Back to Journals » Journal of Pain Research » Volume 18
Device Evaluation, Treatment, and Explantation Recommendations (DETER): Review and Best Practices for Managing Neuromodulation Device Infections
Authors Pritzlaff SG , Goree JH , Dare RK, D’Souza RS , Lee DW, Dudas AA, Kalia H , Orhurhu V, Singh N, Hagedorn JM , Mousavi A, James W, Leong MS, Meacham KW, Gulati A, Sheth SJ, Pena I, Shah JR, Murphy MZ, Nashi SE , Nasseri M, Khoury AM, Dorsi MJ, Falowski SM , Petersen EA , Tomycz ND, Wahezi S, Chakravarthy KV, Pope JE, Schatman ME , Amirdelfan K, Sayed D , Deer TR
Received 2 December 2024
Accepted for publication 2 April 2025
Published 23 April 2025 Volume 2025:18 Pages 2147—2161
DOI https://doi.org/10.2147/JPR.S509623
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Andrea Tinnirello
Scott G Pritzlaff,1 Johnathan Heck Goree,2 Ryan Keith Dare,3 Ryan S D’Souza,4 David W Lee,5 Andrew Adams Dudas,6 Hemant Kalia,7 Vwaire Orhurhu,8 Naileshni Singh,1 Jonathan Michael Hagedorn,4 Arman Mousavi,9 Whitney James,10 Michael Spencer Leong,11 Kathleen W Meacham,12 Amitabh Gulati,13 Samir J Sheth,14 Israel Pena,15 Jarna R Shah,2 Melissa Zhu Murphy,16 Sara E Nashi,17 Morad Nasseri,9 Andrew M Khoury,18 Michael J Dorsi,19 Steven Michael Falowski,20 Erika A Petersen,21 Nestor D Tomycz,22 Sayed Wahezi,23 Krishnan V Chakravarthy,24 Jason E Pope,25 Michael E Schatman,26,27 Kasra Amirdelfan,28 Dawood Sayed,29 Timothy Ray Deer30,31
1Anesthesiology and Pain Medicine, University of California, Davis, Sacramento, CA, USA; 2Anesthesiology, University of Arkansas for Medical Sciences, Little Rock, AR, USA; 3Internal Medicine/Division of Infectious Diseases, University of Arkansas for Medical Sciences, Little Rock, AR, USA; 4Anesthesiology and Perioperative Medicine, Mayo Clinic, Rochester, MN, USA; 5Fullerton Orthopedics, University of California, Fullerton, CA, USA; 6Mays and Schnapp Neurospine and Pain, Memphis, TN, USA; 7Center for Research & Innovation in Spine & Pain (CRISP), Rochester, NY, USA; 8Anesthesiology, University of Pittsburgh Medical Center, Williamsport, PA, USA; 9Neurology and Interventional Pain Medicine, Boomerang Healthcare, Walnut Creek, CA, USA; 10Neurosurgery, James-Marco Health, Prescott, AZ, USA; 11Anesthesiology, Perioperative and Pain Medicine, Stanford University, Stanford, CA, USA; 12Veteran’s Affairs Healthcare System, Saint Louis, MO, USA; 13Anesthesiology and Critical Care, Memorial Sloan Kettering Cancer Center, New York, NY, USA; 14Neurosciences, Sutter Health, Roseville, CA, USA; 15Pain Management, Lakeside Physicians, Granbury, TX, USA; 16North Texas Orthopedics and Spine Center, Dallas Fort Worth, TX, USA; 17Interventional Pain & Spine, TriHealth, Cincinnati, OH, USA; 18Advanced Spine and Pain Specialists, The Woodlands, TX, USA; 19Neurosurgery, University of California Los Angeles, Los Angeles, CA, USA; 20Neurosurgery, Neurosurgical Associates of Lancaster, Lancaster, PA, USA; 21Neurosurgery, University of Arkansas for Medical Sciences, Little Rock, AR, USA; 22Neurological Surgery, Allegheny Health Network, Pittsburgh, PA, USA; 23Rehabilitation Medicine, Montefiore Medical Center, New York, NY, USA; 24Anesthesiology and Pain Medicine, Solaris Research Institute, San Diego, CA, USA; 25Evolve Restorative Center, Santa Rosa, CA, USA; 26Department of Anesthesiology, Perioperative Care and Pain Medicine, NYU Grossman School of Medicine, New York, NY, USA; 27Department of Population Health - Division of Medical Issues, NYU Grossman School of Medicine, New York, NY, USA; 28Clinical Research, Boomerang Healthcare, Inc., Walnut Creek, CA, USA; 29Anesthesiology, The University of Kansas Health System, Kansas City, KS, USA; 30Anaesthesiology and Pain Medicine, West Virginia University School of Medicine, Charleston, WV, USA; 31Pain Services, WVU Medicine – Thomas Hospitals, Charleston, WV, USA
Correspondence: Scott G Pritzlaff, University of California, Davis, 4860 Y Street, Suite 3020, Sacramento, CA, 95817, USA, Email [email protected]
Abstract: Infections related to neuromodulation devices such as spinal cord stimulators (SCS) and intrathecal pumps (ITPs) present complex challenges due to potential complications such as localized infections, deep infections, sepsis, and neurological injury. Prompt diagnosis requires patients and providers to be educated on wound management and sepsis symptoms for immediate medical attention. Antibiotic therapy and duration vary based on infection severity, with deep infections often requiring device removal despite recent improvements in salvage rates with aggressive initial intervention. Deep infections necessitate timely diagnosis through imaging modalities such as magnetic resonance imaging (MRI) or computed tomography (CT), followed by device removal and culture-guided antibiotic therapy, often in collaboration with infectious disease specialists and spine surgeons. ITP infections pose similar challenges along with the risk of meningitis and may require careful management of medication withdrawal symptoms during emergent pump removal. Lab monitoring may aid treatment assessment, although negative cultures can occur due to post-antibiotic exposure. Postoperative recommendations stress standardized guidelines, patient education, and vigilant surveillance, with close follow-up crucial for early infection detection and intervention. Managing device-related infections demands a multi-specialty approach to minimize complications and optimize outcomes. This paper outlines best practices for diagnosing, managing, and treating neuromodulation device infections, focusing on guiding clinical decision-making from the onset of infection through treatment and potential reimplantation.
Keywords: spinal cord stimulation, intrathecal pumps, procedure complications, infection control, surgical site infection
Introduction
The incidence of neuromodulation-related surgical site infections (SSI), including in spinal cord stimulation (SCS), dorsal root ganglion stimulation (DRG-S), and intrathecal pumps (ITP), poses significant challenges to patients and healthcare professionals. Infection-related complications can lead to increased healthcare costs due to prolonged hospital stays and device explantation; the latter is primarily for treating patient discomfort and, infrequently, preventing infection migration through frequent assessments. This analysis provides comprehensive recommendations for managing neuromodulation device infections, specifically focusing on current evidence for recognition, treatment, potential reimplantation or salvage, and long-term surveillance.
Methods
Development Process
The Device Evaluation, Treatment, and Explantation Recommendations (DETER) best practices group was developed using a systematic approach modeled after prior American Society of Pain and Neuroscience (ASPN) guideline and best practice efforts. Under the guidance of ASPN leadership, a diverse, multidisciplinary panel was convened, including experts in anesthesiology, pain medicine, infectious diseases, neurosurgery, and physical medicine and rehabilitation, all with extensive experience in neuromodulation and infection management. Panelists were selected based on clinical expertise, scholarly contributions, and practical experience. A steering committee identified key clinical questions focused on infection risk, diagnosis, and treatment in neuromodulation. Through iterative discussion, these were refined into 14 core topics. Panelists were divided into working groups, drafted recommendations, and engaged in group consensus to finalize content.
Given the limited availability of high-level evidence such as randomized controlled trials, recommendations were informed by the best available data, including observational studies and expert consensus, when appropriate.
Search Strategy
A structured literature search was performed using MEDLINE, Embase, Cochrane Central, and Scopus, supplemented by manual reference checks. Eligible studies included randomized trials, observational studies, and relevant case series. Case reports and preclinical studies were considered when higher-level evidence was unavailable. Non-peer-reviewed publications were excluded.
Oversight of Bias and Conflicts of Interest
All panelists disclosed potential conflicts before participation. Members with relevant conflicts recused themselves from related discussions and voting. Conflicts were managed according to ASPN policies, and at least one primary author of each section remained free of relevant conflicts to ensure integrity.
The Reality: Infections in Neuromodulation
Of the estimated 34,000 SCS implants performed annually,1 2.3% to 6.1% of patients will develop an infection.1–8 In a meta-analysis of patients pooled from 10 studies who underwent DRG-S implant or revision surgery, the infection rate was similar, at 4.7%.9 The infection rate for ITP implants ranges from 2.4% to 6.4%.5,10–12
Surgical infections in all specialties create a significant healthcare burden, with an estimated annual direct medical cost of $9.57 to $15.17 billion.13,14 Each patient who develops an SCS infection after initial implantation and replacement surgery will accrue an average of $59,716 and $64,833 in annual healthcare expenditures, respectively.15 Among patients hospitalized following an SCS or ITP implant, each admission costs a median of $14,118.7
Beyond healthcare costs, SSIs can lead to significant morbidity and mortality for the patient. In a large study of 11,041 patients with SSIs, epidural or intraspinal abscess formation occurred in 5.08% of cases, meningitis in 4.93%, paralysis in 2.77%, osteomyelitis in 1.5%, and death in 1.85%.7 Treatment for SSIs typically requires systemic antibiotics and may necessitate the explantation of the device, further complicating patient care.7
Risks associated with treatment using antibiotics alone without explantation include increased length of stay and hospital cost, sepsis, meningitis, paralysis, epidural or intraspinal abscess, osteomyelitis, septic shock, and death.7 Additionally, patients treated with conservative therapy generally require antibiotics for a significantly longer duration than those who undergo explantation.16 However, device explantation results in temporary, and often permanent, loss of therapeutic benefit from the device.5,6,17,18 Following explantation for SCS infections, reimplantation occurs in a minority (26%) of cases.15
Furthermore, neuromodulation device infections also have medicolegal implications. Among pain medicine claims for ITP, SCS, and peripheral nerve stimulation (PNS) implants, the most damaging event among surgical device procedures was an infection, which occurred in 23% (25/107) of these cases. Among these 25 infection-associated claims, three claims involved permanent, severe injuries, including finger amputation (4%), severe brain damage (4%), and death (4%).19
 |
Table 1 Risk Factors for Infection and Poor Wound Healing |
Infection Risk Factors
Potential risk factors for postoperative infection and poor wound healing are illustrated in Table 1. Patients being considered for implantation should be screened for risk factors such as smoking, obesity, uncontrolled diabetes mellitus, alcohol consumption, anemia, poor hygiene, history of methicillin-resistant Staphylococcus aureus (MRSA), pre-existing infections, and previous post-operative wound infections. In neuromodulation, the generator pocket site is the most common site of postoperative infection (54%), followed by SCS leads (14%) and lumbar incision sites (8%).12,20–22 However, there is some inconsistency in the literature. Mekhail et al reported no increased infection rate in people with diabetes compared to those with other comorbidities.8 Engle et al reported no significant difference in infection rate between patients with and without cancer.5 These findings highlight the need for further research to better understand the relationship between diabetes and postoperative infection risk.
Preoperative and Intraoperative Management
Implementation of standardized infection-prevention protocols into practice is the optimal strategy for preventing postoperative wound infections. Such protocols involve systematized utilization of pre-operative checklists, patient screening questionnaires identifying risk factors for infection, MRSA and methicillin-susceptible Staphylococcus aureus (MSSA) screening and decolonization protocols, weight-based antibiotic prophylaxis, surgeon training, strict adherence to proper prepping and draping and surgical techniques with an emphasis on wound closure and wound care education for patients.22–24
Preoperative antibiotic selection is based on expected organisms known to cause infections in neuromodulation cases and is informed by a patient’s MRSA status. For SCS cases, gram-positive Staphylococcus species account for most of the infections, and these species may be methicillin-sensitive or resistant.21,25 Studies show that gram-negative bacteria can also cause infections, although these are infrequent.21,25,26 Patients known to be colonized with MRSA may be decolonized with nasal mupirocin, which has been demonstrated to decrease the risk of positive clinical cultures or post-discharge infection by 18–30%.27,28
Although no specific prophylactic antibiotic has been definitively established as superior, weight-based first-generation cephalosporins, such as cefazolin, are generally preferred for SSI prevention in neuromodulation procedures. The American Society of Health-System Pharmacists recommends 2 g for patients under 120 kg and 3 g for those weighing 120 kg or more.26
If there is a significant cephalosporin allergy, vancomycin or clindamycin are recommended as second-line alternatives.25 In patients with confirmed MRSA or a higher likelihood of MRSA infection (ie, high community presence), vancomycin is recommended. Additionally, antibiotics should be given within 30–60 minutes of incision for cephalosporins and clindamycin or within 120 minutes of incision for vancomycin.25,26 The need for redosing of these preoperative antibiotics is unlikely due to the short surgical times associated with neuromodulation.
There has been controversy surrounding adding vancomycin for SSI prophylaxis when placing surgical hardware. Still, given the rise of methicillin resistance in the community, this should be considered.29 Some of the most robust data on implant infection prevention have been extrapolated from the arthroplasty literature. In a randomized controlled trial, in patients undergoing arthroplasty without known MRSA colonization status, adding vancomycin to cefazolin for preoperative prophylaxis was not superior to cefazolin alone.30 Furthermore, in another study by Courtney et al, the addition of vancomycin to cefazolin was a risk factor for acute renal injury following total joint arthroplasty.31 For SSI prophylaxis in arthroplasty, the American Academy of Orthopedic Surgeons Clinical Practice Guidelines recommend the use of first-generation cephalosporins (eg, cefazolin), second-generation cephalosporins (eg, cefuroxime) or a glycopeptide (eg, vancomycin).32 A 2024 study found that S. aureus isolates from spinal cord stimulator infections formed robust biofilms and showed high tolerance to vancomycin, trimethoprim/sulfamethoxazole, and levofloxacin. In contrast, rifampin, oxacillin, and teicoplanin were more effective, supporting their use when biofilm infection is suspected.33
The Role of Intra-Operative Topical Vancomycin and Other Antibiotic Strategies
In recent years, vancomycin powder has been placed into the surgical wound to decrease deep SSIs. While vancomycin powder has been used in non-neuromodulation surgeries to reduce SSIs, research for its use in neuromodulation implants is sparse. Amrani’s study reported a 0% infection risk with vancomycin powder versus a 2.6% risk without vancomycin powder during paddle lead implantation.25,26,34,35 Yet, the Centers for Disease Control and Prevention (CDC) recommends against the use of vancomycin powder to prevent SSI.36 A review of the deep brain stimulator literature has shown that using locally administered antibiotics, such as neomycin and polymyxin, in addition to systemic prophylaxis, can reduce infection rates in hardware implantation procedures compared to systemic antibiotics alone.39–42 Randomized-controlled data are needed to assess the efficacy of vancomycin powder for both paddle and percutaneous SCS implants.
Antibiotic-eluting envelopes (AEEs) encompassing the implantable pulse generator have been cleared by the USA Food and Drug Administration (FDA) since 2013. Used primarily in cardiac implantable electronic devices (CIEDs), these bioabsorbable polypropylene envelopes (TYRX, Medtronic Inc, Minneapolis, MN) release minocycline and rifampin for seven days, and the entire envelope is absorbed over approximately nine weeks (see Figure 1).25,41 Initial data from the cardiac literature for CIED devices has shown a 60% decrease in infection in all patients but up to 87% decrease in infections for those at high risk. Kolek’s study determined that those who received an AEE during cardiac device surgery reported an infection rate of only 0.4% compared to a control group that did not receive the envelope and had an infection rate of 3%.42 In a follow-up study with patients at high risk of infection, using the envelope reduced infection to 0%.43 The WRAP-IT trial was a multicenter, randomized, controlled clinical trial that assessed the efficacy and safety of using an absorbable, antibiotic-eluting envelope to reduce major infections associated with CIEDs. Among 6983 patients randomized to receive the envelope or standard care, the infection rate was significantly lower in the envelope group (0.7% vs 1.2%, P=0.04), demonstrating a 40% reduction in relative risk. Safety outcomes showed no increase in complications between the groups, supporting the envelope’s role as an effective adjunctive strategy for preventing CIED-related infections.44 Therefore, AEEs may be considered in high-risk patients, and future prospective trials should be conducted to determine whether antimicrobial envelopes are appropriate for neuromodulation implants.45 Lastly, some practitioners may irrigate the surgical wounds with an antibiotic solution during skin closure. A scoping review of 17 neurosurgical studies showed decreased SSIs amongst 13 studies using antibiotic irrigation (vancomycin, gentamicin, or streptomycin).46
Despite a considerable body of evidence discouraging the use of antibiotics in the postoperative period, many clinicians continue to prescribe them post-operatively.25,47,48 The most recent Neurostimulation Appropriateness Consensus Committee (NACC) recommendations are to avoid post-operative antibiotics unless clinically warranted or if there is concern for a patient at high risk for infection.26 A more detailed discussion regarding the use of postoperative antibiotics, including clinician prescribing trends and management of high-risk patients is provided in the Postoperative Period section below.
Postoperative Period
Implementation of standardized guidelines aims to improve overall patient care and decrease patient morbidity and mortality. The 2017 NACC guidelines recommend using a sterile occlusive dressing over the wounds for 24–48 hours.25 Routine postoperative antibiotics beyond 24 hours and topical antimicrobial agents are not recommended. The patient and family should be well educated on the care of the incision and techniques for wound surveillance, along with a two-week follow-up. If the surgical dressing needs to be changed, it should be done using hand washing and sterile technique.25 A study of nearly 200 pain physicians demonstrated that 44% of clinicians utilize antibiotics up to 24 hours following implant, 24% use antibiotics for 3–5 days, and 14% for more than five days.48 Four percent of physicians reported not administering any antibiotics for the permanent implant.
Despite advances in infection control measures such as sterile precautions, operating room negative pressure ventilation, surgical barriers, and antimicrobial prophylaxis, surgical site and implanted device infection remain a significant cause of SCS failure, loss of efficacy, device explantation, and subsequent morbidity and mortality.51–53 Surveillance programs have been implemented in surgical settings to effectively risk-stratify patients and reduce SSI. For example, patients should be evaluated within 10–14 days following surgery to assess for adequate wound healing and to identify signs of SSI. Further, patients should be educated to recognize the signs and symptoms of an SSI, as vigilance and early recognition are critical to improved outcomes.
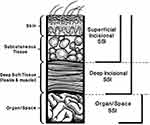 |
Figure 2 CDC Classification of SSI. Superficial incisional SSI involves the skin and subcutaneous tissue. Deep incisional SSI involves fascia and muscle layers. Organ/space SSIs are deep and involve viscera or body cavities.53 Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13(10):606–608. ©Cambridge University Press, reproduced with permission.79 |
Initial Assessment and Management of Infection
Even with the best surgical technique, a post-surgical infection may still occur. There are three SSI categories – superficial, deep, and organ/space (see Figure 2).52,53 The main differences between superficial and deep SSI are location and the presence of an abscess.53 Abscesses are more likely to be caused by deep infections and can track into the neuraxis. The focus for neuromodulatory devices will be on superficial and deep SSIs, as organ/space infections are uncommon in SCS procedures. Appropriate treatment of superficial and deep infections should prevent more severe systemic infections like sepsis.21,53
The initial step in managing suspected infection is completing an in-person history and physical exam. Patient vitals should be obtained, and the wound should be directly visualized and examined for rubor (erythema), calor (warmth to touch), dolor (pain to touch), and tumor (edema of surrounding tissues). Hyper- or hypothermia, tachycardia, tachypnea, hypotension, night sweats, chills, and other constitutional signs should be assessed. Localized pruritus, purulent discharge, and wound dehiscence are all concerns for underlying infection.22 If the patient appears well and vital signs are within normal limits, an attempt should be made to collect a wound culture from purulent drainage for microbial analysis and baseline labs. Depending on the severity and depth of the infection, frequent in-person wound checks, as well as intermittent labs, are recommended.22,24 Recent data for SSI with SCS devices has demonstrated increased salvage rates with appropriate and aggressive initial intervention.6,21
Depth of Infection (Superficial Vs Deep)
Superficial
Superficial infections occur at the epidermis and dermis layers and generally respond well to oral antibiotics.54 Device-related spinal infections occur in subcutaneous tissue layers where leads, catheters, implantable pulse generators (IPGs), and ITPs are implanted, typically in the fat between the dermis and superficial muscle fascia. Superficial infections can be identified by erythema, swelling, warmth, and tenderness in a visually distinct area surrounding a wound. It is helpful to outline the border of erythema using a permanent surgical marker to monitor for spreading infection. Superficial infections often have little or no purulent exudate and are not typically associated with an underlying abscess.8 A recent large retrospective review reaffirmed that localized tenderness and erythema were the most common initial presentations of SSI.6 Therefore, ensuring frequent postoperative visits (ie, 5-day, 2-week, 6-week) is helpful for early identification of any potential SSI. With superficial SSIs, there may be purulent drainage, although this is less common, and the patient may appear clinically well.21,53
Attempts should always be made to culture any purulent drainage, which will assist in tailoring the antibiotic regimen. This should be attempted aseptically before initiating antibiotic therapy.55 Once there is a suspected superficial SSI, empiric oral antibiotics should be started to cover the suspected bacterial organism, including S. aureus, as it is the most frequently isolated organism from SCS SSIs.6,21,25,53 For a 7-day treatment course, a first-generation cephalosporin, such as cephalexin, would be appropriate. However, if the patient is at high risk for MRSA colonization or if local community MRSA rates are high, it is recommended that an additional second oral antibiotic with MRSA coverage, such as doxycycline or trimethoprim/sulfamethoxazole, should be added.55 If oral antibiotics successfully treat the infection, the SSI’s erythematous border should serially decrease. Alternatively, if oral antibiotics fail, erythema will begin to extend beyond the drawn border, and in this instance, an infectious disease specialist should be consulted, and device explantation should be considered.
However, it is crucial to distinguish superficial wound infection from an inflammatory reaction to skin glue or adhesive, suture allergy, and superficial skin irritation. The former may require antibiotics or explantation. In contrast, the latter will require the removal of the triggering agent and possibly the application of a topical steroid. Diagnostic imaging, such as ultrasound, is not necessarily recommended for superficial SSI, but can assist with determining whether there is deeper tissue involvement (see Figure 3).21 If a deep SSI is suspected, advanced imaging such as computed tomography (CT) and/or magnetic resonance imaging (MRI) may be indicated (the latter if the neuromodulation system is MRI conditional).21
Deep Infections and Sepsis
Deep infections involve the soft tissue and communicate with and below the muscle fascia. They can develop anytime within the first 12 months post-implantation.22 Deep infections are more prone to spread to vital organs, including the spinal cord and brain. They can rapidly develop into life- and limb-threatening conditions, including spinal meningitis, vertebral osteomyelitis, and spinal epidural or subdural abscess formation. A CT or MRI should be obtained if a deep infection is suspected, as the physical exam is not always sufficient.20 A point-of-care ultrasound is a quick and readily available tool that can assess depth, detect fluid in the IPG pocket or subcutaneous spaces, and assist in determining the need for advanced imaging. MRI is the preferred imaging modality for infections involving the subdural or epidural space, vertebra, or intervertebral discs.20 Fever with nuchal rigidity and hyperleukocytosis may indicate meningitis, and a lumbar puncture for microbial analysis of cerebrospinal fluid (CSF) should be performed if not contraindicated.20,56 If a deep infection is identified, an emergent and complete explantation of the implanted device, appropriate tissue and fluid bacterial cultures with sensitivities, admission to the hospital for observation and treatment, consultation with an infectious diseases physician, and initiation of broad spectrum antibiotics (including intravenous [IV]) are recommended. The appropriate antibiotic duration depends on the infection’s severity and extent, such as associated neurological or constitutional symptoms. Antibiotic selection should target MSSA, MRSA, and Streptococcus A, thus a regimen such as vancomycin and ceftriaxone could be considered. A spine surgeon should also be consulted if an infection involves the epidural space or spinal canal.52
Notably, the patient and their family should be educated on the symptoms and signs of sepsis and instructed to immediately go to the nearest emergency room should these appear. Some clinicians may prefer to explant at presentation or with worsening symptoms and concurrently initiate IV antibiotics.21 Studies have determined that 78–94% of SCS infections require device explantation.6,12,25 There should not be delays in treating deep infections since complications such as epidural abscesses and neurological motor deficits have a high rate of morbidity and potential mortality.25
Suspected Infection of an Intrathecal Drug Pump
If the patient has a suspected infection of an ITP pocket or catheter site and the wound is not deep, a safe and gradual wean of the daily intrathecal medication dose may be appropriate before the system is explanted to avoid abrupt withdrawal. This is particularly important when a patient has baclofen as a component of an ITP, as acute baclofen withdrawal in the case of an emergent pump explant can be life-threatening.57,58 Boster et al recommend weaning a patient’s baclofen pump by 20–30% weekly until a preoperative target daily dose of 100 mcg/day is achieved prior to surgical explant.59,60 Other reports describe more aggressive weaning of intrathecal baclofen. Hwang et al used an externalized pump to taper baclofen by 20% daily while increasing oral baclofen and tizanidine, with a further 20%–50% reduction each day.61 There is no consistent ratio for converting oral to intrathecal baclofen, as patient tolerance can vary significantly. While some may tolerate high ITP doses, they may struggle with even low oral doses. A 10–20 mg starting regimen every six hours is suggested, and individual responses can vary widely. Limitations of oral baclofen include inconsistent absorption, short duration, slow onset, delayed peak effect, variable renal elimination, and limited blood-brain barrier penetration, making it less reliable for addressing severe underdosing or early withdrawal symptoms.62
If a baclofen ITP needs to be removed emergently in the case of deep infection or sepsis, patients should be admitted to an intensive care unit for observation postoperatively, and attempts should be made to compensate for the loss of intrathecal baclofen with oral baclofen dosing.58 Alternatively, if an opioid ITP needs to be explanted emergently, the patient should be advised of the risks of removal, including a high likelihood that they will experience symptoms of opioid withdrawal. This can be mitigated by the re-initiation of IV or oral opioid therapy until the patient is stabilized.
Laboratory Test Monitoring
Initial laboratory testing should be considered when facing an infectious condition, which often includes white blood cell (WBC) counts, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), wound culture, and blood culture if systemic symptoms are present. Notably, elevations in ESR and CRP are typical in the immediate postoperative setting, often peaking on the second or third postoperative day for CRP levels and the fourth or fifth postoperative day for ESR levels.25 A recent literature review indicates that laboratory results must be considered in the context of the overall clinical presentation, and clinical decision-making should not revolve solely around these values. WBC, CRP, and ESR should be a part of the initial workup. However, not all SSIs have elevated laboratory findings; therefore, clinical judgment is essential, and laboratory values can be used to monitor the infection over time.6 In a multicenter, retrospective study by Bendel et al, approximately half of SCS-related infections were associated with elevated WBC (45.6%), ESR (44.8%), or CRP (53.3%), suggesting the low sensitivity of these studies.6 Yusuf et al reported elevated WBC count in 53% of SCS infections, although CRP was elevated much more frequently (94.7%).63 With meningeal signs, lumbar puncture for CSF analysis should also be obtained.
Gram stain and bacterial cultures of the infected site are indicated and, in some cases, can lead to identifying the culprit bacterial agent. Microbial culture should direct the choice of antibiotic and can be altered according to antibiotic sensitivities.20,22,24 Wound cultures have a high yield of positivity (76%-86%), as noted in several studies.5,63 In instances of device explantation, tissue specimens and device/pocket swabs should be sent for gram stain and bacterial culture. If cultures are negative, 16S ribosomal RNA polymerase chain reaction/sequencing (if available) may lead to isolation of the pathogenic agent.21 However, if cultures are not definitive for a particular organism, broad-spectrum antibiotics, and an infectious disease consultation should be considered.
Monitoring inflammatory markers can help determine treatment success. However, there are currently no specific laboratory guidelines regarding SCS-related infections. Trending WBC counts and changes in the differential can identify trends in response to treatment. With successful treatment, CRP and ESR are expected to decrease, and cultures should become negative.
Decision to Explant - Recommendations and Strategies
When an infection is suspected, swab or blood microbial cultures are crucial in choosing appropriate antibiotics. However, initiating empirical antibiotics should not be delayed due to pending culture results and can be adjusted based on culture sensitivity.26 There is unequivocal evidence that intraoperative incision and drainage of an abscess are superior to targeted antibiotic treatment alone.64
When there is a deep infection or unsuccessful treatment of a superficial infection, the entire implanted system should be removed entirely. This includes the ITP and catheter or pulse generator and leads, regardless of whether the lead is percutaneous or paddle-type. Partial explantation is not advised as it can lead to delayed spinal infections, which pose significant risks. Comprehensive explantation is essential to ensure effective infection management and prevent serious complications.
After removing infected tissue and purulent material, copious low-pressure irrigation is recommended.25 As discussed earlier in this review, while the current pain-specific literature has not found strong evidence supporting the use of antibiotic irrigation in neuromodulation procedures, some practitioners have adopted its use based on its demonstrated utility in neurosurgical practice.26 Achieving adequate hemostasis before wound closure is crucial, as blood or venous fluid can impede wound healing and serve as a nidus for bacterial growth and infections.65
Depending on the severity of the infection, there are various ways to close the incisions. The NACC guideline recommends using triclosan-coated Vicryl and PDS II sutures for contaminated wounds.26 For mild cases, the incisions can be closed primarily. For complicated infections, consideration should be given to closing via secondary intention with serial packing or drain placement.66
Negative pressure wound therapy (NPWT) has demonstrated benefits in reducing wound complications in high-risk surgeries, such as oncoplastic breast surgery and cardiac procedures. For example, a multicenter breast surgery study found a 5.6% wound infection rate with NPWT, significantly lower than the 25% reported in larger studies. Similarly, cardiac surgery data show a 40% reduction in deep surgical site infections. The evidence supporting NPWT’s routine use for neuromodulation patients is limited. More data are needed to establish its effectiveness in reducing infection risks in this specific population.67,68 Consultation with a general surgeon may be obtained if the wound closure is complicated. Close follow-up with the surgeon, infectious disease, and wound care specialists are recommended.22
A gadolinium-enhanced spine MRI may be considered following the explantation of SCS devices in cases of SCS-related infections to assess infection resolution; however, data regarding the effectiveness of advanced imaging post-system removal is limited.6 Considering the expansion of MRI-conditionality among SCS devices, pre-explantation evaluation with MRI studies is recommended (if not contraindicated) in SCS-related infections to assess the extent of the infection and for surgical planning. Neuraxial imaging can help identify fluid accumulation within or around the epidural space and adjacent tissues, with imaging accounting for the diagnosis of 80% to 90% of epidural abscesses.69
Consultation with Infectious Disease
Role of Consultation
Early involvement of infectious diseases consultants could assist with patient assessment, appropriate workup, and management of neuromodulation device infections. In the setting of deep or complicated infections involving an implant device, epidural space, or vertebral bodies, infectious diseases consultation is recommended to assist with antimicrobial selection, duration, and timing of device re-implantation.21,25
Culture Interpretation
Most wound infections are initially diagnosed clinically, with laboratory testing providing further data to guide management. For the pain management physician, laboratory testing typically requires obtaining a swab sample from within the wound. When specimens are obtained for analysis, most laboratories will provide information on the bacteria cultured (qualitative analysis), the number of organisms grown (quantitative or semi-quantitative analysis), and the antibiotic susceptibility of the grown organisms. All this information can be used to provide targeted treatments. Wound specimens should be cultured for both aerobic and anaerobic microorganisms. Qualitative analysis can be rendered in 24–48 hours for aerobic bacteria but may require 2–5 days for anaerobic bacteria.70
Reimplantation and Wound Management Strategies
Advanced neuromodulation surgical treatments are well-established options for chronic pain management. Some variables that can affect the rates of revision and reimplantation include the location of the IPG or ITP reservoir, percutaneous vs paddle lead location or intrathecal catheter location, patient co-morbidities, and types of sutures.71 AEEs may be utilized with reimplantation, and strong consideration should be given to selecting a new pocket site during reimplantation.
Surgical sutures remain the preferred method for closure in SCS and ITP implants. Surgical pockets are typically closed with 1–2 deep dermal layers followed by skin re-approximation with an absorbable monofilament suture or staples. Complications associated with traditional sutures often stem from knots, such as breakage, slippage, suture extrusion, splitting, and infection. Additionally, tightly closed wounds and overly tense sutures can cause ischemia at the wound edge, inflammation, dehiscence, weakened wound integrity, and scarring.72
Unidirectional barbed sutures feature barbs arranged in a spiral pattern with a welded loop end. This arrangement enables self-anchoring of the suture, facilitating precise tissue approximation while preventing migration, which can occur due to swelling. Barbed sutures eliminate the need for knots during closure and help reduce some complications associated with knots. Preclinical studies suggest that knotless barbed sutures offer advantages over smooth sutures, including shorter operative time, improved tissue apposition, and more evenly distributed tension along the incision.73,74
The choice between percutaneous and paddle leads for re-implantation will ultimately depend on the patient’s comfort level with surgical procedures. However, percutaneous lead placement is less invasive and typically requires less anesthesia time.
No direct studies have compared percutaneous versus paddle lead infection rates. Compared to spine surgery, SCS is intuitively safer. One study suggests that infection rates are higher in patients with failed back surgery syndrome (FBSS), although this was not statistically significant. The authors hypothesized this was due to an undiagnosed infection following back surgery.75
Consider shared decision-making with specialists for patients with underlying medical and surgical co-morbidities, including, but not limited to, diabetes, cardiovascular or hematological conditions requiring long-term antiplatelet or anticoagulant therapy, active malignancy, and rheumatological/immunological conditions affecting wound healing and the implantation or reimplantation of neuromodulation devices.
Long-Term Follow-Up and Surveillance
While treatment protocols for identifying and acutely addressing an SCS-related infection are critical, long-term surveillance is equally vital for patient safety. Due to the lack of neurostimulation therapy post-operative infection data, recommendations regarding post-infection patient care are inferred from the literature regarding de novo, non-spinal, or surgery-related spine infections.
Length of surveillance is dependent on the type of infection (ie, superficial SSI, deep SSI, device infection).76 While superficial SSI may be treated with antibiotics alone, deep SSI with the involvement of implanted components warrants device removal. As a result, device infection and deep SSI usually require an infectious disease specialist’s involvement and longer, more vigilant follow-up—not only while on antibiotics but also subsequently to ensure that the infection has fully resolved. This generally entails periodically following the patient over a four-to-eight-week period after completion of antibiotics.
Should one elect to reimplant the neurostimulation device—or a patient requires a future spinal procedure (interventional or surgical)—the timing is essentially anecdotal. The NACC recommends waiting at least 90 days after resolving infection symptoms before considering reimplantation. Follow-up labs should show normalization of ESR and CRP levels. Consulting with an infectious disease expert may be beneficial. With no formal data on reinfection rates or optimal timing, decisions should be individualized based on the previous infection’s severity, potential complications, the system’s effectiveness, and the patient’s readiness for reimplantation.25,26 Clearance by an infectious disease specialist is recommended to mitigate recurrence.25 This is particularly critical, as the risk of reinfection and repeat need for debridement in deep SSI is over 25%.77 Underlying risk factors such as diabetes and tobacco use should be optimized before consideration of reimplantation.78 As with any other infectious process, visits should include assessing constitutional changes, wound inspection, neurological examination, and monitoring inflammatory markers (complete blood count [CBC] with differential, ESR, and CRP).76 For these needs, infectious disease specialists play an integral part in any deep or recurrent SSI case.
Conclusions
SSIs with implantable neuromodulation therapies may be associated with significant morbidity and medical costs. As with any surgical procedure, physicians performing implantable neurostimulation therapy should be well-versed in identifying and managing postoperative sequelae, including infection. Managing infections—particularly those that involve implantable devices—requires a multidisciplinary approach involving support staff, surgeons, neuroradiologists, infectious disease specialists, and implanting physicians. The recommendations summarized in this paper provide a comprehensive review of the current evidence on strategies for reducing the occurrence, identification, and management of infectious complications for neuromodulation devices. This article aims to improve clinical practice, safety, and patient outcomes by consolidating the existing knowledge and offering evidence-based, best-practice recommendations.
Abbreviations
AEE, Antibiotic-eluting envelope; ASPN, American Society of Pain and Neuroscience; CBC, Complete blood count; CDC, Centers for Disease Control and Prevention; CIED, Cardiac implantable electronic device; CRP, C-reactive protein; CSF, Cerebrospinal fluid; CT, Computed tomography; DRG-S, Dorsal root ganglion stimulation; ESR, Erythrocyte sedimentation rate; FBSS, Failed back surgery syndrome; FDA, Food and Drug Administration; IPG, Implantable pulse generators; ITP, Intrathecal pump; IV, Intravenous; MRI, Magnetic resonance imaging; MRSA, Methicillin-resistant Staphylococcus aureus; MSSA, Methicillin-susceptible Staphylococcus aureus; NACC, Neurostimulation Appropriateness Consensus Committee; NPWT, Negative pressure wound therapy; PNS, Peripheral nerve stimulation; SCS, Spinal cord stimulation; SSI, Surgical site infection; USA, United States of America; WBC, White blood cell.
Acknowledgments
The authors thank the American Society of Pain and Neuroscience (ASPN) for supporting this project. Editing was provided by Allison Foster, PhD, of Foster Medical Communications.
Funding
An unrestricted educational grant from Medtronic Inc. supported open-access publication fees and final editing of the paper.
Disclosure
Dr Scott Pritzlaff reports grants from Medtronic, during the conduct of the study; personal fees from SPR Therapeutics, Nalu Medical, Bioventus; royalties from Wolters Kluwer; educational grants from Abbott, Medtronic, Nevro, Biotronik, outside the submitted work. Dr Johnathan Goree reports personal fees from Saluda, Stratus Medical, and Abbott, outside the submitted work. Dr Ryan D’Souza reports grants from Nevro Corp and Saol Therapeutics, outside the submitted work. Dr David Lee reports consultation for Boston Scientific, Abbott, Mainstay Medical, Johnson & Johnson, Vertos Medical, Biotronik. Dr Hemant Kalia is a consultant for Nalu, SPR, Abbott, and Curonix, outside the submitted work. Dr Amitabh Gulati is a consultant for Medtronic, AIS healthcare, SPR therapeutics, Nalu Medical, Neurovasis, Hinge Health, Menda Health, Edenos, Smart MS3, during the conduct of the study. Dr Samir Sheth reports personal fees from SPR, during the conduct of the study; personal fees from Vertos, Boston scientific, Medtronic, and SPR, outside the submitted work. Dr Melissa Murphy reports grants for speaking and consulting fees from Medtronic; advisory board of Nervonik and Pacira, outside the submitted work. Dr Steven Falowski reports personal fees for research and consulting from Abbott, Medtronic, Shiratronics, Saluda, CornerLoc; equities from SPR, Aurora, Backstop Neural, Painteq, Synerfuse, Surgentec, and Thermaquil, outside the submitted work. Dr Erika Petersen reports personal fees from Biotronik, Medtronic Neuromodulation, Nevro, Presidio Medical, Saluda; owns stock options and is a former board of directors of SynerFuse, outside the submitted work. Dr Nestor Tomycz reports personal fees from Abbott Medical, outside the submitted work. Dr Krishnan Chakravarthy is a consultant for Medtronic and Mainstay Medical, outside the submitted work. Dr Jason Pope reports personal fees for consultancy from Abbott, Medtronic, Saluda, Flowonix, SpineThera, Painteq, Vertos, Vertiflex, SPR Therapeutics, Tersera, Aurora, Spark, Ethos, Biotronik, Mainstay, WISE, Boston Scientific, Thermaquil; grants for research from Abbott, Flowonix, Saluda, Aurora, Painteq, Ethos, Muse, Boston Scientific, SPR Therapeutics, Mainstay, Vertos, AIS, Thermaquil; shareholder of Vertos, SPR Therapeutics, Painteq, Aurora, Spark, Celeri Health, Neural Integrative Solutions, Pacific Research Institute, Thermaquil, Saluda, Abbott, SpineThera, Axonics. Dr Michael Schatman is a senior medical advisor for APURANO Pharma, outside the submitted work. Dr Dawood Sayed reports personal fees from Abbott and Saluda, outside the submitted work. Dr Timothy Deer reports personal fees, other from Abbott, personal fees, other from SpineThera, personal fees, other from Saluda, personal fees from Cornerloc, personal fees, other from Boston Scientific, personal fees, other from Pain Teq, personal fees from Spinal Simplicity, personal fees, other from SPR Therapeutics, personal fees from Biotronik, personal fees, other from Aurora, personal fees, other from Nervonik, outside the submitted work; In addition, Dr Timothy Deer reports personal fees for consultancy, research, and/or stock options from Abbott, SpineThera, Saluda, Cornerloc, Boston Scientific, Pain Teq, Spinal Simplicity, SPR Therapeutics, Biotronik, Aurora, and Nervonik, outside the submitted work. In addition, he also has a pending patent to Abbott. The authors report no conflicts of interest in this work.
References
1. Thomson S. Spinal Cord Stimulation’s Role in Managing Chronic Disease. 2019. Available from: https://www.neuromodulation.com/spinal-cord-stimulation.
2. Rauck RL, Loudermilk E, Thomson SJ, et al. Long-term safety of spinal cord stimulation systems in a prospective, global registry of patients with chronic pain. Pain Manag. 2023;13(2):115–127. doi:10.2217/pmt-2022-0091
3. Falowski SM, Provenzano DA, Xia Y, Doth AH. Spinal Cord Stimulation Infection Rate and Risk Factors: results From a United States Payer Database. Neuromodulation Technol Neural Interface. 2019;22(2):279–289. doi:10.1111/ner.12843
4. Hoelzer BC, Bendel MA, Deer TR, et al. Spinal Cord Stimulator Implant Infection Rates and Risk Factors: a Multicenter Retrospective Study. Neuromodulation Technol Neural Interface. 2017;20(6):558–562. doi:10.1111/ner.12609
5. Engle MP, Vinh BP, Harun N, Koyyalagunta D. Infectious complications related to intrathecal drug delivery system and spinal cord stimulator system implantations at a comprehensive cancer pain center. Pain Physician. 2013;16(3):251–257.
6. Bendel MA, O’Brien T, Hoelzer BC. Spinal Cord Stimulator Related Infections: findings From a Multicenter Retrospective Analysis of 2737 Implants. Neuromodulation J Int Neuromodulation Soc. 2017;20(6):553–557. doi:10.1111/ner.12636
7. Goel V, Kumar V, Agrawal SN, et al. Outcomes Associated With Infection of Chronic Pain Spinal Implantable Electronic Devices: insights From a Nationwide Inpatient Sample Study. Neuromodulation J Int Neuromodulation Soc. 2021;24(1):126–134. doi:10.1111/ner.13263
8. Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract. 2011;11(2):148–153. doi:10.1111/j.1533-2500.2010.00407.x
9. Moman RN, Peterson AA, Maher DP, et al. Infectious Complications of Dorsal Root Ganglion Stimulation: a Systematic Review and Pooled Analysis of Incidence. Neuromodulation Technol Neural Interface. 2022;25(7):956–964. doi:10.1111/ner.13473
10. Malheiro L, Gomes A, Barbosa P, Santos L, Sarmento A. Infectious Complications of Intrathecal Drug Administration Systems for Spasticity and Chronic Pain: 145 Patients From a Tertiary Care Center. Neuromodulation: Technology at the Neural Interface. 2015;18(5):421–427. doi:10.1111/ner.12265
11. Taira T, Ueta T, Katayama Y, et al. Rate of Complications Among the Recipients of Intrathecal Baclofen Pump in Japan: a Multicenter Study. Neuromodulation Technol Neural Interface. 2013;16(3):266–272. doi:10.1111/ner.12010
12. Follett KA, Boortz-Marx RL, Drake JM, et al. Prevention and management of intrathecal drug delivery and spinal cord stimulation system infections. Anesthesiology. 2004;100(6):1582–1594. doi:10.1097/00000542-200406000-00034
13. Scott RD The Direct Medical Costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention.; 2009. Available from: https://stacks.cdc.gov/view/cdc/11550.
14. Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039. doi:10.1001/jamainternmed.2013.9763
15. Provenzano DA, Falowski SM, Xia Y, Doth AH. Spinal Cord Stimulation Infection Rate and Incremental Annual Expenditures: results From a United States Payer Database. Neuromodulation Technol Neural Interface. 2019;22(3):302–310. doi:10.1111/ner.12939
16. Van Kroonenburgh I, Tan SKH, Heiden P, et al. Incidence and Management of Hardware-Related Wound Infections in Spinal Cord, Peripheral Nerve Field, and Deep Brain Stimulation Surgery: a Single-Center Study. Stereotact Funct Neurosurg. 2024;102(1):13–23. doi:10.1159/000535054
17. Sayed D, Monroe F, Orr WN, et al. Retrospective Analysis of Intrathecal Drug Delivery: outcomes, Efficacy, and Risk for Cancer-Related Pain at a High Volume Academic Medical Center. Neuromodulation Technol Neural Interface. 2018;21(7):660–664. doi:10.1111/ner.12759
18. Chapman KB, Yang A, Mogilner AY, et al. Dorsal root ganglion stimulation device explantation: a multicenter pooled data analysis. Pain Pract. 2022;22(5):522–531. doi:10.1111/papr.13113
19. Fitzgibbon DR, Stephens LS, Posner KL, et al. Injury and Liability Associated with Implantable Devices for Chronic Pain. Anesthesiology. 2016;124(6):1384–1393. doi:10.1097/ALN.0000000000001122
20. Cherkalin D, Koushik SS, Dua S. A Comprehensive Review of Spinal Cord Stimulator Infections. Curr Pain Headache Rep. 2022;26(12):877–882. doi:10.1007/s11916-022-01090-2
21. Esquer Garrigos Z, Farid S, Bendel MA, Sohail MR. Spinal Cord Stimulator Infection: approach to Diagnosis, Management, and Prevention. Clin Infect Dis off Publ Infect Dis Soc Am. 2020;70(12):2727–2735. doi:10.1093/cid/ciz994
22. Deer TR, Mekhail N, Provenzano D, et al. The appropriate use of neurostimulation: avoidance and treatment of complications of neurostimulation therapies for the treatment of chronic pain. Neuromodulation. 2014;17(6):571–598. doi:10.1111/ner.12206
23. Arocho-Quinones EV, Huang CC, Ward BD, Pahapill PA. Care Bundle Approach to Minimizing Infection Rates after Neurosurgical Implants for Neuromodulation: a Single-Surgeon Experience. World Neurosurg. 2019;128:e87–e97. doi:10.1016/j.wneu.2019.04.003
24. Lim S, Yoo YM, Kim KH. No more tears from surgical site infections in interventional pain management. Korean J Pain. 2023;36(1):11–50. doi:10.3344/kjp.22397
25. Deer TR, Provenzano DA, Hanes M, et al. The Neurostimulation Appropriateness Consensus Committee (NACC) Recommendations for Infection Prevention and Management. Neuromodulation. 2017;20(1):31–50. doi:10.1111/ner.12565
26. Deer TR, Russo MA, Grider JS, et al. The Neurostimulation Appropriateness Consensus Committee (NACC): recommendations for Surgical Technique for Spinal Cord Stimulation. Neuromodulation. 2022;25(1):1–34. doi:10.1016/j.neurom.2021.10.015
27. Huang SS, Singh R, McKinnell JA, et al. Decolonization to Reduce Postdischarge Infection Risk among MRSA Carriers. N Engl J Med. 2019;380(7):638–650. doi:10.1056/NEJMoa1716771
28. Huang SS, Septimus EJ, Kleinman K, et al. Nasal Iodophor Antiseptic vs Nasal Mupirocin Antibiotic in the Setting of Chlorhexidine Bathing to Prevent Infections in Adult ICUs: a Randomized Clinical Trial. JAMA. 2023;330(14):1337–1347. doi:10.1001/jama.2023.17219
29. Spangehl M. Preoperative Prophylactic Antibiotics in Total Hip and Knee Arthroplasty: what, When, and How. J Arthroplasty. 2022;37(8):1432–1434. doi:10.1016/j.arth.2022.01.019
30. Peel TN, Astbury S, Cheng AC, et al. Trial of Vancomycin and Cefazolin as Surgical Prophylaxis in Arthroplasty. N Engl J Med. 2023;389(16):1488–1498. doi:10.1056/NEJMoa2301401
31. Courtney PM, Melnic CM, Zimmer Z, Anari J, Lee GC. Addition of Vancomycin to Cefazolin Prophylaxis Is Associated With Acute Kidney Injury After Primary Joint Arthroplasty. Clin Orthop. 2015;473(7):2197–2203. doi:10.1007/s11999-014-4062-3
32. Tubb CC, Polkowksi GG, Krause B. Diagnosis and Prevention of Periprosthetic Joint Infections. J Am Acad Orthop Surg. 2020;28(8):e340–e348. doi:10.5435/JAAOS-D-19-00405
33. Sivori F, Cavallo I, Truglio M, et al. Biofilm-mediated antibiotic tolerance in Staphylococcus aureus from spinal cord stimulation device-related infections. Microbiol Spectr. 2024;12(12):e0168324. doi:10.1128/spectrum.01683-24
34. Amrani J. Intraoperative powdered vancomycin use with paddle lead placement. Neuromodulation J Int Neuromodulation Soc. 2015;18(3):177–180. doi:10.1111/ner.12216
35. Zarrin DA, Wilson BR, Teton ZE, Sheldon BL, Dorsi MJ. The Role of Vancomycin Powder During Spinal Cord Stimulator Implantation: a Case Series and Review of the Literature. World Neurosurg. 2021;156:e72–e76. doi:10.1016/j.wneu.2021.08.141
36. Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017;152(8):784–791. doi:10.1001/jamasurg.2017.0904
37. Miller JP, Acar F, Burchiel KJ. Significant reduction in stereotactic and functional neurosurgical hardware infection after local neomycin/polymyxin application. J Neurosurg. 2009;110(2):247–250. doi:10.3171/2008.6.17605
38. Kondapavulur S, Burke JF, Volz M, Wang DD, Starr PA. Use of Topical Vancomycin Powder to Reduce Surgical Site Infections after Deep Brain Stimulation Surgery: UCSF Experience and Meta-Analysis. Stereotact Funct Neurosurg. 2022;100(2):130–139. doi:10.1159/000520197
39. Spindler P, Braun F, Truckenmüller P, et al. Surgical Site Infections Associated With Implanted Pulse Generators for Deep Brain Stimulation: meta-Analysis and Systematic Review. Neuromodulation J Int Neuromodulation Soc. 2023;26(2):280–291. doi:10.1016/j.neurom.2022.03.014
40. Cleary DR, Palan MJ, Useinovic N, Burchiel KJ. The effect of delayed-release antibiotics on the rate of postoperative wound infection for implanted neuromodulatory devices. J Neurosurg. 2025;1–10. doi:10.3171/2024.9.JNS241705
41. Xiang K, Catanzaro JN, Elayi C, Esquer Garrigos Z, Sohail MR. Antibiotic-Eluting Envelopes to Prevent Cardiac-Implantable Electronic Device Infection: past, Present, and Future. Cureus. 2021;13(2):e13088. doi:10.7759/cureus.13088
42. Kolek MJ, Dresen WF, Wells QS, Ellis CR. Use of an antibacterial envelope is associated with reduced cardiac implantable electronic device infections in high-risk patients. Pacing Clin Electrophysiol PACE. 2013;36(3):354–361. doi:10.1111/pace.12063
43. Kolek MJ, Patel NJ, Clair WK, et al. Efficacy of a Bio-Absorbable Antibacterial Envelope to Prevent Cardiac Implantable Electronic Device Infections in High-Risk Subjects. J Cardiovasc Electrophysiol. 2015;26(10):1111–1116. doi:10.1111/jce.12768
44. Tarakji KG, Mittal S, Kennergren C. Antibacterial Envelope to Prevent Cardiac Implantable Device Infection. N Engl J Med. 2019;380(20):1895–1905. doi:10.1056/NEJMoa1901111
45. Persad AR, Ahmed SU, Mercure-Cyr R, Waterhouse K, Vitali AM. Use of Antibacterial Envelopes for Prevention of Infection in Neuromodulation Implantable Pulse Generators. Oper Neurosurg Hagerstown Md. 2022;23(5):413–419. doi:10.1227/ons.0000000000000367
46. Duquette E, Bhatti P, Sur S, Felbaum DR, Dowlati E. History and Use of Antibiotic Irrigation for Preventing Surgical Site Infection in Neurosurgery: a Scoping Review. World Neurosurg. 2022;160:76–83. doi:10.1016/j.wneu.2022.01.098
47. Goel V, Kaizer A, Patwardhan AM, et al. Postoperative Oral Antibiotic Use and Infection-Related Complications After Spinal Cord Stimulator Surgery. Neuromodulation J Int Neuromodulation Soc. 2022;25(5):738–744. doi:10.1016/j.neurom.2021.10.012
48. Sarrafpour S, Hasoon J, Urits I, et al. Antibiotics for Spinal Cord Stimulation Trials and Implants: a Survey Analysis of Practice Patterns. Anesthesiol Pain Med. 2021;11(5). doi:10.5812/aapm.120611
49. Hagedorn JM, Lam CM, D’Souza RS, et al. Explantation of 10 kHz Spinal Cord Stimulation Devices: a Retrospective Review of 744 Patients Followed for at Least 12 Months. Neuromodulation. 2021;24(3):499–506. doi:10.1111/ner.13359
50. D’Souza RS, Olatoye OO, Butler CS, Barman RA, Ashmore ZM, Hagedorn JM. Adverse Events Associated with 10-kHz Dorsal Column Spinal Cord Stimulation: a Five-year Analysis of the Manufacturer and User Facility Device Experience (MAUDE) Database. Clin J Pain. 2022. doi:10.1097/AJP.0000000000001026
51. D’Souza RS, Her YF. Stimulation holiday rescues analgesia after habituation and loss of efficacy from 10-kilohertz dorsal column spinal cord stimulation. Reg Anesth Pain Med. 2022;47(12):722–727. doi:10.1136/rapm-2022-103881
52. Deer TR, Lamer TJ, Pope JE, et al. The Neurostimulation Appropriateness Consensus Committee (NACC) Safety Guidelines for the Reduction of Severe Neurological Injury. Neuromodulation. 2017;20(1):15–30. doi:10.1111/ner.12564
53. Young PY, Khadaroo RG. Surgical site infections. Surg Clin North Am. 2014;94(6):1245–1264. doi:10.1016/j.suc.2014.08.008
54. Kumar K, Taylor RS, Jacques L, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63(4):762–770. doi:10.1227/01.NEU.0000325731.46702.D9
55. Mellinghoff SC, Otto C, Cornely OA. Surgical site infections: current management and role of new antibiotics. Curr Opin Infect Dis. 2019;32(5):517–522. doi:10.1097/QCO.0000000000000589
56. Capozza MA, Triarico S, Mastrangelo S, Attinà G, Maurizi P, Ruggiero A. Narrative review of intrathecal drug delivery (IDD): indications, devices and potential complications. Ann Transl Med. 2021;9(2):186. doi:10.21037/atm-20-3814
57. Qureshi AZ, Shacfe H, Ilyas A, et al. Complications of Intrathecal Baclofen Pump Therapy: an Institutional Experience from Saudi Arabia. Healthc Basel Switz. 2023;11(21):2820. doi:10.3390/healthcare11212820
58. Stetkarova I, Brabec K, Vasko P, Mencl L. Intrathecal Baclofen in Spinal Spasticity: frequency and Severity of Withdrawal Syndrome. Pain Physician. 2015;18(4):E633–641.
59. Boster A, Nicholas J, Bartoszek MP, O’Connell C, Oluigbo C. Managing loss of intrathecal baclofen efficacy: review of the literature and proposed troubleshooting algorithm. Neurol Clin Pract. 2014;4(2):123–130. doi:10.1212/cpj.0000000000000000
60. Roth J, Agha H, Davis C. Baclofen bridging, weaning protocol and pain management of a person with T6 paraplegia who required removal of intrathecal baclofen pump due to wound infection. BMJ Case Rep. 2021;14(9):e242686. doi:10.1136/bcr-2021-242686
61. Hwang RS, Sukul V, Collison C, Prusik J, Pilitsis JG. A Novel Approach to Avoid Baclofen Withdrawal When Faced With Infected Baclofen Pumps. Neuromodulation J Int Neuromodulation Soc. 2019;22(7):834–838. doi:10.1111/ner.12873
62. Saulino M, Anderson DJ, Doble J, et al. Best Practices for Intrathecal Baclofen Therapy: troubleshooting. Neuromodulation J Int Neuromodulation Soc. 2016;19(6):632–641. doi:10.1111/ner.12467
63. Yusuf E, Bamps S, Ursi JP. Characteristics of infections associated with a spinal cord stimulator system. J Infect. 2016;73(5):515–517. doi:10.1016/j.jinf.2016.08.012
64. Korownyk C, Allan GM. Evidence-based approach to abscess management. Can Fam Physician Med Fam Can. 2007;53(10):1680–1684.
65. Essebag V, Verma A, Healey JS, et al. Clinically Significant Pocket Hematoma Increases Long-Term Risk of Device Infection: BRUISE CONTROL INFECTION Study. J Am Coll Cardiol. 2016;67(11):1300–1308. doi:10.1016/j.jacc.2016.01.009
66. Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-Sulfamethoxazole versus Placebo for Uncomplicated Skin Abscess. N Engl J Med. 2016;374(9):823–832. doi:10.1056/NEJMoa1507476
67. Fiocco A, Dini M, Lorenzoni G, Gregori D, Colli A, Besola L. The prophylactic use of negative-pressure wound therapy after cardiac surgery: a meta-analysis. J Hosp Infect. 2024;148:95–104. doi:10.1016/j.jhin.2024.04.003
68. Vidya R, Khosla M, Baek K, et al. Prophylactic Use of Negative Pressure Wound Therapy in High-risk Patients Undergoing Oncoplastic and Reconstructive Breast Surgery. Plast Reconstr Surg Glob Open. 2023;11(12):e5488. doi:10.1097/GOX.0000000000005488
69. Deer TR, Russo MA, Sayed D, et al. The Neurostimulation Appropriateness Consensus Committee (NACC)®: recommendations for the Mitigation of Complications of Neurostimulation. Neuromodulation J Int Neuromodulation Soc. 2024;27(6):977–1007. doi:10.1016/j.neurom.2024.04.004
70. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14(2):244–269. doi:10.1128/CMR.14.2.244-269.2001
71. Burke L, Desai MJ. Pocket pain following spinal cord stimulator generator implantation: a narrative review of this under-reported risk. Pain Pract off J World Inst Pain. 2024;24(4):659–669. doi:10.1111/papr.13336
72. Zaruby J, Gingras K, Taylor J, Maul D. An in vivo comparison of barbed suture devices and conventional monofilament sutures for cosmetic skin closure: biomechanical wound strength and histology. Aesthet Surg J. 2011;31(2):232–240. doi:10.1177/1090820X10395010
73. Polland AR, Graversen JA, Mues AC, Badani KK. Polyglyconate unidirectional barbed suture for posterior reconstruction and anastomosis during robot-assisted prostatectomy: effect on procedure time, efficacy, and minimum 6-month follow-up. J Endourol. 2011;25(9):1493–1496. doi:10.1089/end.2010.0668
74. Rubin JP, Hunstad JP, Polynice A, et al. A multicenter randomized controlled trial comparing absorbable barbed sutures versus conventional absorbable sutures for dermal closure in open surgical procedures. Aesthet Surg J. 2014;34(2):272–283. doi:10.1177/1090820X13519264
75. Malinowski MN, Kim CH, Deer TR. Chapter 51 - Complications of Spinal Cord Stimulation. In: Krames ES, Peckham PH, Rezai AR editors. Neuromodulation. Academic Press. 2018:657–668. doi:10.1016/B978-0-12-805353-9.00051-6.
76. Dowdell J, Brochin R, Kim J, et al. Postoperative Spine Infection: diagnosis and Management. Glob Spine J. 2018;8(4 Suppl):37S–43S. doi:10.1177/2192568217745512
77. Freire-Archer M, Sarraj M, Koziarz A, et al. Incidence and Recurrence of Deep Spine Surgical Site Infections: a Systematic Review and Meta-analysis. Spine. 2023;48(16):E269–E285. doi:10.1097/BRS.0000000000004713
78. Martin ET, Kaye KS, Knott C, et al. Diabetes and Risk of Surgical Site Infection: a Systematic Review and Meta-analysis. Infect Control Hosp Epidemiol. 2016;37(1):88–99. doi:10.1017/ice.2015.249
79. Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13(10):606–608.
80. He K, Stolarski A, Whang E, Kristo G. Preventing Surgical Site Infections: a Clinical Perspective. J Surg Open Access. 2020;6(4). doi:10.16966/2470-0991.217
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.