Back to Journals » Journal of Pain Research » Volume 18
Diaphragm Fatigue Does Not Impact Breathing Mechanics or Function in People with Chronic Low Back Pain
Authors Mitchell UH , Robinson LT , Brinkman J, Bruening DA, Bowden AE , Allen SP
Received 21 October 2024
Accepted for publication 5 February 2025
Published 26 February 2025 Volume 2025:18 Pages 963—974
DOI https://doi.org/10.2147/JPR.S496917
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Michael A Ueberall
Ulrike H Mitchell,1,* Lindsey T Robinson,1,* Jared Brinkman,2 Dustin A Bruening,1 Anton E Bowden,2 Steven P Allen3
1Department of Exercise Sciences, Brigham Young University, Provo, UT, USA; 2Department of Mechanical Engineering, Brigham Young University, Provo, UT, USA; 3Department of Electrical and Computer Engineering, Brigham Young University, Provo, UT, USA
*These authors contributed equally to this work
Correspondence: Ulrike H Mitchell, Email [email protected]
Background and Objective: The diaphragm is an important respiratory muscle that also plays a crucial role in developing intra-abdominal pressure and assisting in spinal stability. Chronic low back pain (CLBP) is a complex musculoskeletal condition and has been associated with respiratory problems and altered breathing mechanics. The purpose of this comparative cross-sectional study is to investigate whether diaphragm fatigue affects breathing mechanics and function in individuals with CLBP.
Methods: Volunteers were recruited between the ages of 35– 65 years old with and without CLBP. Participants performed 30 minutes of exercises intended to fatigue the diaphragm. The following respiratory parameters were measured: Breathing mechanics (chest and abdominal movement and respiratory rate), diaphragm function (forced expiratory volume in 1 second (FEV1) using a handheld spirometer), and diaphragm movement as measured by sub-diaphragm (L1 to diaphragm domes) volume changes at the end of exhalation and inhalation using MRI scans.
Results: A total of 36 participants were recruited (n = 21M; n = 15F), with 18 participants suffering from CLBP (n = 10M; 8F) and 18 participants serving as a comparison group (n = 11M; n = 7F). There were no differences in breathing mechanics, diaphragm function or excursion (volumetrics) between people with and without CLBP after 30 minutes of exercises intended to fatigue the diaphragm.
Conclusion: The lack of differences suggests that the diaphragm, regardless of its fatigue status as a spinal stabilizer, will not relinquish or weaken its function as a breathing muscle, though it may be less effective as a spinal stabilizer. Individuals with chronic or recurrent low back pain might therefore be more susceptible to subsequent pain flare-ups than non-symptomatic individuals. A potential clinical implication of these findings is that incorporating diaphragmatic breathing exercises to enhance diaphragm function may offer an effective treatment option for patients with CLBP.
Plain Language Summary: Why was the study done: The diaphragm is a key muscle for breathing but also stabilizes the spine. A prior study has shown that people experiencing chronic low back pain (CLBP) are more susceptible to diaphragm fatigue as a breathing muscle compared to asymptomatic subjects. This fatigue is thought to potentially lead to a decline in respiratory function and compromise the diaphragm’s effectiveness as a spinal stabilizer. This study was performed to see if the opposite is also true: if the diaphragm is fatigued as a spine-stabilizing muscle, will diaphragm movement and breathing be compromised in people with CLBP?
What did the researchers do and find: Thirty-six adults (21 men, 15 women) aged 35– 65, with (10 men, 8 women) and without (11 men, 7 women) CLBP, participated. Participants performed 30 minutes of exercises designed to tire out the diaphragm. Researchers measured breathing patterns (chest and abdominal movement, breathing rate), diaphragm function (using a handheld device to measure air exhaled in one second), and diaphragm movement (using MRI scans to track volume changes during breathing). The breathing patterns and diaphragm function of both groups were compared, but no significant differences were found.
What do these results mean: Even when the diaphragm is tired, it continues to work well for breathing, though it may be less effective in supporting the spine. This could mean people with chronic low back pain are more prone to future flare-ups.
Keywords: back pain, chronic, breathing, mechanics, functional movement, imaging, diaphragm, spine
Introduction
Background/Rationale
Deficiencies in trunk stabilization and postural control are significant factors associated with and even contributing to acute1 and chronic low back pain (CLBP).2 Additionally, trunk stabilization exercises have consistently demonstrated improvements in functional disability for individuals with CLBP.3,4 Furthermore, there is a well-established correlation between low back pain and respiratory disorders.5 These findings suggest a close anatomical relationship between breathing and low back pain, with the diaphragm potentially acting as the connecting element due to its primary roles in breathing and spine stabilization/postural trunk control. When the diaphragm fails to balance these dual functions, spine destabilization may occur, potentially leading to low back pain.6 One possible underlying cause of this imbalance is muscle fatigue, defined as a temporary reduction in muscle function.7 Janssens et al8 showed that increasing the diaphragm’s workload as a breathing muscle (eg, by adding resistance to inspiratory airflow) diminishes its effectiveness in postural control. In addition, it has been suggested that fatigue could potentially lead to a decline in the diaphragm’s respiratory function9 and that diaphragmatic fatigue is more likely to occur in people with CLBP compared to asymptomatic subjects.6
The dual roles of the diaphragm are described below.
Breathing
The diaphragm separates the torso into thoracic and abdominal cavities, serving as the floor of the thoracic cavity and the ceiling of the abdominal cavity. During inhalation, the diaphragm contracts, moves caudally and flattens. This action increases the vertical length of the thoracic cavity and allows for greater lung expansion.10 This is called diaphragmatic or “abdominal” breathing and is typically predominantly used during quiet breathing. In contrast, “chest breathing” involves upward movement of the diaphragm and the activation of accessory breathing muscles that pull the superior rib cage and the shoulder girdle cranially. It is deemed shallower and considered atypical and dysfunctional during quiet breathing if used in an isolated fashion. Chest breathing leads to greater thoracic and smaller abdominal movement, caused by decreased contraction of the diaphragm,10 a smaller tidal volume and higher respiratory rate compared to diaphragmatic breathing.11 Chest breathers are characterized by predominant movement in the thoracic area, while abdominal breathers exhibit more movement in the abdominal area. The most efficient way to quietly breathe is to use both, thoracic and abdominal movements, in a coordinated manner, called “costo-diaphragmatic breathing”.12 Over-reliance on either one could indicate the presence of a dysfunctional breathing pattern and has been associated with people with CLBP.13 A weakened or fatigued diaphragm may impair breathing by preventing the diaphragm muscle from contracting strongly enough to generate adequate negative pressure in the chest cavity, resulting in less air entering the lungs during inhalation. As a result, accessory breathing muscles may need to work harder, leading to fatigue, and individuals may experience shortness of breath during exercise.14
Spine Stabilization
The diaphragm influences lumbar spine stability through direct and indirect mechanisms. It acts directly on the vertebrae via its anatomical tendinous attachments on the lumbar spine at the anterior aspect of the L1 and L2 vertebrae.15 Contraction of this dome-shaped structure results in a “hoop-like” arrangement of the abdominal muscles that reduces the movement of abdominal contents into the thoracic cavity. This creates tension in the thoracolumbar fascia, increasing spinal stability.15
Indirectly, its contraction, in concert with the transverse abdominis and pelvic floor muscles,16 leads to a dramatic increase in intra-abdominal pressure17 and a marked increase in spine stabilization. The increase in intra-abdominal pressure stiffens the spine, offers postural control and prepares it to handle changes in movement and vertebral loading.
Diaphragmatic weakness can destabilize the lumbar spine through several mechanisms. First, the above mentioned reduced intra-abdominal pressure directly diminishes spinal support, thereby increasing stress in the lumbar region. Second, compensatory postural changes from faulty breathing patterns can result in poor spinal alignment, rendering the spine less stable. Third, other lumbar stabilizing muscles may compensate for the weakened diaphragm, potentially causing muscle imbalances and fatigue.1
Pain as a Mediator of Respiratory Function and Breathing Mechanics
Acute pain has been shown to increase respiratory frequency, flow, and volume.18 However, the direct influence of chronic pain on respiration is still unclear. For example, Shah et al19 found no differences in respiratory function, as measured in end-tidal carbon dioxide levels, maximal voluntary ventilation and respiratory rate, in individuals with and without CLBP. Kolar et al1 observed smaller diaphragm excursions and higher diaphragm position with tidal breathing in patients with CLBP during isometric exercise conditions, but not at rest. Their findings confirmed prior research that found that differences in respiratory function20 and breathing mechanics13 become noticeable when assessed during an activity.
Based on these observations, it has been postulated that chronicity or recurrence of symptoms may alter the relationship between low back pain and breathing mechanics, as long-lasting pain can lead to central sensitization, affecting the pain experience based on various contextual, psychological, and emotional factors.21
These previous efforts to probe the relationship between diaphragm fatigue and CLBP expose a key mechanistic gap in our current understanding. Does the diaphragm fatigue in its role as a spine stabilizer first, leading to decreased function with regard to breathing mechanics? Or does depression in breathing mechanics occur first (due to other biopsychosocial factors associated with CLBP), leading to decreased spinal stability? Our study aims to address these questions by investigating the first scenario: if the diaphragm of CLBP individuals is fatigued in its role as a spinal stabilizer, will its function as a breathing muscle also be impaired?
The objective of our study was, therefore, to assess breathing mechanics, diaphragm function, and diaphragm excursion in those who suffer from CLBP compared to healthy individuals after a 30-minute functional spinal movement program intended to fatigue the diaphragm.
We hypothesized that individuals with CLBP exhibited different breathing mechanics, a higher respiratory rate, less diaphragm movement with deep inhalation and exhalation and decreased diaphragm function compared to individuals without CLBP after a movement program intended to fatigue the diaphragm.
Methods
Participants
Sample size was determined based on two studies that used 2022 and 4023 participants for the assessment of diaphragm dimensions and abdominal muscle thickness, respectively, at inspiration and expiration extremes. Adults aged 35–65 years were recruited from the Provo, Utah area, through print and online advertisements between June 2021 and March 2024 for a larger study examining movement patterns in individuals with CLBP compared to a non-LBP comparison group.24 Data collection for this specific sub-study, which was determined a priori, took place between October 2, 2022, and June 21, 2023. Participants were between the ages of 35–65 years. Individuals were excluded if they were diagnosed with scoliosis with a Cobb angle ≥35°, had previous lower extremity injuries or surgeries within the last 6 months prior to data collection, had pain radiating beyond the knee or any current respiratory conditions or diseases (ie, COPD, asthma, etc.). Those who were categorized as being asymptomatic did not have CLBP within the last 6 months prior to data collection. Those who were categorized as having CLBP had low back pain rated at least a “2” on the Numeric Pain Rating Scale (NPRS)25 every day for the last 3 months or 50% of the time within the last 6 months for which they sought treatment.
Variables and Data Sources/Measurement
Data collection occurred at Brigham Young University following a protocol approved by our Institutional Review Board. The participants were informed about the purpose of the study, in accordance with the Declaration of Helsinki and gave written consent which was witnessed by the researcher or MRI operator. They then provided their demographic information, including their current pain intensity and medical history. Testing sequence was always: 1. breathing mechanics (respiratory sensors), 2. diaphragm movement/volumetrics (MRI) followed by 3. forced expiratory volume in one second (spirometry). All participants performed a movement protocol, consisting of non-functional and functional activities designed to fatigue the spinal stabilizers as described below24 immediately before their MRI scan. This was done to capture the diaphragm volumetrics as close as possible after the fatiguing protocol.
Breathing Mechanics
Respiratory sensors (Bio-Medical Instruments’ Respiration Sensor SA9311M) were used to measure axial displacement of the chest and abdomen as well as respiratory rate in a standing position (Figure 1).
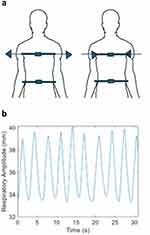 |
Figure 1 Respiratory sensors. (a) Placement of sensors on chest and abdomen. (b) Typical waveform generated by movements of the thorax. |
The thoracic sensor was placed at the midsternal level, and the abdominal sensor was placed at the midline of the abdominal wall just above the umbilicus (Figure 1a).26 While fastening the sensors, the participants were asked to fully inhale and exhale. At the end of full exhalation, the sensors were quickly fastened so that they were fully secured and with slight tension on the band.27 Sensor signaling was recorded using the manufacturer’s software.
Data preparation: For both the thoracic and abdominal sensors, the average peak-to-peak displacement and average peak-to-peak cycle time (ie, surrogate for respiratory rate) were measured (Figure 1b). Displacements were calculated using the manufacturer’s software, while cycle times were calculated using a custom MATLAB (Natik, Massachusetts) script. Changes in individual sensor measurements over time were evaluated as well as differences between the thoracic and abdominal displacement measurements. Resting breaths were recorded for 90 seconds. After these measurements were taken, the sensors were taken off.
Exercise Description
A trained research assistant provided instruction to the participants regarding specific movement patterns and closely monitored their performance throughout the session. The exercise intervention lasted approximately 30 minutes and included a combination of uni-planar and functional movements aimed at promoting movement and stability of the lumbar spine.24 The movements were carried out in a standing position and comprised lumbar sagittal plane flexion and extension, flexion with right and left rotation, extension with right and left rotation, transverse plane lumbar rotation to both sides, and coronal plane lateral flexion of the spine to the right and left. Additionally, participants transitioned from a seated position on a stool to a standing position without the use of their hands. A total of 6 repetitions for each exercise were selected to effectively challenge the stabilizing muscles, including the diaphragm while not exacerbating the pain.28 This is significant as repeated muscle activation can influence neuromuscular function and feedback, potentially leading to a decrease in mechanical sensitivity.29 The sagittal plane movements were performed at two distinct speeds: a self-selected pace and the maximum speed achievable while maintaining safety. The rationale for employing varying speeds in exercise execution was to challenge local dynamic stability, which has been shown to be limited in fast movements.30 The uni-planar movements (flexion/extension, rotation, and lateral bending to both sides) were deliberately chosen for their conceptual simplicity and feasibility, based on evidence indicating that individuals with low back pain frequently exhibit alterations in motor control.31 In contrast, the multiplanar movements (flexion and extension coupled with rotation) were selected to better replicate functional activities, acknowledging that the spine typically engages in multi-planar motion simultaneously. However, they are inherently more difficult to perform because they challenge the body’s awareness of its position in space and motor control.31 The sit-to-stand motion was specifically chosen due to its reliance on lumbar stability, requiring a coordinated contraction of core muscles to transition from a flexed lumbar spine position while sitting to an extended position upon standing.
All participants were instructed to perform each movement at a comfortable pace. As described above, sagittal plane flexion and extension was also performed at the fastest speed possible while staying safe. Symptomatic participants, prone to experiencing pain, were encouraged to only perform the movements within their comfort range. If they expressed any discomfort, they were advised to stop or slow down. All participants were able to complete all movement patterns.
Diaphragm Excursion (Volumetrics)
A Siemens VIDA 3.0T MRI scanner was used to image the diaphragm. Participants were oriented in a supine position with their heads facing the bore. An 18-channel abdominal coil was placed on the participant over the location of their diaphragm, and the subjects lay on an 8-channel spine coil. Imaging of the diaphragm included its distal attachments at the first lumbar vertebrae via the diaphragmatic crura and its most superior point at the level of T6. It also included its anterior and lateral muscle bundles or “diaphragmatic slips” that attach proximally to the lower six ribs and costal cartilages and the xiphoid process.
Participants were asked to perform breath holds at the end of inhalation and the end of exhalation at two non-consecutive time points. The length of each breath hold sequence was 19 seconds. During each breath hold, subjects were scanned using the following MRI sequence parameters. Sequence Type : 3D, Spoiled Gradient Echo, TE/TR: 1.74/4 ms, FOV: 400 × 300 × 288 mm; Image Matrix Size: 320 × 180 × 96; Resolution: 1.3 × 1.3 × 3 mm; Flip Angle: 5°; Fat Suppression: None; Bandwidth: 500 hz/pixel; Acceleration Strategy: CAIPIRINHA; Acceleration Factor: 4; Acquisition Time: 19s. The imaging volume was placed sagittally in the axial topogram going paravertebrally on the right side, midway through the center of the vertebral body and edge of the thoracic wall.32
Data preparation: We measured the volume between L1 and diaphragm domes during inhalation and exhalation. The volume change between exhalation and inhalation was used as a proxy for diaphragm movement. With diaphragmatic breathing, the volume between an imaginary vertical line between the first lumbar vertebra (L1) and the domes of the diaphragms becomes smaller with inspiration and greater with expiration. A chest breather has a smaller volume difference compared to a diaphragmatic breather.
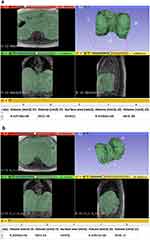
Volumes were measured based on semi-automatic “growth by seeds” segmentation of MRI scans using 3D Slicer Image Computing Software (Figure 2a and b). Each series of data was analyzed blind (the engineer performing the data analysis had no prior knowledge of the files belonging to either a CLBP or control participant), and volume measurements for the diaphragm domes were obtained.
Diaphragm Function
A spirometer (SpiroLink’s handheld Spirometer Model B1) was used to measure forced expiratory volume in one second (FEV1). The device includes a rechargeable unit with a digital display area and removable mouthpieces. Each participant was provided with a new disposable mouthpiece for their test. This test was conducted with the participant in standing position. The participant was given the following instructions: (1) plug your nose, (2) take a deep breath in, (3) place mouth over the mouthpiece, (4) exhale as hard and as quick as you possibly can and (5) relax. This test was performed three times, the FEV1 displayed on the digital screen of the spirometer was recorded and the three trials from each subject were averaged.
Statistical Analyses
All metrics were compared between CLBP and control groups using independent t-tests (α=0.05). A total of thirteen metrics were tested; five were from participant demographics, six from breathing mechanics, and one each from volumetrics and spirometry.
Results
Participants, Descriptive Data and Outcome Data
We recruited 36 participants (n = 21M; n = 15F), of whom 18 were controls (n = 11M; n = 7F) and 18 were CLBP (n = 10M; 8F) subjects (Table 1). The average time between breaths was lost (not saved) for 5 of the control participants. The MRI scan of one individual with CLBP could not be used because the scan did not include L1.
 |
Table 1 Participant Demographics |
Main Results
There was no difference between groups for any of the outcome variables that measured breathing mechanism (abdominal and chest expansion and time between breaths), diaphragm excursion as measured by volumetric changes, or diaphragm function (FEV1) (Table 2, Figure 2a and b) . P-values for the t-test comparisons ranged between 0.34 and 0.97, indicating very little probability that the controls and participants with CLBP represent statistically distinct populations with regard to these respiratory variables under the experimental conditions.
 |
Table 2 Respiratory Variables |
Discussion
The aim of our study was to evaluate and compare breathing mechanics, diaphragm function, and diaphragm excursion in individuals with CLBP and healthy controls following a 30-minute spinal movement program designed to induce diaphragm fatigue. We did not find any differences between any of the variables. As posited in the introduction, a lack of differences is a meaningful result, pointing more specifically towards an alternative hypothesis. Specifically, the diaphragm, regardless of its fatigue status as spinal stabilizer, will not relinquish or weaken its function as a breathing muscle, though since muscle fatigue implies a decline in its function7 it may be less effective as a spinal stabilizer.6 Individuals with chronic or recurrent low back pain might therefore be more susceptible to subsequent pain flare-ups than non-symptomatic individuals. Whether this is a function of a centralized pathway due to separate biopsychosocial triggers or just a mechanical response to fatigue is more challenging to directly address through a small cohort study. Thus, the present work represents a necessary precursor to a much larger and more demanding study intended to address that topic.
Breathing Mechanics
Although there are clear associations between acute pain and some respiration variables,18 the lack of difference in any breathing mechanics metric suggests that this does not seem to directly translate to individuals with CLBP. The average pain in our group with CLBP was 4.1 on the visual analog pain scale. This is considered “mild pain”33 and might not be a strong enough stimulus to alter breathing mechanics, even if tolerated over a prolonged period of time. While there does not appear to be a straightforward linear relationship between pain intensity and altered breathing mechanics, “mild pain” may not be sufficient to trigger the cascade of physiological responses that result in changes to breathing patterns and diaphragm function. Pain, as a multifaceted experience involving both physical and psychological components,34 often correlates with increased anxiety and stress.35 However, only at higher intensities might pain elicit noticeable alterations in breathing patterns, such as shallower breaths primarily engaging the upper chest and rib cage.
Another possible, though unlikely, reason for not detecting any differences between the two groups could be related to the methods used for measurement, ie, we measured anterior/posterior (A/P) and lateral expansion of the rib cage. Chest breathing usually involves the activation of the intercostal muscles, leading to the expansion of the rib cage in the transverse plane. Deep breathing, such as that occurring during exercise, engages the scalenes and sternocleidomastoid muscles, resulting in a more pronounced superior rise of the chest.36 This vertical movement was not captured by the sensors we employed.
Surprisingly, we found that more participants in the control group (12/18) exhibited a thoracic/abdominal movement ratio greater than 1 (ie, chest expansion was greater than abdominal expansion during quiet breathing), while 6 participants had a ratio that was smaller than 1. In the group of individuals with CLBP there was an equal distribution (9/9).
We anticipated a higher respiratory rate for those with CLBP based on the findings by Glynn et al37 who reported that those experiencing pain have shorter and shallower breaths (ie, hyperventilating) than those who do not have pain. The authors surmise that those with pain hyperventilate most likely not because of the pain itself but rather from the individual’s anxiety towards the pain. Those who experience chronic pain develop changes in behavior that lead to fear of movement/reinjury, catastrophizing of their pain, and a general fear of pain.38–40 It is likely that our participants did neither experience the necessary high levels of pain at the time of testing nor the changes in respiratory rate associated with them.
Diaphragm Excursion
We hypothesized that the diaphragm in individuals with CLBP exhibited less movement during inhalation compared to the healthy group based on finding by Kolar et al1 who found that the diaphragm was positioned higher in the thorax in individuals with CLBP when compared to healthy individuals. We measured the volume changes between an imaginary axial line at level L2 and the diaphragmatic domes. We did not find any differences between groups. In fact, the volumetric data were very similar between the two groups, implying that on average the diaphragm moved similarly between inspiration and expiration in individuals with CLBP and controls. This seems to indicate that a functionally fatigued (ie, fatigued in its spine stabilizing function) diaphragm will not moderate its function as a primary breathing muscle in individuals with CLBP. This finding is interesting in light of Janssens et al’s6 conclusions that a diaphragm that was fatigued in its function as a breathing muscle potentially had diminished effectiveness as a spinal stabilizer. We found in our study that the reverse is not true. This is the first study assessing this directional relationship between the diaphragm and CLBP.
Diaphragm Function
The average FEV1 in both of our groups was 3.68 L/s with little variability. These values fall in line with average values in healthy individuals aged 20–60 years that range from 4.5 to 3.5 liters in males and from 3.25 to 2.5 liters in females.41 We expected a difference in FEV1 based on an earlier study20 where individuals with CLBP used 7.2% more of their vital capacity (48.2%) than those without LBP (40.9%) during a functional lifting task of a heavy load. It was hypothesized that individuals with CLBP relied more on respiratory mechanisms for lumbar support during the lift. Because our FEV1 was taken after several minutes of MRI scanning, it is possible and even likely that the diaphragm had recuperated and was no longer fatigued. In addition, the FEV1 measurement was taken with the individual in a quiet standing position and not during an activity, as in the above-named study. Lastly, although unlikely, it is possible that both groups experienced a decrease in FEV1 values due to diaphragm fatigue. Future studies should include pre- and post-testing to address this question.
Conclusion
After 30 minutes of movement exercises meant to fatigue the diaphragm, no differences were observed in breathing mechanics, diaphragm function or excursion (volumetrics) between individuals with and without CLBP. These findings suggest that the diaphragm, regardless of its fatigue status as spinal stabilizer, will not relinquish or weaken its function as a breathing muscle, though it may be less effective as a spinal stabilizer. However, due to the preliminary nature of these results, further research with a larger sample size and alternative methods is needed to confirm these conclusions and explore the potential implications for individuals with CLBP, who may be more susceptible to subsequent pain flare-ups. These findings suggest that incorporating diaphragmatic breathing exercises to improve diaphragm function may provide an effective treatment option for patients with CLBP.42 Furthermore, there appears to be a reciprocal relationship between diaphragm training and the strengthening of other core stabilizing muscles.43,44
Our study addresses just a small aspect of the complex relationship between respiratory mechanics and musculoskeletal health. Proper diaphragm function plays a crucial role, not only in directly influencing CLBP but also in affecting other contributing factors. These include (1) psychological components, as diaphragmatic breathing helps regulate the autonomic nervous system, promoting relaxation and body awareness,14 and (2) physical activity and overall fitness, where exercise encourages deeper breathing and improved tissue oxygenation.
Future studies should adopt a more experimental design to establish causal relationships. These studies should exclude participants with mild CLBP and include measurements of superior/inferior thoracic movement to better distinguish between chest and abdominal breathing. Additionally, future research could explore other factors influencing diaphragm function and breathing mechanics, such as psychological aspects of pain, physical fitness levels, and comorbid conditions.
Limitations and Generalizability
Our study was strengthened by the blinded nature of breathing mechanics and diaphragm excursion analyses. Nonetheless, it is important to acknowledge several limitations. There were no measures of the diaphragm in both a fatigued and a non-fatigued state to provide a self-control for each subject which limits the ability to make definite conclusions about the diaphragm’s performance under varying conditions. Similarly, there was no measure of whether the diaphragm was in a fatigued state. The lack of observed differences in this study may be due to the failure to sufficiently load or fatigue the diaphragm. There are no objective tests specifically for measuring diaphragm strength; instead, functional breathing tests like FEV1 are typically used. Although the participants were MRI scanned immediately after the exercise program, there were several minutes between these two occurrences, which could have been enough to rest the diaphragm. MRI measures were performed with the participants laying down. This could potentially decrease diaphragm use and relieve pain and thus confounded the results. It is also important to acknowledge the inherent limitations of observational studies in as much as they do not have an experimental design and cannot infer causation. Lastly, while we recruited participants of ages 35–65 years to reflect the age range with the greatest prevalence of CLBP,45 the findings may not generalize to adults younger and older.
We need to acknowledge possible confounding factors that could have influenced breathing mechanics and diaphragm function, such as physical fitness levels, comorbidities or psychological factors related to pain.
Acknowledgments
Research funds for this work were provided by the National Institutes of Health, as part of the BACPAC consortium of the HEAL initiative. Grant Number: UH3AR076723. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors thank the participants for taking part in the study. This paper has been uploaded to https://scholarsarchive.byu.edu/etd/10019 as a preprint in June 2023 by the 2nd author, Ms Lindsey Robinson (nee Lindsey Wensel).46
Disclosure
The authors have no financial or non-financial competing interest to report in this work.
References
1. Kolar P, Sulc J, Kyncl M, et al. Postural function of the diaphragm in persons with and without chronic low back pain. J Orthop Sports Phys Ther. 2012;42(4):352–362. doi:10.2519/jospt.2012.3830
2. Lamoth CJ, Meijer OG, Daffertshofer A, Wuisman PI, Beek PJ. Effects of chronic low back pain on trunk coordination and back muscle activity during walking: changes in motor control. Eur Spine J. 2006;15(1):23–40. doi:10.1007/s00586-004-0825-y
3. Hwangbo G, Lee CW, Kim SG, Kim HS. The effects of trunk stability exercise and a combined exercise program on pain, flexibility, and static balance in chronic low back pain patients. J Phys Ther Sci. 2015;27(4):1153–1155. doi:10.1589/jpts.27.1153
4. Porwal S, Rizvi MR, Sharma A, et al. enhancing functional ability in chronic nonspecific lower back pain: the impact of EMG-guided trunk stabilization exercises. Healthcare. 2023;11(15):2153. doi:10.3390/healthcare11152153
5. Beeckmans N, Vermeersch A, Lysens R, et al. The presence of respiratory disorders in individuals with low back pain: a systematic review. Man Ther. 2016;26:77–86. doi:10.1016/j.math.2016.07.011
6. Janssens L, Brumagne S, McConnell AK, Hermans G, Troosters T, Gayan-Ramirez G. Greater diaphragm fatigability in individuals with recurrent low back pain. Respir Physiol Neurobiol. 2013;188(2):119–123. doi:10.1016/j.resp.2013.05.028
7. Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol. 2008;586(1):11–23. doi:10.1113/jphysiol.2007.139477
8. Janssens L, Brumagne S, Polspoel K, Troosters T, McConnell A. The effect of inspiratory muscles fatigue on postural control in people with and without recurrent low back pain. Spine. 2010;35(10):1088–1094. doi:10.1097/BRS.0b013e3181bee5c3
9. Cuenca-Martinez F, Sempere-Rubio N, Munoz-Gomez E, Molla-Casanova S, Carrasco-Gonzalez E, Martinez-Arnau FM. Respiratory function analysis in patients with chronic pain: an umbrella review and meta-analysis of pooled findings. Healthcare. 2023;11(9):1358. doi:10.3390/healthcare11091358
10. Shimozawa Y, Kurihara T, Kusagawa Y, et al. Point prevalence of the biomechanical dimension of dysfunctional breathing patterns among competitive athletes. J Strength Cond Res. 2023;37(2):270–276. doi:10.1519/JSC.0000000000004253
11. Vieira DS, Mendes LP, Elmiro NS, Velloso M, Britto RR, Parreira VF. Breathing exercises: influence on breathing patterns and thoracoabdominal motion in healthy subjects. Braz J Phys Ther. 2014;18(6):544–552. doi:10.1590/bjpt-rbf.2014.0048
12. Higashino M, Miyata K, Kudo K. Coordination dynamics of thoracic and abdominal movements during voluntary breathing. Sci Rep. 2022;12(1):13266. doi:10.1038/s41598-022-17473-9
13. Roussel N, Nijs J, Truijen S, Vervecken L, Mottram S, Stassijns G. Altered breathing patterns during lumbopelvic motor control tests in chronic low back pain: a case-control study. Eur Spine J. 2009;18(7):1066–1073. doi:10.1007/s00586-009-1020-y
14. Bordoni B, Purgol S, Bizzarri A, Modica M, Morabito B. The influence of breathing on the central nervous system. Cureus. 2018;10(6):e2724. doi:10.7759/cureus.2724
15. Kocjan J, Adamek M, Gzik-Zroska B, Czyzewski D, Rydel M. Network of breathing. multifunctional role of the diaphragm: a review. response to the letter to editor of prof bordoni. Adv Respir Med. 2017;85(5):292–293. doi:10.5603/ARM.a2017.0048
16. Akuthota V, Nadler SF. Core strengthening. Arch Phys Med Rehabil. 2004;85(3 Suppl 1):S86–92. doi:10.1053/j.apmr.2003.12.005
17. Hodges PW, Eriksson AE, Shirley D, Gandevia SC. Intra-abdominal pressure increases stiffness of the lumbar spine. J Biomech. 2005;38(9):1873–1880. doi:10.1016/j.jbiomech.2004.08.016
18. Jafari H, Courtois I, Van den Bergh O, Vlaeyen JWS, Van Diest I. Pain and respiration: a systematic review. Pain. 2017;158(6):995–1006. doi:10.1097/j.pain.0000000000000865
19. Shah SG, Choezom T, Prabu Raja G. Comparison of respiratory parameters in participants with and without chronic low back pain. J Bodyw Mov Ther. 2019;23(4):894–900. doi:10.1016/j.jbmt.2019.03.008
20. Hagins M, Lamberg EM. Individuals with low back pain breathe differently than healthy individuals during a lifting task. J Orthop Sports Phys Ther. 2011;41(3):141–148. doi:10.2519/jospt.2011.3437
21. Crofford LJ. Chronic pain: where the body meets the brain. Trans Am Clin Climatol Assoc. 2015;126:167–183.
22. Cassart M, Pettiaux N, Gevenois PA, Paiva M, Estenne M. Effect of chronic hyperinflation on diaphragm length and surface area. Am J Respir Crit Care Med. 1997;156(2 Pt 1):504–508. doi:10.1164/ajrccm.156.2.9612089
23. Arab AM, Sheikhhoseini R, Rasouli O. Altered ultrasonographic activity of abdominal muscles during breathing in males with and without nonspecific chronic low back pain. J Ultrasound. 2021;24(4):457–462. doi:10.1007/s40477-020-00528-w
24. Baker SA, Billmire DA, Bilodeau RA, et al. Wearable nanocomposite sensor system for motion phenotyping chronic low back pain: a BACPAC technology research site. Pain Med. 2023;24(Supplement_1):S160–S174. doi:10.1093/pm/pnad017
25. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798–804. doi:10.1111/j.1365-2702.2005.01121.x
26. Gastinger S, Sefati H, Nicolas G, Sorel A, Gratas-Delamarche A, Prioux J. Estimates of ventilation from measurements of rib cage and abdominal distances: a portable device. Eur. J. Appl. Physiol. 2010;109(6):1179–1189. doi:10.1007/s00421-010-1463-1
27. Ltd. TT. Procomp2 TM hardward manual. 5250 Ferrier, Suite 812, Montreal, Quebec, Canada2021: 36.
28. Park CH, Beom J, Chung CK, et al. Long-term effects of lumbar flexion versus extension exercises for chronic axial low back pain: a randomized controlled trial. Sci Rep. 2024;14(1):2714. doi:10.1038/s41598-024-51769-2
29. Viggiani D, Callaghan JP. Interrelated hypoalgesia, creep, and muscle fatigue following a repetitive trunk flexion exposure. J Electromyogr Kinesiol. 2021;57:102531. doi:10.1016/j.jelekin.2021.102531
30. Granata KP, England SA. Stability of dynamic trunk movement. Spine. 2006;31(10):E271–6. doi:10.1097/01.brs.0000216445.28943.d1
31. van Dieen JH, Reeves NP, Kawchuk G, et al. Motor control changes in low back pain: divergence in presentations and mechanisms. J Orthop Sports Phys Ther. 2019;49(6):370–379. doi:10.2519/jospt.2019.7917
32. Vostatek P, Novák D, Rychnovský T, Rychnovská S. Diaphragm postural function analysis using magnetic resonance imaging. PLoS One. 2013;8(3):e56724. doi:10.1371/journal.pone.0056724
33. Cho S, Kim YJ, Lee M, Woo JH, Lee HJ. Cut-off points between pain intensities of the postoperative pain using receiver operating characteristic (ROC) curves. BMC Anesthesiol. 2021;21(1):29. doi:10.1186/s12871-021-01245-5
34. Raja SN, Carr DB, Cohen M, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi:10.1097/j.pain.0000000000001939
35. de Heer EW, Gerrits MMJG, Beekman MM, et al. The association of depression and anxiety with pain: a study from NESDA. PLoS One. 2014;9(10):e106907. doi:10.1371/journal.pone.0106907
36. Nelson N. Diaphragmatic breathing: the foundation of core stability. Strength Conditioning J. 2012;34(5):34–40. doi:10.1519/SSC.0b013e31826ddc07
37. Glynn CJ, Lloyd JW, Folkhard S. Ventilatory response to intractable pain. Pain. 1981;11(2):201–211. doi:10.1016/0304-3959(81)90005-1
38. Asmundson GJ, Norton PJ, Norton GR. Beyond pain: the role of fear and avoidance in chronicity. Clin Psychol Rev. 1999;19(1):97–119. doi:10.1016/s0272-7358(98)00034-8
39. Linton SJ. A review of psychological risk factors in back and neck pain. Spine. 2000;25(9):1148–1156. doi:10.1097/00007632-200005010-00017
40. Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85(3):317–332. doi:10.1016/s0304-3959(99)00242-0
41. Medical International Research. Understanding normal values in spirometry. Available from: https://spirometry.com.
42. Masroor S, Tanwar T, Aldabbas M, Iram I, Veqar Z. Effect of adding diaphragmatic breathing exercises to core stabilization exercises on pain, muscle activity, disability, and sleep quality in patients with chronic low back pain: a randomized control trial. J Chiropr Med. 2023;22(4):275–283. doi:10.1016/j.jcm.2023.07.001
43. Finta R, Nagy E, Bender T. The effect of diaphragm training on lumbar stabilizer muscles: a new concept for improving segmental stability in the case of low back pain. J Pain Res. 2018;11:3031–3045. doi:10.2147/JPR.S181610
44. Dulger E, Bilgin S, Bulut E, et al. The effect of stabilization exercises on diaphragm muscle thickness and movement in women with low back pain. J Back Musculoskelet Rehabil. 2018;31(2):323–329. doi:10.3233/BMR-169749
45. Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251–258. doi:10.1001/archinternmed.2008.543
46. Wensel L. Does Chronic Low Back Pain Influence Breathing Mechanics and Diaphragm Positioning? A Pilot Study. Brigham Young University; 2023. Available from: https://scholarsarchive.byu.edu/etd/10019.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.