Back to Journals » Drug Design, Development and Therapy » Volume 19
Dexmedetomidine Cannot Attenuate Liver Injury and Improve Outcomes Following Laparoscopic Living Donor Hepatectomy: A Randomised Controlled Trial
Authors Cui LL , Zhang L, Liu S, Zhu Q , Xue FS
Received 22 February 2025
Accepted for publication 15 May 2025
Published 22 May 2025 Volume 2025:19 Pages 4263—4274
DOI https://doi.org/10.2147/DDDT.S524343
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yan Zhu
Ling-Li Cui,1 Liang Zhang,1 Shen Liu,1 Qian Zhu,1 Fu-Shan Xue1,2
1Department of Anesthesiology, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Anesthesiology, Shengli Clinical Medical College of Fujian Medical University, Fuzhou University Affiliated Provincial Hospital, Fujian Provincial Hospital, Fuzhou, People’s Republic of China
Correspondence: Fu-Shan Xue, Department of Anesthesiology, Beijing Friendship Hospital, Capital Medical University, No. 95 Yong-An Road, Xi-Cheng District, Beijing, 100050, People’s Republic of China, Tel +86-13911177655, Fax +86-10-63138362 ; +86-0591-88217841, Email [email protected]
Purpose: To determine the effects of intraoperative dexmedetomidine (DEX) administration on postoperative ischaemia/reperfusion injury (HIRI) and clinical outcomes of patients undergoing the laparoscopic living donor hepatectomy (LLDH).
Patients and Methods: Fifty-five patients who underwent the LLDH were randomly assigned to the DEX or control group. The DEX group received an intravenous infusion of DEX with an bolus dose of 1 μg/kg for 15 min before anaesthesia induction, followed by a continuous infusion at a rate of 0.4 μg/kg/h until the portal branch was disconnected. The control group was given an intravenous infusion of 0.9% saline at same volume and rate. The primary outcome was peak serum aspartate aminotransferase (AST) level during the first 72 h postoperatively. The secondary outcomes included other variables of postoperative liver and kidney function, intraoperative hemodynamic changes, postoperative recovery outcomes and the occurrence of complications.
Results: The peak serum AST level during the first 72 h postoperatively was not significantly different between groups (DEX vs control: 288 [194– 466] vs 324 [194– 437] IU/L; difference, − 1.2 IU/L; 95% CI, − 86.9 to 88.0; P=0.973). The intraoperative mean artery pressure was not significantly different, but intraoperative heart rate was significantly decreased in the DEX group. There were no significant differences between groups in other secondary outcomes.
Conclusion: This study demonstrates that intraoperative DEX administration at the studied dosage regimens cannot attenuate postoperative HIRI and does not improve clinical outcomes in patients undergoing the LLDH.
Clinical Trial Registration: www.chictr.org.cn, ChiCTR2000040629.
Keywords: laparoscopic living donor hepatectomy, hepatic ischemia/reperfusion injury, dexmedetomidine, postoperative recovery
Introduction
In 2002, Cherqui et al reported the first case of laparoscopic living donor hepatectomy (LLDH) for liver transplantation in a child with end-stage liver disease.1 With the improvement of surgical techniques in recent years, laparoscopic donor left lateral sectionectomy has now been recognized as a standard practice and is supported by international consensus.2,3 The adult LLDH has been undergoing for over 20 years in America, with over 4500 surgical cases in total.4 By 2012, pure laparoscopic living left or right hemi-hepatectomy has also been reported in several studies worldwide.5,6 The LLDH has both cosmetic and functional advantages for the donor,7,8 but intraoperative bleeding is still a major risk associated with severe adverse outcomes.9 The intermittent liver inflow occlusion, ie, Pringle’s maneuver, is an effective maneuver commonly used for reducing blood loss during laparoscopic hepatectomy,10 but it may inevitably lead to the development of hepatic ischaemia/reperfusion injury (HIRI).11 For patients undergoing hepatectomy and liver transplantation, HIRI actually is an important pathophysiologic cause of liver injury and may significantly increase the risk of postoperative morbidity and mortality.12–14 Furthermore, abnormal liver function after hepatectomy in healthy living liver donors is significantly associated with prolonged hospital stay, increased financial burden and postoperative morbidity.15,16 Thus, preventing or attenuating the HIRI by hepatectomy to improve the safety and outcomes of donors deserves more attention.
As the mechanisms of HIRI are very complex and have not been fully elucidated,17 effective treatment or preventive strategies of HIRI are still scarce. Dexmedetomidine (DEX) is a highly selective α2-adrenoreceptor agonist that has been extensively used in clinical practice.18 Available literatures indicates that inflammation is an important pathophysiological mechanism of ischemia/reperfusion injury and nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammasome plays a pivotal role in mediating inflammatory responses associated with ischemia/reperfusion injury and post-transplant rejection.19,20 Experimental researches confirm that DEX can alleviate the HIRI by inhibiting the NLRP3 inflammasome and thus enhance liver regeneration and functional recovery after partial hepatectomy.21–23 In recent years, some clinical studies also demonstrate that perioperative DEX administration may provide a protection against the HIRI after hepatectomy and living donor liver transplantation (LDLT).24–28 Furthermore, the results of two retrospective studies from our center show that intraoperative DEX administration has the potential to decrease the risk of HIRI and is associated with a reduced incidence of moderate-to-severe HIRI in the pediatric patients undergoing the deceased liver transplantation.29,30 However, there has been no study determining the effects of intraoperative DEX administration on postoperative liver injury and clinical outcomes of healthy adult patients undergoing the LLDH. Given above the beneficial effects of intraoperative DEX administration on postoperative liver function in patients with LDLT and hepatectomy, we hypothesized in this study that intraoperative DEX administration can provide a protection against the HIRI in the healthy adult patients with LLDH.
Methods
Ethics
This study protocol was approved by the Ethics Committee of Beijing Friendship Hospital at November 2, 2020 (Reference Number:2020-P2-208-02, Chairperson Prof. Miao-Rong Xie), and registered in the Chinese Clinical Trial Registry website (www.chictr.org.cn, ChiCTR2000040629) at December 4, 2020. Each surgery was also approved by the Ethics Committees of Beijing Friendship Hospital and Beijing Municipal Health Commission. The study was performed in accordance with the Consolidated Standards of Reporting Trials (CONSORT 2010) Guidelines31 and the principles of the Declaration of Helsinki.
Participants
This prospective randomised controlled trial was conducted at the Beijing Friendship Hospital, Capital Medical University, Beijing, China, from December 10, 2020 to August 30, 2023. Each living liver donor voluntarily signed an informed consent to donate a portion of her or his liver in accordance with the Declaration of Istanbul. The patients aged 18–60 years with American Society of Anesthesiologists (ASA) grades I–II and undergoing the elective LLDH were screened for eligibility. The exclusion criteria included a history of severe allergy to DEX, conversion to open laparotomy, reduced liver size in vivo, and refusal of informed consent.
Randomisation and Masking
For all included living liver donors, they were told about the details of the study protocol and that they had the right to withdraw from the study at any time. After written informed consent was obtained from the included patients, they were randomly assigned to the DEX or control group at a 1:1 ratio using a computer-generated, random-sequence allocation list. The randomisation process was performed by an investigator who was not involved in the patient eligibility assessment or recruitment processes and data collection. The allocation sequence was concealed from the researchers at all sites by serially numbered, opaque, sealed envelopes. After patients entered into the operating room, a nurse anaesthetist who was not involved in the study opened the envelope containing the group allocation and prepared the study drugs in the identical 50-mL infusion syringes. Anaesthesiologists who did not participate in data collection administered the study drugs. All other related medical staffs, investigators and patients were blinded to the group assignments.
Interventions
Patients in the DEX group received a loading dose of DEX (1 µg/kg) for 15 min followed by a continuous infusion (0.4 µg/kg/h). Intravenous infusion of DEX was started before anaesthesia induction and discontinued after the portal vein branch was disrupted. For patients in the control group, 0.9% saline was intravenously infused at same volume and rate with identical duration.
Anaesthesia and Surgical Management
All participants received the same anaesthetic management and surgical procedures according to the standard protocols of our institution. Other than standard monitoring including non-invasive blood pressure, heart rate (HR), pulse oxygen saturation (SpO2), electrocardiogram and end-tidal carbon dioxide pressure (PETCO2), an arterial catheter was also inserted into the right radial artery under local anaesthesia to monitor invasive blood pressure. Then, anaesthesia was induced with intravenous sufentanil (0.3–0.4 µg/kg), propofol (2 mg/kg) and cisatracurium (0.2 mg/kg). After successful intubation, anaesthesia was maintained with intravenous infusions of propofol (4–8 mg/kg/h), remifentanil (0.1–0.3 µg/kg/min), and cisatracurium (0.1 mg/kg/h). The depth of anaesthesia was monitored with the bispectral index (BIS), which was maintained between 40 and 60. The lungs of patients were mechanically ventilated using a volume-controlled model with a tidal volume of 6–8 mL/kg, 0.6 fraction of inspiration oxygen and respiratory rate of 12–18/min to maintain the PETCO2 at 35 to 45 mmHg. Before surgery started, a double-lumen central venous catheter was inserted via the internal jugular vein under ultrasound guidance for monitoring of central venous pressure (CVP) and infusion of fluid.
During surgery, a low CVP strategy was implemented in all patients by reducing the CVP to less than 5 mmHg in the reverse-Trendelenburg position and limiting infusion of fluid at a rate of 1–1.5 mL/kg/h, with intravenous administration of furosemide (10–20 mg) and/or nitroglycerine (0.2–0.5 µg/kg/min). If mean artery pressure (MAP) was <60 mmHg, ephedrine (6–10 mg) and/or phenylephrine (25–50 µg) were intravenously administered and then noradrenalin was continuously infused at a rate of 0.02–0.1 µg/kg/min if needed. If HR was <50 beats/min, atropine 0.5 mg was intravenously injected. When liver tissue transection was completed, fluid infusion was no longer limited.
According to our routine practice,32 the choice of graft type was determined on the basis of a comprehensive preoperative imaging assessment using the IQQA®-3D liver system. Key decision factors included a predicted graft-to-recipient weight ratio (GRWR) and remnant liver volume to standard liver volume (RLV/SLV). All donor hepatectomies were performed by the same surgical team under carbon dioxide pneumoperitoneum with a pressure of 10–13 mmHg. Furthermore, consistent surgical procedures for laparoscopic donor hepatectomy were carried out throughout the study period. During the liver resection, the intermittent Pringle’s maneuver with one cycle of hepatic inflow occlusion for 10 min and reperfusion for 5 min was performed, and the number of occlusion cycles was determined by the surgeons, as needed. Hepatic parenchymal transection was performed by the same technique, and bile duct alignment was confirmed via the cholangiography before dissection. After the completion of hepatic parenchymal resection, a transverse incision was made 3 cm above the symphysis pubis, the vessels and biliary branches were clamped and dissociated sequentially, and the graft was preserved with histidine-tryptophan-ketoglutarate solution immediately after removal. At the end of surgery, all patients received wound infiltration with 20 mL of 0.5% ropivacaine for postoperative multimodal analgesia. After the closure of peritoneum, both tramadol hydrochloride (100 mg) and tropisetron (5 mg) were intravenously administered as postoperative analgesic and anti-emetic drugs. Extubation was carried out when patient was able to follow the instructions and spontaneous breathing was adequately resumed. Then patient was transferred to the post-anaesthesia care unit (PACU) for close observation until discharge criteria were reached.
After surgery, all patients received the standard postoperative care regimens. Flurbiprofen axetil 50 mg was intravenously administered twice a day. Additionally, patient-controlled analgesia was carried out with analgesic solution containing sufentanil 200 µg and ondansetron 32 mg in 0.9% sodium chloride of 200 mL, and analgesic pump was set up at a background infusion of 2 mL/h and a single bolus dose of 2 mL with a lockout time of 10 min. The Visual Analogue Scale scores of postoperative pain were kept to 4 or less.
Primary Outcome and Secondary Outcomes
The primary outcome was the peak serum aspartate aminotransferase (AST) level during the first 72 h postoperatively. The secondary outcomes were the serum alanine aminotransferase (ALT) and lactic dehydrogenase (LDH) levels within the first 72 h postoperatively, other variables related to liver and kidney function, anaesthesia-related data, surgical data, hemodynamic changes, postoperative recovery variables, length of hospital stay and postoperative complications.
Assessments
The biomarkers of liver injury, such as the serum AST, ALT, LDH and total bilirubin (TBIL) levels and coagulation parameters including the prothrombin time (PT) and activated partial thromboplastin time (APTT), were measured at five time points: day before surgery, at arrival in the PACU and daily for 3 days postoperatively. Kidney function variables including serum urea nitrogen (BUN) and creatinine (Cr) were also measured at the above mentioned time points. Demographic data and fatty liver disease status were noted preoperatively. Anaesthesia time, surgery time, graft weight, graft type, liver splitting time, ischemic time by the Pringle’s maneuver, dosages of propofol and remifentanil, and intraoperative dosage of phenylephrine, fluid volume, urine output, blood loss and blood transfusion volume were measured. Hemodynamic changes including MAP and HR, and BIS were recorded before surgery, before intubation, 5 min after intubation, 30 min and 1 hour after the initiation of surgery, at the end of the first Pringle’s maneuver, 5 min after resection, at the end of surgery and at discharge of the PACU. Postoperative complications during the first 30 days after surgery were recorded and categorized based on the Clavien-Dindo classification.33 Minor complications were defined as grade I to II, whereas major ones were defined as grade III to V complications. The delayed recovery of hepatic function was defined as follows: the presence of a PT > 20 s or serum TBIL > 50 μmol/L on postoperative days 1–5 (excluding obstructive jaundice).34
Statistical Analysis
The sample size was calculated based on the primary outcome, that is, the peak serum AST level during the first 72 h postoperatively. According to the results of a previous study in patients undergoing hepatectomy,24 the postoperative peak serum AST level was 195.8 (110.4) IU/L in the DEX group and 255.1 (140.8) IU/L in the control group, respectively. A difference of 40 IU/L in the postoperative peak serum AST is considered to be clinically significant. Using the PASS 15.0 software, assuming a power of 80% with an alpha of 0.025 to detect a difference of 40 IU/L in the postoperative peak serum AST level, 26 patients in each group were required. Considering a potential dropout rate of 5%, a final sample size of 56, ie, 28 patients in each group, was determined.
Quantitative data are presented as the mean (standard deviation, SD) or median (interquartile range, IQR) and their between-group comparisons were performed using Student’s t test or the Mann‒Whitney U-test according to the normality of the data. Qualitative data are presented as frequencies or percentages and their between-group comparisons were done using the Chi-square test or Fisher’s exact test. The Mann‒Whitney U-test was used to compare the between-group difference in the primary outcome. Generalized estimating equations were used to compare the between-group differences in the TBIL, AST, ALT, LDH, renal function, coagulation parameters and hemodynamic variables. The pseudomedian difference was calculated using the Hodges–Lehmann estimate, which is based on the Mann–Whitney U-test. The odds ratios (or difference) and 95% CI were also calculated. For all analyses, a two-sided P < 0.05 was considered to indicate statistical significance. All the statistical analyses were performed by The SPSS software 23.0 and PRISM 10.0 and completed by the specialized statisticians who were from the Clinical Research Institute of Beijing Friendship Hospital and blinded to grouping assignment of patients.
Results
Patients’ Characteristics, Anaesthesia and Surgery Data
The flowchart of included and excluded patients according to the Consolidated Standards of Reporting Trials statement is displayed in Figure 1. A total of 74 donors were screened for eligibility, 16 of them did not meet the inclusion criteria. The remaining 58 donors were randomly allocated to the DEX group (n = 29) or control group (n = 29). In the DEX group, two donors were further excluded after randomisation because one had the biliary tract injury during surgery, and another was lost of postoperative follow-up. In the control group, one donor was also excluded after randomisation because of intraoperative major bleeding from a ruptured vena cava. Finally, 55 donors (27 in the DEX group and 28 in the control group) were included in the final data analysis.
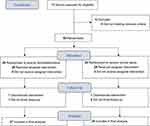 |
Figure 1 Consolidated Standards of Reporting Trials (CONSORT) flowchart. |
Two groups were comparable with respect to the demographic and baseline characteristics of patients (Table 1). Anaesthesia time, surgery time, graft types, graft weights, ischemic time, liver split times, blood loss and transfusion volumes, and extubation time were not significantly different between groups, but intraoperative dosages of propofol and remifentanil were significantly decreased and dosage of phenylephrine and urine output were significantly increased in the DEX group compared with the control group (Table 2).
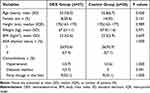 |
Table 1 Demographic and Baseline Characteristics of Patients |
 |
Table 2 Anaesthesia and Surgery Data |
Primary and Important Secondary Outcomes
There was no significant difference in the peak serum AST level during the first 72 h postoperatively between groups. The peak serum ALT and LDH levels during the first 72 h postoperatively, and proportion of patients with delayed recovery of liver function were also not significantly different between groups (Table 3).
 |
Table 3 Primary and Important Secondary Outcomes |
Secondary Outcomes
The liver and renal function variables at different time points are shown in Figure 2 and Supplement Table 1. The serum AST, ALT, LDH, TBIL, Cr and BUN levels, PT and APTT during the first three postoperative days were not significantly different between groups.
The changes of hemodynamic parameters at different time points are shown in Figure 3. Compared to the control group, the DEX group had a significantly higher MAP during tracheal intubation and a significantly lower MAP during the PACU stay (P = 0.004). Heart rate was significantly lower in the DEX group than in the control group throughout the surgical procedure and during the PACU stay (P = 0.000). The BIS values before and after intubation were significantly decreased in the DEX group.
The length of hospital stays, time to first flatus and the occurrence of postoperative complications were not significantly different between groups (Supplement Table 2).
Discussions
This study aimed to determine the effects of intraoperative DEX administration on postoperative HIRI and clinical outcomes in the healthy patients who underwent the LLDH. The results showed that intraoperative DEX administration did not provide any favorable effect on early postoperative liver function variables. Furthermore, extubation time, the incidence of delayed recovery of liver function, postoperative hospital stay and time to first flatus were not significantly different between groups. These results indicate that intraoperative DEX administration does not product a significant protection against the HIRI during the LLDH and cannot improve the postoperative outcomes. Thus, this study does not validate our hypothesis.
Available experimental evidence indicates that HIRI is characterized by significant oxidant stress and inflammatory response35,36 and DEX can attenuate ischaemia/reperfusion injury and provide a protection against organ injury by anti-inflammatory and antioxidant effects.37 Most important, several clinical trials also provide evidence that perioperative DEX administration product a protection against the HIRI after hepatectomy. A propensity score-matched retrospective study including 494 patients with liver disease who underwent partial hepatectomy showed that intraoperative DEX administration significantly decreased the serum ALT and LDH levels in the early postoperative period, indicating that DEX exerts a protective effect on postoperative liver function.38 In a prospective, randomised double-blind clinical trial including 44 patients who underwent elective hepatectomy with hepatic inflow occlusion maneuver, Wang et al25 demonstrated that perioperative DEX administration with a loading dose of 1 μg/kg over 10 min followed by a maintenance dose of 0.3 μg/kg/h was able to significantly mitigate intestinal and hepatic injuries. In another prospective, randomised, single-blind controlled study including 58 patients who underwent hepatectomy, Zhang et al24 similarly showed that regardless of with or without hepatic inflow occlusion maneuver during surgery, intraoperative DEX administration with a loading dose of 0.5 μg/kg for 10 min and a continuous infusion at a rate of 0.5 μg/kg/h until resection of liver lobes significantly decreased the serum level of α-glutathione S-transferase at 0.5 h after resection of liver lobe, and the serum levels of ALT and AST at 2 h and 24 h postoperatively, suggesting that DEX provides a protection against postoperative liver injury. In a prospective controlled, double-blinded, randomised study including 50 patients who underwent partial hepatectomy with inflow occlusion, Taman et al26 showed that continuous infusion of DEX at a rate of 0.3 μg/kg/h without a loading dose from the completion of tracheal intubation to the end of surgery exerted a protective effect on postoperative liver function, shown by decreased serum TBIL, AST and ALT levels and increased serum albumin level. Especially, a meta-analysis of 8 randomised controlled trials including 468 patients also confirm that perioperative DEX administration can produce a protective effect on the HIRI after hepatectomy, with decreased serum levels of ALT, AST, TBIL and malondialdehyde and increased serum superoxide dismutase activity.27
However, the results of our clinical study in patients undergoing the LLDH are not in agreement with the findings of above clinical trials. In this study, the serum enzymatic biomarkers including AST, ALT and LDH were applied as primary and important secondary outcomes to determine postoperative liver injury. The results showed that the peak serum levels of these liver injury biomarkers within first 72 h postoperatively were not significantly different between groups. Furthermore, there were no significant between-group differences in other liver function variables obtained at different postoperative time points, as well as proportion of patients with delayed recovery of liver function.
The detailed reasons of different results among previous and our studies are unclear, but they may be attributable to several factors. First, different study populations may be an important contributing factor. The participants of previous studies are the patients undergoing partial hepatectomy because of hepatic tumors or parenchymatous diseases. Except for older ages, these patients often suffer from hepatic cirrhosis, steatosis or structure disorders with significant sinusoidal hemodynamic changes, microcirculation impairment and metabolic dysfunction. Thus, they maybe more vulnerable to the liver injury by surgical procedures.39,40 In contrast, the living liver donors are often healthy young individuals who are identified by rigorous preoperative assessments. For example, only one-third of the donors in this study have a mild hepatic steatosis. Consequently, these patients may have a stronger tolerance to the liver injury by surgical procedures, and the extent of liver injury induced by intraoperative ischaemia might not be severe enough to cause significantly increases of serum liver injury biomarkers. Furthermore, mild liver injury may obscure the opportunity to identify the protective effect that DEX provides by inhibiting inflammatory and oxidative stress responses. This could be a major reason of no significant differences in postoperative serum levels of liver injury biomarkers between groups in this study. In a randomised, controlled trial study of 62 patients undergoing the elective LDLT, Shin et al41 used the peak serum levels of liver transaminases including ALT and AST during the first 7 days after surgery as primary outcomes and failed to find any protective benefit of propofol on the postoperative HIRI. They consider that a possible reason for this negative result also is that the HIRI induced by the LDLT is not enough to produce significant changes of serum liver injury biomarkers. Thus, for healthy patients with a low risk of severe liver injury during surgery, the protective effect of intraoperative DEX may be clinical insignificant. In fact, our study also demonstrated that intraoperative DEX administration did not improve postoperative recovery parameters including the time to first flatus and length of hospital stay, and did not decrease the severity of postoperative complications. These results are consistent with the findings of previous studies, in which intraoperative DEX administration can significantly decrease the postoperative serum levels of liver injury biomarkers, but does not improve postoperative outcomes of patients undergoing partial hepatectomy.24–27,38
Second, available evidence indicates that DEX provides dose-dependent organ protection.42–44 Thus, the dose and timing of DEX administration may have influenced the outcomes of various studies. It is generally believed that pharmacological preconditioning remains a promising therapeutic strategy for attenuating HIRI.45 Furthermore, the early phase of reperfusion injury is defined as the first 2 h after reperfusion, and involves the activation of inflammatory cells, excessive release of proinflammatory cytokines and significant production of reactive oxygen species, which are responsible for more severe tissue damage in the late phase of reperfusion injury.46 Given that the half-life of DEX is approximately 2 h,47 the protocol of this study was to start DEX administration before induction of anaesthesia or the onset of intraoperative HIRI and then maintain intravenous infusion until the completion of portal branch dissection. In the previous works,25–27,38 however, DEX administration was often continued to the end of surgery. It has been shown that a prolonged use of DEX or a 24 h perioperative infusion of DEX may reduce the incidence of delayed graft function after kidney donation after cardiac death.48 Thus, more researches are needed to determine whether a continuous infusion of DEX throughout the surgical procedure or perioperative period would produce more favorable outcomes on postoperative liver function of patients undergoing the LLDH.
Third, previous works used more observed time points in the first postoperative day, such as 2 h, 6 h, 12 h and 24 h postoperatively, and showed that intraoperative DEX administration significantly reduced serum levels of liver injury biomarkers within 24 h postoperatively.24–26,38 However, our study measured serum levels of liver injury biomarkers at arrival in the PACU and daily for 3 days postoperatively. It is unclear if this design of less observed time points in the first postoperative day could miss the opportunity to reveal the protective effects of intraoperative DEX administration on early postoperative liver function of patients undergoing the LLDH.
It is worth noting that postoperative serum Cr and BUN levels were not significantly different between groups, but intraoperative urine output was significantly increased in the DEX group compared with the control group. This may be attributable to the ability of DEX to act as a diuretic and improve renal function.49 As previously reported,50 moreover, this study showed that intraoperative DEX administration significantly reduced the dosages of propofol and remifentanil without affecting the time to emergence or extubation.
It is reported that common adverse effects of DEX are hypotension and bradycardia.51 In this study, MAP during anaesthesia induction and intubation was significantly decreased in the DEX group. This may be due to the use of a loading dose DEX. The MAP during surgery was not significantly different between groups, albeit intraoperative dosage of phenylephrine was significantly increased in the DEX group. Furthermore, HR was significantly decreased throughout surgery in the DEX group, but no severe bradycardia was observed in the two groups. These results indicates that the use of DEX as a general anaesthetic adjuvant for the LLDH may be helpful to enhance surgical stress inhibition and stabilize intraoperative hemodynamic variables.
This clinical study has several limitations that deserve special attention. First, this was a single-center randomised controlled trial with a small sample size. It may be not power to show significant between-group differences of clinical outcomes, as the sample size was evaluated based on the primary outcome, that is, postoperative serum AST level. As surgical procedures, liver resection weight and surgical complications can significantly affect the occurrence and severity of postoperative liver injury, the findings of this study should not be extrapolated into the other surgical patients who are different from the participants of our study. Second, other than liver injury, the serum AST level may also increase when injuries of myocardium, skeletal muscles and kidneys occur. Thus, the primary outcome of the study, the peak serum AST level during the first 72 h postoperatively, is a surrogate endpoint of liver injury, rather than a gold standard variable of liver injury. This may decrease the ability to validate beneficial effects of intraoperative DEX administration on postoperative liver function. Third, both oxidative stress and excessive inflammatory responses have been regarded as the two pivotal mechanisms of HIRI after hepatectomy and liver transplantation.35 Furthermore, perioperative DEX administration has been shown to attenuate oxidative stress and systemic inflammatory responses associated with HIRI in patients undergoing elective hepatectomy with inflow occlusion.25,52 Given that this study mainly focused on postoperative liver function and clinical outcomes of patients, we did not further evaluate the possible influences of DEX on postoperative oxidant stress and inflammatory biomarkers. Fourth, a single-dose scheme of intraoperative DEX administration was designed. The important questions that this study cannot answer are whether protective effect of DEX on the HIRI are the dose-dependent and the duration of DEX administration can significantly affect protective effect of DEX on the HIRI. Fifth, the mechanisms of HIRI by surgical procedures are complex and alone intraoperative DEX administration may not adequately protect against HIRI in the healthy patients undergoing the LLDH. Recently, a randomised controlled trial conducted in patients with cirrhosis undergoing laparoscopic hepatectomy for liver cancer demonstrates that ulinastatin combined with DEX compared with alone administration of two drugs can provide an enhanced protection against HIRI possibly through a synergistic effect against oxidative stress and inflammatory response.52 Evidently, this study cannot provide any clues regarding the potential benefits of DEX combined with other interventions on postoperative HIRI of living liver donors. Thus, further studies with well design and large sample sizes are still needed to address above issues.
Conclusions
In conclusion, this randomised controlled trial does not demonstrate the evidence that intraoperative DEX administration can attenuate postoperative HIRI and improve clinical outcomes in healthy patients undergoing the LLDH. This finding does not support the routine use of intraoperative DEX for preventing HIRI in the patients undergoing LLDH, but future multicenter studies with larger sample size and longer follow-up period are needed to determine the potential benefits and risks of different-dose DEX or DEX combined with other protective strategies in the patients who undergo the LLDH and are at a high risk for HIRI.
Data Sharing Statement
Individual deidentified participant-level data underlying the results reported in this article, as well as the study protocol and statistical analysis plan, will be made available upon reasonable request. Data are available beginning 3 months after online publication and for up to 24 months thereafter. To request data, please contact the corresponding author (Prof. Fu-Shan Xue). Requests must include a methodologically sound proposal, and approval will be at the discretion of the study investigators.
Acknowledgments
The authors thank the patients undergoing laparoscopic living donor hepatectomy included in the study and all the researchers. Furthermore, the authors thank Prof. San-San Wu and Na Zeng, the Clinical Research Institute of Beijing Friendship Hospital, for their statistical consultations in our data analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cherqui D, Soubrane O, Husson E, et al. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet. 2002;359(9304):392–396. doi:10.1016/S0140-6736(02)07598-0
2. Soubrane O, Eguchi S, Uemoto S, et al. Minimally invasive donor hepatectomy for adult living donor liver transplantation: an international, multi-institutional evaluation of safety, efficacy and early outcomes. Ann Surg. 2022;275(1):166–174. doi:10.1097/SLA.0000000000003852
3. Soubrane O, de Rougemont O, Kim KH, et al. Laparoscopic living donor left lateral sectionectomy: a new standard practice for donor hepatectomy. Ann Surg. 2015;262(5):757–761. doi:10.1097/SLA.0000000000001485
4. Abu-Gazala S, Olthoff KM. Status of adult living donor liver transplantation in the United States: results from the adult-to-adult living donor liver transplantation cohort study. Gastroenterol Clin North Am. 2018;47(2):297–311. doi:10.1016/j.gtc.2018.01.004
5. Samstein B, Cherqui D, Rotellar F, et al. Totally laparoscopic full left hepatectomy for living donor liver transplantation in adolescents and adults. Am J Transplant. 2013;13(9):2462–2466. doi:10.1111/ajt.12360
6. Soubrane O, Perdigao Cotta F, Scatton O. Pure laparoscopic right hepatectomy in a living donor. Am J Transplant. 2013;13(9):2467–2471. doi:10.1111/ajt.12361
7. Lee J-M, Shehta A, Lee K-W, et al. Donor wound satisfaction after living-donor liver transplantation in the era of pure laparoscopic donor hepatectomy. Surg Endosc. 2021;35(5):2265–2272. doi:10.1007/s00464-020-07640-2
8. Samstein B, Griesemer A, Cherqui D, et al. Fully laparoscopic left- sided donor hepatectomy is safe and associated with shorter hospital stay and earlier return to work: a comparative study. Liver Transpl. 2015;21(6):768–773. doi:10.1002/lt.24116
9. Egger ME, Gottumukkala V, Wilks JA, et al. Anesthetic and operative considerations for laparoscopic liver resection. Surgery. 2017;161(5):1191–1202. doi:10.1016/j.surg.2016.07.011
10. Mise Y, Sakamoto Y, Ishizawa T, et al. A worldwide survey of the current daily practice in liver surgery. Liver Cancer. 2013;2(1):55–66. doi:10.1159/000346225
11. Donadon M, Molinari AF, Corazzi F, et al. Pharmacological Modulation of Ischemic-Reperfusion Injury during Pringle Maneuver in Hepatic Surgery. A Prospective Randomized Pilot Study. World J Surg. 2016;40(9):2202–2212. doi:10.1007/s00268-016-3506-1
12. Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59(5):1094–1106. doi:10.1016/j.jhep.2013.06.017
13. Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11(8):1563–1569. doi:10.1111/j.1600-6143.2011.03579.x
14. Black GE, Sokol KK, Moe DM, et al. Impact of a novel phosphoinositol-3 kinase inhibitor in preventing mitochondrial DNA damage and damage-associated molecular pattern accumulation: results from the biochronicity project. J Trauma Acute Care Surg. 2017;83(4):683–689. doi:10.1097/TA.0000000000001593
15. Abecassis MM, Fisher RA, Olthoff KM, et al. Complications of living donor hepatic lobectomy-a comprehensive report. Am J Transplant. 2012;12(5):1208–1217. doi:10.1111/j.1600-6143.2011.03972.x
16. Song GW, Lee SG. Living donor liver transplantation. Curr Opin Organ Transplant. 2014;19(3):217–222. doi:10.1097/MOT.0000000000000088
17. Liu J, Man K. Mechanistic insight and clinical implications of ischemia/reperfusion injury post liver transplantation. Cell Mol Gastroenterol Hepatol. 2023;15(6):1463–1474. doi:10.1016/j.jcmgh.2023.03.003
18. Weerink MAS, Struys MMRF, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. doi:10.1007/s40262-017-0507-7
19. Yu Y, Cheng Y, Pan Q, et al. Effect of the selective NLRP3 inflammasome inhibitor mcc950 on transplantation outcome in a pig liver transplantation model with organs from donors after circulatory death preserved by hypothermic machine perfusion. Transplantation. 2019;103(2):353–362. doi:10.1097/TP.0000000000002461
20. Xu KY, Tong S, Wu CY, et al. Nlrp3 inflammasome inhibitor MCC950 ameliorates obliterative bronchiolitis by inhibiting Th1/Th17 response and promoting treg response after orthotopic tracheal transplantation in mice. Transplantation. 2020;104(6):e151–e163. doi:10.1097/TP.0000000000003208
21. Wu Y, Qiu G, Zhang H, et al. Dexmedetomidine alleviates hepatic ischaemia-reperfusion injury via the PI3K/AKT/Nrf2-NLRP3 pathway. J Cell Mol Med. 2021;25(21):9983–9994. doi:10.1111/jcmm.16871
22. Yu W, Lyu J, Jia L, Sheng M, Yu H, Du H. Dexmedetomidine ameliorates hippocampus injury and cognitive dysfunction induced by hepatic ischemia/reperfusion by activating SIRT3-mediated mitophagy and inhibiting activation of the NLRP3 inflammasome in young rats. Oxid Med Cell Longev. 2020;2020:7385458. doi:10.1155/2020/7385458
23. Lv M, Zeng H, He Y, Zhang J, Tan G. Dexmedetomidine promotes liver regeneration in mice after 70% partial hepatectomy by suppressing NLRP3 inflammasome not TLR4/NFκB. Int Immunopharmacol. 2018;54:46–51. doi:10.1016/j.intimp.2017.10.030
24. Zhang Y, Liu M, Yang Y, Cao J, Mi W. Dexmedetomidine exerts a protective effect on ischemia-reperfusion injury after hepatectomy: a prospective, randomized, controlled study. J Clin Anesth. 2020;61:109631. doi:10.1016/j.jclinane.2019.109631
25. Wang ZX, Huang CY, Hua YP, Huang WQ, Deng LH, Liu KX. Dexmedetomidine reduces intestinal and hepatic injury after hepatectomy with inflow occlusion under general anaesthesia: a randomized controlled trial. Br J Anaesth. 2014;112(6):1055–1064. doi:10.1093/bja/aeu132
26. Taman HI, Elhefnawy E. Hepatic protective effect of dexmedetomidine after partial hepatectomy surgery: a prospective controlled study. Anesth Essays Res. 2019;13(1):132–137. doi:10.4103/aer.AER_106_18
27. Huang YQ, Wen RT, Li XT, Zhang J, Yu ZY, Feng YF. The protective effect of dexmedetomidine against ischemia-reperfusion injury after hepatectomy: a meta-analysis of randomized controlled trials. Front Pharmacol. 2021;12:747911. doi:10.3389/fphar.2021.747911
28. Fayed NA, Sayed EI, Saleh SM, Ehsan NA, Elfert AY. Effect of dexmedetomidine on hepatic ischemia-reperfusion injury in the setting of adult living donor liver transplantation. Clin Transplant. 2016;30(4):470–482. doi:10.1111/ctr.12713
29. Zhang L, Cui LL, Yang WH, Xue FS, Zhu ZJ. Effect of intraoperative dexmedetomidine on hepatic ischemia-reperfusion injury in pediatric living-related liver transplantation: a propensity score matching analysis. Front Surg. 2022;9:939223. doi:10.3389/fsurg.2022.939223
30. Zhang L, Li N, Cui LL, Xue FS, Zhu ZJ. Intraoperative low-dose dexmedetomidine administration associated with reduced hepatic ischemia-reperfusion injury in pediatric deceased liver transplantation: a retrospective cohort study. Ann Transplant. 2021;26:e933354. doi:10.12659/AOT.933354
31. Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT Statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11(1):32. doi:10.1186/1745-6215-11-32
32. Zhang HM, Wei L, Li HY, et al. Impact of pure laparoscopic surgery on bile duct division of living donor left lateral section procurement. Hepatobiliary Surg Nutr. 2023;12(3):328–340. doi:10.21037/hbsn-21-418
33. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi:10.1097/SLA.0b013e3181b13ca2
34. Alkozai EM, Nijsten MW, de Jong KP, et al. Immediate postoperative low platelet count is associated with delayed liver function recovery after partial liver resection. Ann Surg. 2010;251(2):
35. Singh K, Gupta JK, Kumar S, Anurag, Mukherjee S, Patel A. Hepatic Ischemia-reperfusion Injury: protective Approaches and Treatment. Curr Mol Pharmacol. 2024;17:e030823219400. doi:10.2174/1874467217666230803114856
36. Huang Y, Xu Q, Zhang J, et al. Prussian Blue Scavenger Ameliorates Hepatic Ischemia-Reperfusion Injury by Inhibiting Inflammation and Reducing Oxidative Stress. Front Immunol. 2022;13:891351. doi:10.3389/fimmu.2022.891351
37. Bao N, Tang B. Organ-protective effects and the underlying mechanism of dexmedetomidine. Mediators Inflamm. 2020;2020:6136105. doi:10.1155/2020/6136105
38. Wang X, Li YR, Shi Y, et al. Dexmedetomidine ameliorates liver injury and maintains liver function in patients with hepatocellular carcinoma after hepatectomy: a retrospective cohort study with propensity score matching. Front Oncol. 2023;13:1108559. doi:10.3389/fonc.2023.1108559
39. Jia C, Dai C, Wang H, et al. Differential effects of three techniques for hepatic vascular exclusion during resection for liver cirrhosis on hepatic ischemia-reperfusion injury in rats. Gastroenterol Res Pract. 2018;2018:5309286. doi:10.1155/2018/5309286
40. Reddy SK, Marsh JW, Varley PR, et al. Underlying steatohepatitis, but not simple hepatic steatosis, increases morbidity after liver resection: a case-control study. Hepatology. 2012;56(6):2221–2230. doi:10.1002/hep.25935
41. Shin S, Joo DJ, Kim MS, et al. Propofol intravenous anaesthesia with desflurane compared with desflurane alone on postoperative liver function after living-donor liver transplantation: a randomised controlled trial. Eur J Anaesthesiol. 2019;36(9):656–666. doi:10.1097/EJA.0000000000001018
42. Hu Q, Liu XM, Liu ZR, et al. Dexmedetomidine reduces enteric glial cell injury induced by intestinal ischaemia-reperfusion injury through mitochondrial localization of TERT. J Cell Mol Med. 2022;26(9):2594–2606. doi:10.1111/jcmm.17261
43. Chen Y, Zhang X, Zhang B, He G, Zhou L, Xie Y. Dexmedetomidine reduces the neuronal apoptosis related to cardiopulmonary bypass by inhibiting activation of the JAK2-STAT3 pathway. Drug Des Devel Ther. 2017;11:2787–2799. doi:10.2147/DDDT.S140644
44. Fan X, Du J, Wang MH, et al. Irisin contributes to the hepatoprotection of dexmedetomidine during intestinal ischemia/reperfusion. Oxid Med Cell Longev. 2019;2019:7857082. doi:10.1155/2019/7857082
45. Benoit L, Dieu A, Foguenne M, Bonaccorsi-Riani E. Experimental and clinical aspects of sevoflurane preconditioning and postconditioning to alleviate hepatic ischemia-reperfusion injury: a scoping review. Int J Mol Sci. 2023;24(3):2340. doi:10.3390/ijms24032340
46. Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver Transpl. 2005;11(9):1031–1047. doi:10.1002/lt.20504
47. Chen Y, Bian W, Xu B. Pretreatment with dexmedetomidine alleviates lung injury in a rat model of intestinal ischemia reperfusion. Mol Med Rep. 2020;21(3):1233–1241. doi:10.3892/mmr.2020.10942
48. Shan X-S, Hu L-K, Wang Y, et al. Effect of perioperative dexmedetomidine on delayed graft function following a donation-after-cardiac-death kidney transplant: a randomized clinical trial. JAMA Netw Open. 2022;5(6):e2215217. doi:10.1001/jamanetworkopen.2022.15217
49. Nakashima T, Miyamoto K, Shima N, et al. Dexmedetomidine improved renal function in patients with severe sepsis: an exploratory analysis of a randomized controlled trial. J Intensive Care. 2020;8(1):1. doi:10.1186/s40560-019-0415-z
50. Moon J, Chun DH, Kong HJ, et al. The intraoperative administration of dexmedetomidine alleviates postoperative inflammatory response in patients undergoing laparoscopy-assisted gastrectomy: a double-blind randomized controlled trial. Biomedicines. 2023;11(12):3253. doi:10.3390/biomedicines11123253
51. Lee S. Dexmedetomidine: present and future directions. Korean J Anesthesiol. 2019;72(4):323–330. doi:10.4097/kja.19259
52. Ou Y, Liu G, Yin F, Yang Y, Zhang F. Protective effect of ulinastatin combined with dexmedetomidine against hepatic ischemia-reperfusion injury in laparoscopic hepatectomy for liver cancer and cirrhosis: a randomized controlled trial. Nan Fang Yi Ke Da Xue Xue Bao. 2022;42(12):1832–1838. doi:10.12122/j.issn.1673-4254.2022.12.11
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



