Back to Journals » Journal of Inflammation Research » Volume 18
Development of a Nomogram Integrating Modified Inflammation-Based Indexes for Predicting Overall Survival in Pancreatic Cancer: A Retrospective Study
Authors Qin D , Huang K, Yao Z , Xi P , Jiang L, Wei R, Li S
Received 27 January 2025
Accepted for publication 29 March 2025
Published 7 April 2025 Volume 2025:18 Pages 4813—4830
DOI https://doi.org/10.2147/JIR.S519779
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Dailei Qin,* Kewei Huang,* Zehui Yao,* Pu Xi, Lingmin Jiang, Ran Wei, Shengping Li
State Key Laboratory of Oncology in South China, Guangdong Provincial ClinicalResearch Center for Cancer, Department of Hepatobiliary and Pancreatic Surgery, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ran Wei; Email [email protected] Shengping Li, Email [email protected]
Background: Pancreatic cancer (PCA) is a highly malignant tumor with a 5-year survival rate of < 10%. It is characterized as a cold tumor with an immunosuppressive microenvironment. Liver dysfunction due to biliary obstruction can affect the inflammation index, an indicator of immune status. Adjusting inflammation indices for liver function may enhance their clinical utility for predicting overall survival (OS) in PCA patients.
Methods: Resected PCA cases were selected using specific criteria. Liver function indicators identified by Spearman’s analysis were integrated into a covariance analysis to refine inflammation indices, including modified neutrophil-to-lymphocyte ratio (mNLR), modified platelet-to-lymphocyte ratio (mPLR), modified lymphocyte-to-monocyte ratio (mLMR), modified systemic immune-inflammation index (mSII), and modified C-reactive protein (mCRP). These modified indices and clinicopathological factors were analyzed to identify independent OS predictors. A nomogram was developed and compared with a primary inflammation-based model using calibration curves, decision curve analysis (DCA), and the concordance index (C-index).
Results: Liver function indicators including direct bilirubin (DBIL), indirect bilirubin (IBIL), alanine aminotransferase (ALT), and aspartate aminotransferase (AST), lactate dehydrogenase (LDH), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), and albumin (ALB) were integrated to refine inflammation indices. In PCA patients, higher mNLR, mSII, CA19-9, T stage, and N stage were associated with worse OS, while higher mLMR or PNI levels correlated with better OS. Vascular invasion predicted poor OS, whereas chemotherapy improved OS. The nomogram model’s clinical utility surpassed that of the primary inflammation-based model.
Conclusion: The nomogram incorporating modified inflammation indices demonstrated superior clinical utility. Adjusting inflammation indices for liver function is recommended for prognostic prediction, especially in PCA patients with biliary obstruction. For patients with advanced T and N staging or poorly differentiated tumors, intraoperative margin nanoknife ablation and timely postoperative adjuvant chemotherapy are recommended to enhance prognosis.
Keywords: pancreatic cancer, modified inflammation indexes, nomogram, overall survival
Introduction
PCA is a malignant tumor with a poor prognosis, characterized by an alarmingly low 5-year OS rate of only 7%.1 Despite its relatively low incidence, PCA is the fourth leading cause of cancer-related deaths in the United States.2 It has been suggested that PCA will surpass breast and colorectal cancers to become the second-most common cause of cancer - related death by 2030.3 Surgical resection remains the cornerstone of curative treatment for PCA; however, even among patients who undergo successful resection, the 5-year OS rate improves only modestly to 20–30%.4 Early recurrence within 12 months of curative resection is common, occurring in up to 80% of patients, and significantly contributes to the poor prognosis.5
The TME of PCA is often characterized by an immunosuppressive situation, which facilitates tumor cells’ evasion of immune surveillance.6 Immunosuppressive cells, such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs), infiltrate the tumor site, secreting immunosuppressive cytokines like TGF-β and IL-10. These cytokines inhibit the function of cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells, thereby dampening the antitumor immune response.7,8 The neutrophil-to-lymphocyte ratio (NLR) is a systemic inflammatory marker that reflects the balance between pro-inflammatory neutrophils and anti-inflammatory lymphocytes.9 In the presence of an immunosuppressive microenvironment, the recruitment and activation of neutrophils are often enhanced, while lymphocyte function is suppressed.10,11 This shift leads to an elevated NLR, which has been associated with poor prognosis in various cancers, including PCA.12 The immunosuppressive microenvironment can also drive the expression of genes associated with a high NLR, further exacerbating the inflammatory state.13 Moreover, the immunosuppressive microenvironment can influence other inflammatory markers such as the platelet-to-lymphocyte ratio (PLR) and the systemic immune inflammation index (SII).11 These markers are similarly affected by the presence of immunosuppressive cells and cytokines, leading to a systemic inflammatory response that correlates with disease progression and treatment outcomes.14 Recent studies have explored the prognostic value of combining these inflammatory markers with clinical and pathological features to develop nomograms for personalized risk stratification in PCA patients undergoing various treatments.14–16
However, in clinical practice, many PCA patients often present with preoperative biliary obstruction, leading to abnormal liver function.17 This condition can further trigger systemic inflammatory responses, thereby interfering with the levels of inflammatory markers in laboratory tests.18 As a result, the prognostic value of these inflammatory markers may be compromised. Therefore, correcting clinical inflammatory markers to eliminate the impact of abnormal liver function may further enhance their prognostic predictive power in PCA patients.
To address this challenge, we used Spearman’s analysis to identify liver function indicators affecting the inflammation index, and then introduced them into covariance analysis to obtain the modified inflammation index. The modified indices, along with tumor-related factors, such as T and N stages, were incorporated into the Cox analysis to identify independent risk factors associated with OS. Based on these independent risk factors, we developed a nomogram model. The modified model exhibited superior OS prediction capability compared to traditional models. This nomogram model can assist clinicians in preliminarily estimating the postoperative OS of PCA patients, especially for cases with preoperative obstructive jaundice. However, gene markers related to PCA are not included in this model. Future studies could integrate genetic and molecular markers, such as KRAS and TP53 mutations, holds promise for refining survival predictions in PCA patients.
Materials and Methods
Patient Enrollment, Grouping, and Ethical Considerations
We retrospectively collected data from patients who underwent radical resection for PCA between January 2008 and December 2019 from our medical records (Figure 1). To ensure the validity of the subsequent statistical analysis, we will adjust the total sample size of the study based on the 10 EPV principle.19,20 Prior to surgery, a mandatory departmental discussion was conducted to meticulously identify and minimize potential surgical risks. For cases with ambiguous clinical diagnoses, the definitive treatment plan was typically formulated through a multidisciplinary team (MDT) approach, with computed tomography (CT), magnetic resonance imaging (MRI), and pathological reports serving as the primary references for MDT deliberations. Notably, the radical surgeries were performed by three senior chief physicians, with participation from attending physicians and residents. The specific surgical approaches, including open surgery, laparoscopic-assisted, and robotic-assisted procedures, were determined based on the tumor stage and the patient’s physical condition and were implemented only with the patient’s full consent.
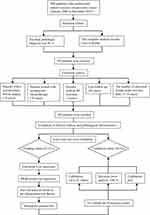 |
Figure 1 Patient enrollment and analysis workflow. |
In this study, we obtained informed consent from all participants for the use of their medical records. All procedures involving human participants were conducted in strict accordance with the Helsinki Declaration. Additionally, the study was granted approval by the hospital’s ethics committee for the retrospective analysis that was performed.
Inclusion and Exclusion Criteria
Patients were included in the study if they met the following criteria: (1) pathological diagnosis of PCA and (2) availability of complete medical records. Conversely, exclusion criteria included: (1) presence of a second primary tumor prior to surgery, (2) receipt of neoadjuvant chemotherapy, (3) lack of R0 resection (defined as a margin of 1.5–2 mm in a previous study), (4) loss to follow-up, and (5) fewer than 15 lymph nodes dissected.21–23 Following the application of these criteria, patients were assigned to training and validation cohorts using the leave-one-out cross-validation (LOOCV) method. Specifically, the screening protocol allocated one case to the validation cohort in each iteration, with the remaining cases forming the training set. The predictive accuracy of the model was then evaluated and recorded using analytical tools. This iterative process continued until all subjects had been assessed in the validation set. Optimal grouping strategies were subsequently determined based on the mean accuracy of the outcomes².
Acquisition of Modified-Inflammation Indexes
In the present study, we comprehensively evaluated a panel of inflammation indexes, including the NLR, PLR, SII, LMR, CRP. To investigate the potential associations between hepatic function indicators and these inflammation indexes, Spearman correlation analysis was conducted. Specifically, the hepatic function indicators examined encompassed DBIL, IBIL, AST, ALT, ALP, GGT, ALB. Subsequently, hepatic function indicators that exhibited significant positive correlations with the inflammation indexes were further incorporated into an analysis of covariance (ANCOVA) to adjust and refine the inflammation indexes.
Collection of Clinicopathological Characteristics
In this study, the selection of clinicopathological factors was guided by a previous study focusing on prognostic analyses. Specifically, the pathological factors examined included tumor size, tumor differentiation, lymph node metastasis, venous invasion, and lymphatic invasion. Additionally, clinical factors such as chemotherapy, carbohydrate antigen 19–9 (CA19-9), carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), and diabetes were incorporated into the analysis.
It is important to note that the chemotherapy regimens used in this study were administered in accordance with the recommendations from the NCCN guidelines for PCA patients (2021 Ver. 2.0). Prior to initiating chemotherapy, patients’ preferences and Eastern Cooperative Oncology Group Performance Status (ECOG PS) were considered. For patients with good physical condition (ECOG PS 0–1), FOLFIRINOX (oxaliplatin, irinotecan, calcium folinate, fluorouracil), AG (nab-paclitaxel, gemcitabine), GS (gemcitabine, tegafur) or monotherapy with tegafur were preferred. In contrast, for patients with poor overall physical condition (ECOG PS 2–5), gemcitabine or tegafur was recommended.
Follow-up
Follow-up commenced within the first month following discharge. Patients were scheduled for tri-monthly outpatient reviews to monitor their postoperative recovery and disease status. During each review, routine assessments included postoperative abdominal and chest CT scans, as well as serum evaluations of CA19-9, CA125, and CEA. For patients with limitations in accessing outpatient services, telephonic follow-ups were employed as an alternative method to ensure continuous monitoring and support. The follow-up period extended from the time of study enrollment until the occurrence of loss to follow-up, mortality, or the final patient contact.
Statistical Analysis
The characteristics of the training and validation groups were compared using the chi-square test. Multivariate Cox regression analysis was employed to identify independent prognostic factors for OS. The relationship between these independent risk factors and OS was further explored using Kaplan-Meier methods. Specifically, the Log rank test was used when survival curves did not intersect, whereas landmark analysis was applied in cases where survival curves intersected. The predictive accuracy of the nomogram based on the modified inflammation indexes was assessed by comparing it with the nomogram based on the primary inflammation indexes using C-indexes, calibration plots, and DCA. A two-tailed p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 22 and R software version 4.2.2 (R Development Core Team; http://www.r-project.org).
Nomogram Construction and Validation
The nomogram is constructed using R packages such as rms, survival, regplot, nomogramFormula, and SvyNomv. To evaluate its validity, we employed the C - index, calibration curves, and decision curve analysis (DCA). The C - index, ranging from 0.5 to 1, assesses the model’s ability to rank individuals by their event risk, with higher values indicating better performance. Calibration curves show the match between predicted probabilities and observed outcomes, with deviations from the ideal line suggesting miscalibration. Decision curve analysis evaluates the model’s clinical utility by plotting net benefit against threshold probability, with a higher area under the curve (AUC) indicating better utility.
Results
Patient’s Enrollment and Grouping
A total of 394 PCA patients underwent radical surgery from January 2008 to December 2019, with 358 cases meeting the inclusion criteria. 56 cases were excluded per the exclusion criteria: pre-surgical second tumors (15 cases), neo-adjuvant chemotherapy (10 cases), no R0 resection (2 cases), lost to follow-up (20 cases), and fewer than 15 dissected lymph nodes (9 cases). Ultimately, 302 PCA patients were included in the study. The patients were then split into a training cohort (211 cases) and a validation cohort (91 cases) via LOOCV (Figure 1). For the entire cohort, median OS was 28.7 months, with a five-year survival rate of 37.2%. The raw data of the patients enrolled in this study can be found in supplementary Table 1: Training and validation cohort.
Modified Inflammation Indexes and Clinicopathological Factors
As presented in Table 1, a comprehensive analysis of hepatic function indicators revealed significant correlations with inflammation indexes. The ANCOVA was conducted to modify inflammation indexes, the Quantile-Quantile plot (QQ plot) and residual plot were used to detect the efficacy and statistical validity of the corrected model. The strong calibration ability of the ANCOVA model was evidenced by the high concordance of the QQ plot as well as the p-value derived from the residual plot for CRP (Figure 2A), LMR (Figure 2B), NLR (Figure 2C), PLR (Figure 2D), SII (Figure 2E), indicating a robust adjustment for confounding factors. Furthermore, the receiver operating characteristic (ROC) were made for evaluating the performance of binary classification models. The results shown that the ROC model based on the mCRP own the stronger discrimination ability when compared with the model based on the CRP (Figure 3A and 3B), the same results were estimated in the group of mLMR VS LMR (Figure 3C and D), mNLR VS NLR (Figure 3E and F), mPLR VS PLR (Figure 3G and 3H), mSII VS SII (Figure 3I and J). Meanwhile, the ROC of PNI was also conducted for the subsequently binary classification (Figure 3K). Based on the results summarized in the baseline table (Table 2), significant differences were observed between the training and validation cohorts for several indicators, including mNLR, mLMR, mSII, and CA19-9.
 |
Table 1 Correlation Analysis Between Inflammatory and Biochemical Indicators |
 |
Table 2 Baseline of 302 Resected PCA Patients |
Prognostic Factors for OS in PCA Patients
In the present study, a total of 20 indicators, encompassing both inflammation-related and clinicopathological factors, were initially subjected to univariate Cox regression analysis (Table 3). Of these, 12 factors were identified as significantly associated with OS and were subsequently included in the multivariate Cox regression analysis. Ultimately, tumor differentiation, venous invasion, CA19-9, mNLR, mLMR, mSII, chemotherapy, and PNI were determined to be independent risk factors for OS in patients with pancreatic cancer (PCA) following surgical resection (Table 4).
 |
Table 3 Univariate Cox Analysis of Inflammatory and Clinicopathological Factors |
 |
Table 4 Multivariate Cox Analysis of Inflammatory and Clinicopathological Factors |
Survival Analysis for Independent Prognostic Factors
The independent risk factors identified through multivariate Cox regression analysis were incorporated into the survival analysis. The results demonstrated that PCA patients with higher grade of the T stage (Figure 4A), N stage (Figure 4B), CA19-9 (Figure 4E), mNLR (Figure 4H), and mSII (Figure 4I) exhibited significantly poorer OS compared to those with lower values. Conversely, patients with higher values of the mLMR (Figure 4G) and PNI (Figure 4J) had significantly better OS compared to those with lower values. The presence of vascular invasion was associated with a shorter OS (Figure 4D). PCA patients who received adjuvant chemotherapy had improved OS compared to those who did not receive treatment (Figure 4F). Well-differentiated tumors were associated with better OS compared to poorly differentiated tumors.
Construction of Nomogram Model
A nomogram for predicting OS in resected PCA patients was constructed based on the independent risk factors identified through multivariate Cox regression analysis (Figure 5). As illustrated in Figure, the T stage was the most influential factor affecting OS in PCA patients, followed by chemotherapy, N stage, mSII, venous invasion, CA19-9, mNLR, mLMR, and PNI. Additionally, this nomogram model can be utilized to predict survival rates at 1, 3, and 5 years following radical resection.
 |
Figure 5 Nomogram based on the modified inflammation indexes. |
Comparison of Predictive Abilities Between the Modified and Primary Nomograms
The results of calibration curves demonstrated that the predicted survival rates at 1, 3, and 5 years generated by the nomogram model closely approximated the actual survival in the training cohort (Figure 6A–C). These findings were consistently replicated in the validation cohort (Figure 6D–F). Moreover, DCA indicated that the nomogram incorporating modified inflammatory indices exhibited a higher clinical benefit compared to the primary model (Figure 6G). This superiority was further confirmed in the DCA of the validation cohort (Figure 6H). Additionally, the modified nomogram demonstrated a significantly higher C-index compared to the primary model, a finding that was also consistently observed in the validation cohort (Figure 6I).
Discussion
PCA is currently the fourth leading cause of cancer-related mortality and is projected to become the second most common cause of cancer death by 2030.3 Although surgical resection remains the only potentially curative treatment, only 19–22% of patients achieve 5-year survival, primarily due to local or distant recurrence.24 To date, various prognostic factors for overall survival in resected PCA patients have been identified, including lymph node involvement, lymphatic vascular and perineural invasion, tumor grade, portal vein and mesenteric artery involvement, positive resection margins, and inflammation-based biomarkers.25,26
PCA is characterized as an immune-cold tumor, with a TME predominantly infiltrated by immunosuppressive cells.27 Inflammation-based biomarkers reflect the systemic immune status and are closely associated with the immune cell composition within the TME.28 In detail, neutrophils, platelets, lymphocytes, and monocytes are key regulators of tumor growth and recurrence.29 Neutrophils can transition from an anti-tumor phenotype to a pro-tumorigenic state, thereby promoting inflammation and immunosuppression.30 Platelets support tumor proliferation and metastasis by protecting cancer cells and facilitating angiogenesis.31 Lymphocytes, particularly tumor-infiltrating lymphocytes, are essential for anti-tumor immunity, with higher counts often correlating with better prognosis.32 Monocytes contribute to the tumor microenvironment by differentiating into tumor-associated macrophages, which secrete pro-inflammatory cytokines and support tumor growth.33,34 Consequently, elevated NLR and PLR values are typically associated with poorer clinical outcomes, as they suggest a weakened immune response and increased tumor burden.
In the present study, we employed covariance analysis to adjust for the influence of abnormal liver function on inflammation-based biomarkers mentioned above, and we observed that higher values of the mNLR and mSII predicted poorer OS in PAC patients compared to those with lower values. Conversely, higher mLMR values were associated with better OS compared to those with lower values. Moreover, the nomogram constructed based on the modified inflammatory indices demonstrated superior predictive ability for OS compared to the primary model. Based on the results of this study, the use of adjusted inflammatory indices can better predict the prognosis of pancreatic cancer, particularly in cases with preoperative obstructive jaundice. Additionally, these markers reflect the systemic inflammatory status and immune response, helping to identify patients who may benefit most from immunotherapy.21 Therefore, for those PCA patients with high mSII, mNLR and low mLMR, using immunomodulators and immune checkpoint inhibitors may be more effective.35–37
The T stage, which denotes tumor dimensions as determined by pathologists, serves as a critical indicator of tumor burden in pancreatic cancer.38 Variations in tumor size are attributed to differences in genetic mutations, tumor microenvironment, and the presence of growth factors.39 For instance, mutations in the KRAS oncogene, which are highly prevalent in pancreatic cancer, can drive rapid tumor growth by promoting cell proliferation and survival.40 A higher T stage often correlates with an increased likelihood of drug-resistant clones within the tumor, thereby contributing to poorer OS.41 In the present study, we also identified that a higher T stage predicted a shorter OS in pancreatic cancer patients. To address this challenge, additional therapies such as intraoperative radiotherapy or targeted interventions like nano knife ablation, focusing on resection margins should be recommended for reducing local recurrence rates and improve prognosis in patients with large tumor volumes. Furthermore, neoadjuvant chemotherapy is increasingly recommended for patients with higher T stages to diminish tumor size and invasion, facilitate radical resection, and minimize residual disease, thereby potentially prolonging postoperative survival.
Epithelial-mesenchymal transition (EMT) is a critical process in pancreatic cancer, endowing tumor cells with enhanced migratory and invasive capabilities, thereby facilitating their entry into the lymphatic system.42 Upon reaching the lymph nodes, these tumor cells interact with the local microenvironment, including immune cells and stromal cells, ultimately establishing secondary tumors.43 Numerous studies have established the tumor N stage as a robust predictor of tumor progression in PCA.44 The Japanese Pancreatic Society has further emphasized that metastasis to specific lymph node regions, such as N9 and N16, is closely associated with tumor relapse and distant metastasis.45,46 Consistent with these findings, our study demonstrated that a higher N stage is significantly correlated with diminished OS in PCA patients. Given this correlation, PCA patients with a higher N stage may derive substantial benefits from adjuvant chemotherapy, which has the potential to improve OS outcomes.47,48
Tumor cell invasion into the venous system facilitates hematogenous dissemination, thereby increasing the likelihood of distant metastases, particularly to the liver.49,50 Once tumor cells enter the circulation as circulating tumor cells (CTCs) or via extracellular vesicle (EV), they can colonize distant organs, thus perpetuating the metastatic cascade.51,52 The presence of venous invasion typically indicates a more advanced stage of disease, often precluding complete surgical resection and significantly increasing the risk of recurrence.52 This underscores the importance of venous invasion as a critical consideration for postoperative management. In our study, we observed a significant correlation between venous invasion and poor OS in PCA patients. Given the heightened risk of recurrence and metastasis associated with vascular invasion, we recommend that PCA patients with venous invasion promptly initiate adjuvant chemotherapy following surgical resection to optimize therapeutic outcomes in this high-risk cohort.
Poorly differentiated tumors are more aggressive, metastasize more readily, and are associated with worse survival outcomes.53 Recent studies have highlighted the roles of transcription factors (TFs) and epigenetic regulators in driving differentiation status and aggressiveness. For example, FOXA1 reprograms enhancer landscapes to promote metastasis by activating foregut developmental genes, while EVI1 activates superenhancers to upregulate tumorigenic and metastatic genes.54 Consistent with these findings, our study observed that PCA patients with poorly differentiated tumors had worse OS compared to those with well or moderately differentiated tumors. Therefore, for patients with poorly differentiated tumors, genetic testing to identify molecular targets, combined with targeted therapy and systemic chemotherapy, may improve prognosis.
CA19-9 is expressed on the surface of tumor cells and can interact with immune cells, contributing to immune evasion and tumor progression.55,56 Single-cell sequencing studies have revealed that CA199 expression is associated with specific tumor cell subpopulations and immune microenvironmental changes, including the presence of pro-tumorigenic macrophages and upregulation of IL-6 signaling pathways.57 In clinical practice, CA19-9 is not only a diagnostic marker but also a valuable tool for monitoring treatment response and predicting outcomes in pancreatic cancer. Our study also demonstrated that higher levels of CA199 were significantly associated with shorter OS in pancreatic cancer patients. Elevated preoperative CA19-9 levels, particularly when combined with a large primary tumor or positive lymph nodes on CT imaging, may indicate advanced disease and poor prognosis. For such patients, neoadjuvant chemotherapy followed by radical surgery is recommended. This approach can downstage the tumor, improve resectability, and potentially enhance long-term survival outcomes.
Adjuvant chemotherapy is pivotal in preventing recurrence and improving prognosis in pancreatic cancer by targeting residual micrometastases and enhancing OS.58,59 Adjuvant chemotherapy directly targeting circulating tumor cells and micrometastases that may be undetectable at the time of surgery.60 By eliminating these residual cancer cells, adjuvant chemotherapy reduces the risk of local and distant recurrence, thereby prolonging disease-free survival (DFS) and OS. Moreover, adjuvant chemotherapy modulates the tumor microenvironment by reducing the density of cancer-associated fibroblasts (CAFs) and enhancing the efficacy of subsequent therapies.61 This approach also enhances immune surveillance and supporting the body’s natural defense mechanisms against cancer recurrence.27 Consistent with these mechanisms, our study identified adjuvant chemotherapy as a significant protective factor for OS in pancreatic cancer patients.
There are some limitations in the present study. Firstly, selection bias is a significant potential confounder in this analysis. In detail, patients with PCA and a history of stroke or coronary artery disease were less likely to be readmitted due to increased surgical risk, as compared to those with better physical conditions. This factor may constrain the nomogram’s predictive power in PCA patients with poor physical status. Additionally, the investigation was conducted using a single-center retrospective design, which may limit the generalizability of our findings due to regional variations in clinical practice. To estimate the predictive capacity of the nomogram developed in this study, a multi-center, controlled study is needed. Furthermore, the cut-off values mentioned in this article are proposed based on the data from our center. To further enhance the applicability of the model, it is necessary to conduct further clinical practice to determine cut-off values that are more clinically practical.
In clinical research, nomograms have become increasingly popular due to their ability to provide clear and mathematically sound predictive models. They are especially useful for turning complex data into easily understood info for clinical decisions. By combining various factors like patient details, clinical traits, and imaging, nomograms create personalized risk assessments or outcome predictions. There is published literature on the use of nomograms for prognosis prediction in pancreatic cancer patients with obstructive jaundice. However, some literature indicates that when patients have excessively high bilirubin levels, the accuracy of the prognostic model will significantly decrease. In the present study, the nomogram model based on the modified inflammation indexes as well as the clinicopathological factors showed better prediction capacity when compare with primary nomogram model. Therefore, this modified model may have a better predictive role in the prognosis of pancreatic cancer patients with obstructive jaundice in clinical practice.
In clinical research, nomograms have gained increasing favor due to their capacity to deliver clear and mathematically reliable predictive models.62 They are particularly adept at transforming complex data into easily digestible information for clinical decision-making.63 By synthesizing various factors, including patient details, clinical characteristics, and imaging findings, nomograms generate personalized risk assessments or outcome predictions.64 At present, the application of nomograms in PCA patients with obstructive jaundice has been documented in the literature.65 However, some studies have shown that when bilirubin levels are excessively high, the accuracy of prognostic models, such as those using CA199 and SII, will significantly decrease.66–68 In our current study, the nomogram model, which is based on modified inflammation indices as well as clinicopathological factors, demonstrated superior predictive ability compared to the original nomogram model. Hence, this modified model is expected to play a more effective predictive role in the prognosis of PCA patients with obstructive jaundice in clinical practice.
There are certain limitations in this study. First, selection bias is a notable potential source of confusion in this analysis. Specifically, patients with PCA and a history of coronary artery disease were less likely to be readmitted compared to those in better physical condition due to increased surgical risks. This factor may limit the predictive power of the nomogram for PCA patients with poor physical status. In addition, the study was conducted using a single-center retrospective design, which may limit the generalizability of our findings due to regional differences in clinical practice. To assess the predictive ability of the nomogram developed in this study, a multi-center, controlled study is required. Moreover, the cut-off values discussed in this article are based on data from our center. To further improve the applicability of the model, it is necessary to carry out further clinical practice to establish cut-off values that are more clinically practical.
Conclusion
In PCA patients, high mSII and mNLR are linked to worse OS, while a high mLMR suggests better OS. For those with high mSII, mNLR and low mLMR, using immunomodulators and immune checkpoint inhibitors may be more effective. Also, the nomogram with these modified inflammatory markers forecasts OS better than the original one. So, for PCA patients with obstructive jaundice, this modified model may better predict prognosis. Furthermore, advanced T and N stages, high CA19-9 levels, and venous invasion are associated with poor OS. For patients with these bad prognostic factors, adding chemotherapy or trying IRE margin treatment might help improve OS outcomes.
Data Sharing Statement
The raw data has been uploaded as supplementary material: Training and validation cohort.
Ethical Approval and Consent to Participate
The studies involving human participants were reviewed and approved by Institutional Review Board of Sun Yat-sen University Cancer Center.
Author Contributions
All authors have substantially contributed to the work reported, be it in the conception, study design, execution, data acquisition, analysis and interpretation, or in all these aspects; they have participated in drafting, revising, or critically reviewing the article; granted final approval for the publication of the version; reached a consensus on the journal to which the article has been submitted; and agreed to be answerable for all aspects of the work.
Consent for Publication
The writing informed were obtained from all of the patients/participants in this study.
Funding
Cancer Innovative Research Program of Sun Yat-sen University Cancer Center 308-Program for Clinical Research of Sun Yat-sen University Cancer Center National Natural Science Funds (Nos. 81972299 and 81672390) National Key Research and Development Plan (No. 2017YFC0910002).
Disclosure
All authors in the present study declare that they have no conflicts of interest.
References
1. Sung H, Ferlay J, Siegel R, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 Countries. Ca a Cancer J Clini. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca a Cancer J Clini. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Rahib L, Smith B, Aizenberg R, Rosenzweig A, Fleshman J, Matrisian L. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi:10.1158/0008-5472.Can-14-0155
4. Kamisawa T, Wood L, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388(10039):73–85. doi:10.1016/s0140-6736(16)00141-0
5. Groot V, Rezaee N, Wu W, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018;267(5):936–945. doi:10.1097/sla.0000000000002234
6. Falcomatà C, Bärthel S, Schneider G, Rad R, Schmidt-Supprian M, Saur D. Context-specific determinants of the immunosuppressive tumor microenvironment in pancreatic cancer. Cancer Discov. 2023;13(2):278–297. doi:10.1158/2159-8290.Cd-22-0876
7. Vilbois S, Xu Y, Ho PC. Metabolic interplay: tumor macrophages and regulatory T cells. Trends Cancer. 2024;10(3):242–255. doi:10.1016/j.trecan.2023.11.007
8. Mirlekar B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: its implications in cancer immunotherapy. SAGE Open Med. 2022;10:20503121211069012. doi:10.1177/20503121211069012
9. Iwai N, Okuda T, Sakagami J, et al. Neutrophil to lymphocyte ratio predicts prognosis in unresectable pancreatic cancer. Sci Rep. 2020;10(1):18758. doi:10.1038/s41598-020-75745-8
10. Xiang ZJ, Hu T, Wang Y, Wang H, Xu L, Cui N. Neutrophil-lymphocyte ratio (NLR) was associated with prognosis and immunomodulatory in patients with pancreatic ductal adenocarcinoma (PDAC). Biosci Rep. 2020;40(6). doi:10.1042/bsr20201190
11. Maggiorani D, Le O, Lisi V, et al. Senescence drives immunotherapy resistance by inducing an immunosuppressive tumor microenvironment. Nat Commun. 15(1):2435. doi:10.1038/s41467-024-46769-9
12. Asaoka T, Miyamoto A, Maeda S, et al. Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology. 2016;16(3):434–440. doi:10.1016/j.pan.2015.10.006
13. Cheng H, Long F, Jaiswar M, Yang L, Wang C, Zhou Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis. Sci Rep. 2015;5:11026. doi:10.1038/srep11026
14. Wang J, Yang J, Narang A, et al. Consensus, debate, and prospective on pancreatic cancer treatments. J Hematol Oncol. 2024;17(1):92. doi:10.1186/s13045-024-01613-x
15. Yifan Y, Shaoqi Z, Yongqiang H. Nomogram for prognosis prediction in metastatic pancreatic cancer patients undergoing intra-arterial infusion chemotherapy: incorporating immune-inflammation scores and coagulation indicators. BMC Cancer. 2025;25(1). doi:10.1186/s12885-025-13523-3
16. Huifen S, Fei Z. Prognostic role of systemic inflammation response index (SIRI) in patients with pancreatic cancer: a meta-analysis. Front Oncol. 2024;14. doi:10.3389/fonc.2024.1465279
17. Lee PJ, Podugu A, Wu D, Lee AC, Stevens T, Windsor JA. Preoperative biliary drainage in resectable pancreatic cancer: a systematic review and network meta-analysis. HPB. 2018;20(6):477–486. doi:10.1016/j.hpb.2017.12.007
18. Sasahira N, Hamada T, Togawa O, et al. Multicenter study of endoscopic preoperative biliary drainage for malignant distal biliary obstruction. World J Gastroenterol. 2016;22(14):3793–3802. doi:10.3748/wjg.v22.i14.3793
19. Steyerberg EW, Eijkemans MJ, Harrell FE Jr, Habbema JD. Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Med Decis Making. 2001;21(1):45–56. doi:10.1177/0272989x0102100106
20. Steyerberg EW, Eijkemans MJ, Harrell FE Jr, Habbema JD. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19(8):1059–1079. doi:10.1002/(sici)1097-0258(20000430)19:8
21. Wang Z, Xu B, Wang L, et al. Platelet-to-lymphocyte ratio predicts tumor response and survival of patients with hepatocellular carcinoma undergoing immunotherapies. Int Immunopharmacol. 2024;131:111863. doi:10.1016/j.intimp.2024.111863
22. Werner H, V CM, Abe F, et al. Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS). Surgery. 2014;156(1). doi:10.1016/j.surg.2014.02.009
23. S MB, C DC, John LC, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2007;15(1). doi:10.1245/s10434-007-9587-1
24. Chen X, Liu F, Xue Q, Weng X, Xu F. Metastatic pancreatic cancer: mechanisms and detection (Review). Oncol Rep. 2021;46(5). doi:10.3892/or.2021.8182
25. Karamitopoulou E. Emerging prognostic and predictive factors in pancreatic cancer. Mod Pathol. 2023;36(11):100328. doi:10.1016/j.modpat.2023.100328
26. Lei H, Quanli H, Liangchao Z, Zhikuan W, Guanghai D, Yan S. Development and validation of the predictive and prognostic chemoresist signature in resected pancreatic ductal adenocarcinoma: multicenter study. Ann Surg. 2024. doi:10.1097/sla.0000000000006610
27. Tang R, Xu J, Wang W, et al. Targeting neoadjuvant chemotherapy-induced metabolic reprogramming in pancreatic cancer promotes anti-tumor immunity and chemo-response. Cell Rep Med. 2023;4(10):101234. doi:10.1016/j.xcrm.2023.101234
28. Ho W, Jaffee E, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17(9):527–540. doi:10.1038/s41571-020-0363-5
29. Gautam SK, Batra SK, Jain M. Molecular and metabolic regulation of immunosuppression in metastatic pancreatic ductal adenocarcinoma. mol Cancer. 2023;22(1):118. doi:10.1186/s12943-023-01813-y
30. Wu G, Pan B, Shi H, et al. Neutrophils’ dual role in cancer: from tumor progression to immunotherapeutic potential. Int Immunopharmacol. 2024;140:112788. doi:10.1016/j.intimp.2024.112788
31. Garcia-Leon MJ, Liboni C, Mittelheisser V, et al. Platelets favor the outgrowth of established metastases. Nat Commun. 2024;15(1):3297. doi:10.1038/s41467-024-47516-w
32. Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169(5):2756–2761. doi:10.4049/jimmunol.169.5.2756
33. Elewaut A, Estivill G, Bayerl F, et al. Cancer cells impair monocyte-mediated T cell stimulation to evade immunity. Nature. 2025;637(8046):716–725. doi:10.1038/s41586-024-08257-4
34. Kzhyshkowska J, Shen J, Larionova I. Targeting of TAMs: can we be more clever than cancer cells? Cell mol Immunol. 2024;21(12):1376–1409. doi:10.1038/s41423-024-01232-z
35. Rojas LA, Sethna Z, Soares KC, et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature. 2023;618(7963):144–150. doi:10.1038/s41586-023-06063-y
36. Faget J, Peters S, Quantin X, Meylan E, Bonnefoy N. Neutrophils in the era of immune checkpoint blockade. J Immunother Cancer. 2021;9(7). doi:10.1136/jitc-2020-002242
37. Alexander GR, Ilenia P, Carmine FP, et al. Cytotoxic chemotherapy potentiates the immune response and efficacy of combination CXCR4/PD-1 inhibition in models of pancreatic ductal adenocarcinoma. bioRxiv. 2024. doi:10.1101/2023.12.24.573257
38. My Linh T, Maia Blomhoff H, Caroline Sophie V. Tumour size and t-stage in pancreatic cancer resection specimens depend on the pathology examination approach. Cancers. 2022;14(10). doi:10.3390/cancers14102471
39. Mo CK, Liu J, Chen S, et al. Tumour evolution and microenvironment interactions in 2D and 3D space. Nature. 2024;634(8036):1178–1186. doi:10.1038/s41586-024-08087-4
40. Qian Y, Gong Y, Fan Z, et al. Molecular alterations and targeted therapy in pancreatic ductal adenocarcinoma. J Hematol Oncol. 2020;13(1):130. doi:10.1186/s13045-020-00958-3
41. Ibiayi D-J, Alice TS. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2017;15(2). doi:10.1038/nrclinonc.2017.166
42. Xiaofeng Z, C JL, Jiha K, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579). doi:10.1038/nature16064
43. Paiella S, Sandini M, Gianotti L, Butturini G, Salvia R, Bassi C. The prognostic impact of para-aortic lymph node metastasis in pancreatic cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2016;42(5):616–624. doi:10.1016/j.ejso.2016.02.003
44. Wang Y, Qin C, Zhao B, et al. EGR1 induces EMT in pancreatic cancer via a P300/SNAI2 pathway. J Transl Med. 2023;21(1):201. doi:10.1186/s12967-023-04043-4
45. Usefulness of Japanese staging in the prognosis of patients treated operatively for adenocarcinoma of the head of the pancreas. J Am Coll Surg. 1996;182(1).
46. Liu C, Chen R, Chen Y, et al. Should a standard lymphadenectomy during pancreatoduodenectomy exclude para-aortic lymph nodes for all cases of resectable pancreatic head cancer? A consensus statement by the Chinese Study Group for Pancreatic Cancer (CSPAC). Int j Oncol. 2015;47(4):1512–1516. doi:10.3892/ijo.2015.3128
47. L CY, James M, Jeffrey E, et al. Clinical implications of extensive lymph node metastases for resected pancreatic cancer. Ann Surg Oncol. 2018;25(13). doi:10.1245/s10434-018-6763-4
48. van Roessel S, van Veldhuisen E, Klompmaker S, et al. Evaluation of Adjuvant Chemotherapy in Patients With Resected Pancreatic Cancer After Neoadjuvant FOLFIRINOX Treatment. JAMA Oncol.;6(11):1733–1740. doi:10.1001/jamaoncol.2020.3537
49. Wenchao X, Jianzhou L, Qiaofei L, et al. NFE2-driven neutrophil polarization promotes pancreatic cancer liver metastasis progression. Cell Rep. 2025;44(2). doi:10.1016/j.celrep.2024.115226
50. Chathura BBR, Nehal S, Benjamin L, John AW, Sanjay P. The impact of the depth of venous invasion on survival following pancreatoduodenectomy for pancreatic cancer: a meta-analysis of available evidence. J Gastrointest Cancer. 2019;51(2). doi:10.1007/s12029-019-00248-3
51. Jee-Soo L, Sung Sup P, Young Kyung L, Jeffrey AN, Stefanie SJ. Liquid biopsy in pancreatic ductal adenocarcinoma: current status of circulating tumor cells and circulating tumor DNA. mol Oncol. 2019;13(8). doi:10.1002/1878-0261.12537
52. Lanxiang H, Yaqi X, Na W, et al. Next-generation preclinical functional testing models in cancer precision medicine: CTC-derived organoids. Small Methods. 2023;8(1). doi:10.1002/smtd.202301009
53. Linara G-C, Aizhan S, Suraj P, et al. Cholesterol pathway inhibition induces TGF-β signaling to promote basal differentiation in pancreatic cancer. Cancer Cell. 2020;38(4). doi:10.1016/j.ccell.2020.08.015
54. Lei X, Xiao M, Xiuzhong Z, et al. hsa_circ_0007919 induces LIG1 transcription by binding to FOXA1/TET1 to enhance the DNA damage response and promote gemcitabine resistance in pancreatic ductal adenocarcinoma. mol Cancer. 2023;22(1). doi:10.1186/s12943-023-01887-8
55. Daniel Vasile B, Flavius Stefan M, George M, et al. Clinical characteristics and outcomes in carbohydrate antigen 19-9 negative pancreatic cancer. World J Clin Oncol. 2022;13(7). doi:10.5306/wjco.v13.i7.630
56. Mohsen A, Anish B, Mohsen S, et al. CA19-9 and CEA biosensors in pancreatic cancer. Clin Chim Acta. 2024;554. doi:10.1016/j.cca.2024.117788
57. Deyu Z, Fang C, Kailian Z, et al. Single-cell RNA sequencing reveals the process of CA19-9 production and dynamics of the immune microenvironment between CA19-9 (+) and CA19-9 (-) PDAC. Chin Med J (Engl). 2024;137(20). doi:10.1097/cm9.0000000000003130
58. William FR, Ross AA. Adjuvant therapy for pancreatic cancer: current status, future directions. Semin Oncol. 2006;33. doi:10.1053/j.seminoncol.2006.10.005
59. Marta H-V, Elizabeth H, Angel C, Luis B. Adjuvant and neoadjuvant treatment in pancreatic cancer. World J Gastroenterol. 2012;18(14). doi:10.3748/wjg.v18.i14.1565
60. B NC, G MUJE, C GA, et al. Perspectives of the medical oncologist regarding adjuvant chemotherapy for pancreatic cancer: an international expert survey and case vignette study. Eur J Surg Oncol. 2024;51(3). doi:10.1016/j.ejso.2024.109544
61. L BL, Daiyao Z, Carla H-L, et al. Engineered matrices reveal stiffness-mediated chemoresistance in patient-derived pancreatic cancer organoids. Nat Mater. 2024;23(8). doi:10.1038/s41563-024-01908-x
62. Cristina RF, Michael WK, James ST, Sarah PT, Murray FB, Andrew LW. Validation of a postresection pancreatic adenocarcinoma nomogram for disease-specific survival. J Clin Oncol. 2005;23(30). doi:10.1200/jco.2005.01.8101
63. Alessandra P, J AA, Luca L, et al. Multi-institutional development and external validation of a nomogram to predict recurrence after curative resection of pancreatic neuroendocrine tumors. Ann Surg. 2019;274(6). doi:10.1097/sla.0000000000003579
64. A MA, Carlos F-DC, Mohammad AE, et al. Development and validation of a multi-institutional preoperative nomogram for predicting grade of dysplasia in Intraductal Papillary Mucinous Neoplasms (IPMNs) of the pancreas: a report from the pancreatic surgery consortium. Ann Surg. 2017;267(1). doi:10.1097/sla.0000000000002015
65. Ziyun S, Zhiwei X, Weishen W, et al. A novel nomogram for predicting the risk of major complications after pancreaticoduodenectomy in patients with obstructive jaundice. Clin Chim Acta. 2021;517. doi:10.1016/j.cca.2021.02.018
66. Aziz MH, Sideras K, Aziz NA, et al. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels: a retrospective multicenter cohort study. Ann Surg. 2019;270(1):139–146. doi:10.1097/sla.0000000000002660
67. Anger F, Lock JF, Klein I, et al. Does concurrent cholestasis alter the prognostic value of preoperatively elevated CA19-9 serum levels in patients with pancreatic head adenocarcinoma? Ann Surg Oncol. 2022;29(13):8523–8533. doi:10.1245/s10434-022-12460-w
68. Boyd LNC, Ali M, Comandatore A, et al. Prediction model for early-stage pancreatic cancer using routinely measured blood biomarkers. JAMA Network Open.;6(8):e2331197. doi:10.1001/jamanetworkopen.2023.31197
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





