco E, Moharir M, Beal D, Thorpe K, Martino R. Feeding and swallowing impairment in children with stroke and unilateral cerebral palsy: a systematic review. Dev Med Child Neurol. 2019;61(7):761–769. doi:10.1111/dmcn.14094
2. Romano C, van Wynckel M, Hulst J, et al. European society for paediatric gastroenterology, hepatology and nutrition guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with neurological Impairment. J Pediatr Gastroenterol Nutr. 2017;65(2):242–264.
3. Fernando T, Goldman RD. Management of gastroesophageal reflux disease in pediatric patients with cerebral palsy. Can Fam Physician. 2024;65(11):796–798.
4. Lezo A, Capriati T, Spagnuolo MI, et al. Paediatric Home Artificial Nutrition in Italy: report from 2016 survey on behalf of Artificial Nutrition Network of Italian Society for Gastroenterology, Hepatology and Nutrition (SIGENP). Nutrients. 2018;10(9). Available from: http://www.ncbi.nlm.nih.gov/pubmed/30223620. Accessed February 18, 2025.
5. Pearce CB, Duncan HD. Enteral feeding. nasogastric, nasojejunal, percutaneous endoscopic gastrostomy, or jejunostomy: its indications and limitations. Postgrad Med J. 2002;78(918):198–204.
6. Sleigh G. Mothers’ voice: a qualitative study on feeding children with cerebral palsy. Child Care Health Dev. 2005;31(4):373–383.
7. Ferluga ED, Sathe NA, Krishnaswami S, Mcpheeters ML. Surgical intervention for feeding and nutrition difficulties in cerebral palsy: a systematic review. Dev Med Child Neurol. 2014;56(1):31–43.
8. Batra A, Marino LV, Beattie RM. Feeding children with neurodisability: challenges and practicalities. Arch Dis Child. 2022;107(11):967–972.
9. Bell KL, Benfer KA, Ware RS, et al. Development and validation of a screening tool for feeding/swallowing difficulties and undernutrition in children with cerebral palsy. Dev Med Child Neurol. 2019;61(10):1175–1181.
10. Cuerda MC, Apezetxea A, Carrillo L, et al. Development and validation of a specific questionnaire to assess health-related quality of life in patients with home enteral nutrition. NutriQoL® Development Patient Prefer Adherence. 2016;10;2289–2296.
11. Baxter JP, Fayers PM, McKinlay AW. The clinical and psychometric validation of a questionnaire to assess the quality of life of adult patients treated with long-term parenteral nutrition. JPEN J Parenter Enteral Nutr. 2010;34(2):131–142.
12. Di Leo G, Pascolo P, Hamadeh K, et al. Gastrostomy placement and management in children: a single-center experience. Nutrients. 2019;11(7):1555. doi:10.3390/nu11071555
13. Balogh B, Kovács T, Saxena AK. Complications in children with percutaneous endoscopic gastrostomy (PEG) placement. World J Pediatr. 2019;15(1):12–16.
14. Yi DY. Enteral nutrition in pediatric patients. Pediatr Gastroenterol Hepatol Nutr. 2018;21(1):12–19.
15. Seyedhejazi M, Hamidi M, Sheikhzadeh D, Aliakbari Sharabiani B. Nasogastric tube placement errors and complications in pediatric intensive care unit: a case report. J Cardiovasc Thorac Res. 2011;3(4):133–134.
16. Bodoky G, Kent-Smith L. Basics in clinical nutrition: complications of enteral nutrition. E Spen Eur E J Clin Nutr Metab. 2009;4(5):e209–11.
17. Madre C, Serhal L, Michaud L, et al. Prolonged enteral feeding is often required to avoid long-term nutritional and metabolic complications after esophagogastric dissociation. J Pediatr Gastroenterol Nutr. 2010;50(3):280–286.
18. Ojo O, Keaveney E, Wang XH, Feng P. the effect of enteral tube feeding on patients’ health-related quality of life: a systematic review. Nutrients. 2019;11(5). Available from: http://www.ncbi.nlm.nih.gov/pubmed/31083338. Accessed February 18, 2025.
19. Mahmoud NF, Mohammed Z, Mohammed HO, Lotfy AMM. Validation of the Arabic Version of Feeding Handicap Index for Children with developmental disabilities (A-FHI-C). 2024;J Autism Dev Disord. 1–9.
20. Day T. Home enteral feeding and its impact on quality of life. Br J Community Nurs. 2017;22(Sup7):S14–6.
21. Chen W, Codreanu I, Yang J, Li G, Servaes S, Zhuang H. Tube feeding increases the gastric-emptying rate determined by gastroesophageal scintigraphy. Clin Nucl Med. 2013;38(12):962–965.
22. Quitadamo P, Thapar N, Staiano A, Borrelli O. Gastrointestinal and nutritional problems in neurologically impaired children. Eur J Paediatr Neurol. 2016;20(6):810–815.
23. Trivić I, Hojsak I. Evaluation and treatment of malnutrition and associated gastrointestinal complications in children with cerebral palsy. Pediatr Gastroenterol Hepatol Nutr. 2019;22(2):122–131.
24. Melunovic M, Hadzagic-Catibusic F, Bilalovic V, Rahmanovic S, Dizdar S. Anthropometric parameters of nutritional status in children with cerebral palsy. Mater Sociomed. 2017;29(1):68–72.
25. Sullivan PB, Juszczak E, Bachlet AME, et al. Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy. Dev Med Child Neurol. 2004;46(12):796–800.
26. Craig GM, Scambler G, Spitz L. Why parents of children with neurodevelopmental disabilities requiring gastrostomy feeding need more support. Dev Med Child Neurol. 2003;45(3):183–188.
27. Matuszczak E, Hermanowicz A, Klek S, et al. Parents’ perceptions of gastrostomy feeding for children with neurological disabilities. J Hospice Palliative Nurs. 2014;16(8):521–525.
28. Cerchiari A, Tofani M, Giordani C, et al. Development and pilot study of a pediatric screening for feeding and swallowing disorders in infants and children: the Pediatric Screening–Priority Evaluation Dysphagia (PS–PED). Children. 2023;10(4):638.
29. Myr RK, Bere E, Øverby NC. Test-retest reliability of a new questionnaire on the diet and eating behavior of one year old children. BMC Res Notes. 2015;8(1):16.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

