Back to Journals » Journal of Pain Research » Volume 18
Differences in the Effectiveness of Different Physical Therapy Modalities in the Treatment of Delayed-Onset Muscle Soreness: A Systematic Review and Bayesian Network Meta-Analysis
Authors Chen J, Hu Q, Hu J, Liu S, Yin L
Received 17 February 2025
Accepted for publication 5 June 2025
Published 15 June 2025 Volume 2025:18 Pages 2993—3008
DOI https://doi.org/10.2147/JPR.S519242
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alaa Abd-Elsayed
Jing Chen,1– 3,* Qing Hu,1,2,* Jiajie Hu,1,2 Songtao Liu,1,2 Linyu Yin1,2
1Department of Rehabilitation Medicine, Affiliated Hospital (Clinical College) of Xiangnan University, Xiangnan University, Chenzhou, Hunan, People’s Republic of China; 2College of Medical Imaging Laboratory and Rehabilitation, Xiangnan University, Chenzhou, Hunan, People’s Republic of China; 3School of Acupuncture, Tuina and Rehabilitation, Hunan University of Chinese Medicine, Changsha, Hunan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Linyu Yin; Songtao Liu, Department of Rehabilitation Medicine, Affiliated Hospital (Clinical College) of Xiangnan University, No. 31, West Renmin Road, BeiHu District, Chenzhou, Hunan Province, 42300, People’s Republic of China, Tel +86 15173509798 ; +86 13875515541, Email [email protected]; [email protected]
Purpose: Delayed-onset muscle soreness (DOMS) is a common clinical condition that frequently affects various populations. Physical therapy offers distinct advantages in managing this condition. However, many recently published studies have produced conflicting results and lack compelling evidence, complicating clinicians’ decision making. We employed a Bayesian meta-analysis to assess the efficacy and safety of physical therapy modalities (PTMs) for DOMS, aiming to provide robust, evidence-based medical insights for clinical application.
Patients and Methods: We conducted a comprehensive search for randomized controlled trials (RCTs) evaluating PTMs for DOMS across databases, including CNKI, CSCD, CCD, CBM, PubMed, EMbase, Cochrane Library, and Web of Science, until October 22, 2024. The included studies were assessed for risk of bias using the Cochrane Risk of Bias Assessment tool, tailored for RCTs. A network meta-analysis was performed using R v.4.2.2.
Results: At 24 hours post-intervention, photobiomodulation therapy(PBMT) demonstrated a significant advantage over placebo (− 3.91 [− 5.57, − 2.17],P< 0.05). The effects of other therapies were not significant (Cryotherapy: − 0.58 (− 1.20, 0.11), Cryotherapy combined with PBMT: 0.48 (− 1.09, 2.01), ES: − 0.98 (− 2.82, 0.89), Irradiated: − 0.10 (− 1.71, 1.53), STM: − 0.89 (− 2.63, 0.85), UT: − 0.61 (− 1.92, 0.84)).At 48 hours post-intervention, both PBMT (− 5.24 [− 6.95, − 3.20],P< 0.05) and sauna (− 3.29 [− 6.21,-0.33],P< 0.05) exhibited significant effects compared to placebo.The effects of other therapies were not statistically significant.; However, beyond 48 hours, there was no notable benefit from PTMs when compared with placebo, indicating that PTMs are more effective within the initial 48 hours, with PBMT yielding superior outcomes.
Conclusion: The findings from this investigation indicate that PBMT and sauna treatment produce significant effects within the first 48 hours; however, beyond this period, the impact of photobiomodulation diminishes significantly. Overall, physical therapy modalities are the most effective within the 48-h window.
Keywords: delayed-onset muscle soreness, physical therapy modalities, efficacy, systematic review, bayesian network meta-analysis
Introduction
Delayed-onset muscle soreness (DOMS) is characterized by transient muscular injury resulting from high-intensity eccentric contractions or unfamiliar exercise modalities.1 Symptoms typically manifest 6–12 hours post-exercise, peak between 24 and 72 hours, and gradually resolve within 5–7 days.2,3 Common symptoms of DOMS include muscle pain, reduced strength, stiffness, and swelling. The associated decrease in muscle strength and joint range of motion can negatively affect athletic performance during training or competition.4 This may lead to athletes requiring additional medical intervention, such as physical therapy, massage, and cold and hot therapy. However, these treatments are expensive, and thus, DOMS increases economic burdens. For instance, massage is believed to accelerate recovery and reduce the duration of interruptions to training. However, its cost may pose a financial burden on individuals or teams. DOMS may prevent athletes from participating in training or competitions on time, thereby affecting the overall performance and event results of the team. Such interruptions may require additional compensation or fines, further increasing the economic burden. In high-intensity sports, such as rugby, DOMS may cause long-term muscle injuries and inflammation, requiring a longer period of rehabilitation. Studies have shown that even after adequate rest, DOMS may persist, which increases the cost of long-term treatment.5 DOMS reduces athletes’ strength, flexibility, and endurance, thereby limiting their training intensity and frequency. This limitation may prevent athletes from reaching their best competitive state, thereby affecting the length of their careers. Long-term DOMS can trigger psychological stress and burnout in athletes, particularly in high-intensity competitive environments, which may accelerate the end of their careers.6
The current interventions for DOMS include anti-inflammatory pharmacotherapy, physical therapy, acupuncture, and massage.7 While nonsteroidal anti-inflammatory drugs are frequently used to alleviate DOMS symptoms, prolonged use may have detrimental effects on muscle hypertrophy.8 Consequently, there is an urgent need for alternative therapeutic approaches to facilitate recovery. In recent years, physical therapy modalities(PTMs) has gained prominence in the management of DOMS because of its noninvasive and painless nature. Modalities, such as massage, lymphatic drainage techniques, thermotherapy (including cryotherapy), stretching exercises, vibration therapy, and laser treatment, share a common mechanism aimed at mitigating exercise-induced muscle damage and inflammation. This mechanism limits edema formation by reducing the available space for fluid accumulation in interstitial areas while enhancing the transport of metabolites/neutrophils/injury-related proteins from muscles into the circulation through alterations in blood flow dynamics, thereby diminishing exercise-induced muscular injury and inflammation.9,10 Previous studies have validated the efficacy of various physical therapy modalities, including cold therapy, light therapy, and alternating heat and cold treatments,11–13 however, the optimal therapeutic approach remains undetermined.
Numerous randomized controlled trials (RCTs) investigating treatments for DOMS have emerged in recent decades,10,14–19 however, these studies often yield conflicting results without convincingly establishing the effectiveness of distinct physical therapy modalities, complicating clinical decision-making processes. Therefore, we conducted a network meta-analysis aimed at evaluating the efficacy of diverse physical therapy modalities for treating DOMS, while performing probability rankings based on outcome indicators to identify superior physical therapy modalities that can enhance informed clinical decision-making.
Materials and Methods
Study Registration
This study has been registered with PROSPERO (ID: CRD42024597844).
Eligibility Criteria
Inclusion Criteria
Population
Patients with Delayed-onset muscle soreness(DOMS). Race, nationality, sex, age, and course of the disease were not restricted.
Intervention
Physical therapy modalities included cold therapy, heat therapy, alternating hot and cold therapy, electrical stimulation(ES), light therapy, shock wave therapy, electromagnetic field therapy, and ultrasonic wave.
Comparison
Placebo.
Outcome
The Visual Analog Scale (VAS),20 a widely used instrument for DOMS, that quantifies pain intensity on a 10-cm linear continuum, where 0 signifies “no pain” and 10 denotes “extreme pain”. Patients were instructed to indicate their perceived level of discomfort by marking a line, with higher scores corresponding to greater levels of pain. This scale is applicable across various types of pain and patient populations, offering a precise reflection of patients’ subjective experiences.
Study Design
Randomized controlled trial.
Exclusion Criteria
Studies were excluded if the full text was unavailable, necessary outcome indicators were missing, or data for these indicators were incomplete. Additionally, studies with significant design flaws, animal experiments, systematic reviews, case reports, observational study, conference proceedings or those in which the control group received active recovery or other treatments affecting recovery were excluded.
Data Sources and Search Strategy
RCTs evaluating physical therapy modalities for Delayed-onset muscle soreness(DOMS) were searched in CNKI, CSCD,CCD,CBM, PubMed, Embase, Cochrane Library, and Web of Science until October 22, 2024. The search strategy is provided in the Supplementary Table 1.
Study Selection
Two researchers independently searched and screened the literature, and extracted data. In the literature screening process, the title and abstract were read first, and the full text was read to determine whether the literature was included after obviously irrelevant literature was excluded. In cases of disagreement, a third researcher was consulted for resolution.
Data Extraction
Two researchers independently designed and populated data extraction tables.The extracted information included:
1. Basic information: First author, publication date, country, DOMS location, treatment measures, treatment duration, outcome indices.
2. Demographic characteristics: Sample size, age and sex.
3. Methodological information: Randomization method, allocation concealment, and blinding methods, etc.
Discrepancies in extracted data were discussed; unresolved issues were adjudicated through further discussion.
Risk of Bias in Studies
Two investigators assessed the bias risk using the Cochrane Randomized Controlled Trial Bias Risk Assessment tool for RCTs.21 The assessment tool included the following seven items: random sequence generation, allocation concealment, blinding of participants and providers, blinding of outcome evaluators, incomplete outcome data, selective outcome reporting, and other sources of bias, each of which assessed the outcome as low, high, or unclear.
Synthesis Methods
A Bayesian random-effects model was used to compare intervention effects. The Markov chain Monte Carlo method was used for modeling, with four Markov chains running simultaneously. The model was annealed for 20,000 iterations, and simulation was completed after 50,000 iterations. The Deviation Information Standard (DIC) was used to compare the model fit and global consistency. The node-splitting method used to analyze local consistency in the case of a closed-loop network. In addition, the interventions were ranked based on SUCRA, and the league tables were generated to compare differences in effects across the interventions. Subgroup and sensitivity analyses were performed to further analyze the results. If ≥10 studies were included in the outcome measure, funnel plots were used to assess intuitive response publication bias. The analyses was completed using Stata 15 and R v.4.2.2.
Results
Study Selection
A preliminary search identified 1,339 literatures. After eliminating 138 duplicate entries using EndNoteX9.1, 1,163 articles were excluded based on the title and abstract of the article. Further 23 studies were excluded after reading the full text, and 15 RCTs were finally included. Figure 1 presents a flowchart of the screening process.
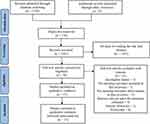 |
Figure 1 Flow chart of literature screening. |
Study Characteristics
This study included 15 studies,22–36 involving a total of 447 patients from several countries: China, the United States, Brazil, Belgium, South Africa, Germany, Australia, Saudi Arabia, the United Kingdom, and Thailand. Nine interventions were evaluated, including irradiation, cryotherapy, sauna, photobiomodulation therapy(PBMT), electrical stimulation(ES), ultrasonic therapy(UT), soft tissue mobilization (STM),cryotherapy combined with PBMT, cryotherapy combined with ES. Table 1 summarizes the basic characteristics of the included studies.
 |
Table 1 The Basic Characteristics Included in the Study |
Risk of Bias in Studies
Seven studies23–25,31–33,35 used the correct randomization method, whereas six studies23–25,31,33,35 used the correct concealment method. Four studies23,24,32,33 were single-blinded and three25,31,36 were double-blinded. Data were complete for all studies, and no selective reporting or other publication biases were observed. Figures 2 and 3 shows the results of the risk of bias analysis involving the included studies.
 |
Figure 2 Risk of bias graph. |
 |
Figure 3 Risk of bias summary. |
Meta Analysis
Subgroup analysis were conducted based on the time of detection.
24 hours
Associations Between Interventions
Fourteen studies reported the effectiveness of eight interventions: irradiation, cryotherapy, sauna, Electrical stimulation(ES), Ultrasonic therapy(UT), Photobiomodulation therapy(PBMT), Short contrast immersion, and cryotherapy combined with Photobiomodulation therapy(PBMT) Heterogeneity test results showed an I2 value of 1%, indicating a low heterogeneity. This figure shows the presence of a closed loop. The consistency test results showed that when P > 0.05, the surface network was consistent, and the consistency model was used for the analysis (see Figure 4).
 |
Figure 4 Correlation diagram between different physical therapy modalities (24h). |
Synthesized Results
Compared with placebo, Photobiomodulation therapy(PBMT) demonstrated a significant effect (P < 0.05) among various physical therapy modalities, PBMT was the most effective. (Figure 5, Supplementary Table 2 League table). The top three SUCRA rankings were PBMT (0.99), cryotherapy and PBMT (0.63), and SCI (0.61) (Table 2).
 |
Table 2 SUCRA Sort |
 |
Figure 5 Forest map of the effectiveness of different physical therapy modalities compared to placebo (24h). |
48 hours
Associations Between Interventions
Twelve studies reported effectiveness of eight types of interventions: irradiation, cryotherapy, sauna, Electrical stimulation(ES), Ultrasonic therapy(UT), Photobiomodulation therapy(PBMT), Short contrast immersion, and cryotherapy combined with PBMT. The heterogeneity test results showed an I2 value of 1%, indicating a low heterogeneity. This figure shows the presence of a closed loop. The consistency test results showed that when P > 0.05, the surface network was consistent, and the consistency model was used for the analysis (see Figure 6).
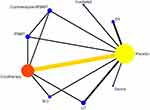 |
Figure 6 Correlation diagram between different physical therapy modalities (48h). |
Synthesized Results
Photobiomodulation therapy(PBMT) and sauna had significant effects compared with the placebo (P < 0.05). Among the treatments, PBMT had the greatest effect (Figure 7, Supplementary Table 2 League table). The top three SUCRA treatments were PBMT (0.98), Sauua (0.84), cryotherapy and PBMT (0.57) (see Table 2).
 |
Figure 7 Forest map of the effectiveness of different physical therapy modalities compared to placebo (48h). |
72 hours
Associations Between Interventions
Twelve studies reported efficient intervention by eight types: irradiation, cryotherapy, sauna, Electrical stimulation(ES), Ultrasonic therapy(UT), Photobiomodulation therapy(PBMT), Short contrast immersion, and cryotherapy combined with Photobiomodulation therapy(PBMT). The heterogeneity test results showed an I2 value of 5%, indicating a low heterogeneity. This figure shows the presence of a closed loop. The consistency test results showed that when P > 0.05, the surface network was consistent, and the consistency model was used for the analysis (see Figure 8).
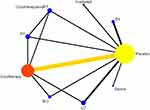 |
Figure 8 Correlation graph between different physical therapy modalities (72h). |
Synthesized Results
Physical therapy modalities had no significant advantage over placebo (P > 0.05) (Figure 9 and Supplementary Table 2 League table) (see Table 2).
 |
Figure 9 Forest plot of effectiveness of different physical therapy modalities compared to placebo (72h). |
96 hours
Associations Between Interventions
Six studies reported the efficacy of six types of interventions: irradiation, cryotherapy, sauna, Photobiomodulation therapy(PBMT), Short contrast immersion, and cryotherapy combined with Photobiomodulation therapy(PBMT). The heterogeneity assay results showed an I2 value of 7%, indicating a low heterogeneity. This figure shows the presence of a closed loop. The consistency test results showed that when P > 0.05, the surface network was consistent, and the consistency model was used for the analysis (see Figure 10).
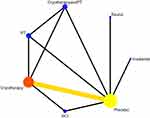 |
Figure 10 Correlation diagram between different physical therapy modalities(96h). |
Synthesized Results
Physical therapy modalities had no significant advantage over placebo (P > 0.05) (Figure 11 and Supplementary Table 2 League table) (see Table 2).
 |
Figure 11 Forest plot of effectiveness of different physical therapy modalities compared to placebo (96h). |
Publication Bias Analysis and Sensitivity Analysis
The Egger test, contour-weighted funnel plot, and clip-complement method were applied to the 24-h, 48-h and 72-h results, and the results showed that there was a small possibility of publication bias (P=0.70 for 24h: Egger test, P=0.96 for 48h: Egger, P=0.79 for 72h: Egger). Figure 12 shows the contour-weighted funnel plots. Sensitivity analysis results were stable (Supplementary Table 3).
 |
Figure 12 Contour weighted funnel plot (24h,48h,72h). |
Discussion
This study conducted a network meta-analysis to assess the efficacy of various physical therapy modalities for the treatment of Delayed-onset muscle soreness(DOMS). Fifteen studies were included, encompassing nine distinct physical therapy modalities: irradiated therapy, cryotherapy, sauna, photobiomodulation therapy (PBMT), electrical stimulation (ES), ultrasound therapy (UT), soft tissue mobilization (STM), cryotherapy combined with PBMT, and Cryotherapy combined with ES. In terms of pain reduction at 24 hour post-intervention, PBMT demonstrated significant effectiveness compared to the other treatments. At the 48 hour mark, both PBMT and sauna treatment exhibited notable effects; however, beyond this period, no significant differences were observed among therapies when compared to placebo controls. Notably, the SUCRA ranking indicated that PBMT maintained its position was the top intervention. These findings suggest that physical therapy modalities are more effective within the initial 48 hour post-treatment and highlight that PBMT yields superior outcomes. Furthermore, these results remained stable without any evidence of significant publication bias.
Photobiomodulation therapy(PBMT) emerged in its contemporary form as a low-energy laser therapy shortly after the invention of ruby lasers in 1960 and He-Ne lasers in 1961, and has since evolved into a versatile therapeutic approach encompassing light therapies utilizing various light sources and parameter settings.37 This modality is characterized by three primary therapeutic effects: reduction in inflammation and edema, promotion of wound healing and deep tissue repair, facilitation of nerve regeneration, and alleviating of pain.38 Typically involving the exposure of cells or tissues to light within the visible to near-infrared spectrum, photobiomodulation therapy(PBMT) does not induce structural changes owing to elevated tissue temperatures when compared with other forms of light therapy. Consequently, it represents an ideal treatment option for numerous conditions associated with inflammatory states or those necessitating stimulation of growth and repair.39
Photobiomodulation Therapy(PBMT) exerts beneficial effects on alleviates pain and enhances functional mobility in patients. For instance, PBMT effectively mitigates the pain associated with rest, activities of daily living, stiffness, muscle shortening, and exercise, particularly in the knee joints of middle-aged and olderly women suffering from osteoarthritis (OA).40 Additionally, PBMT reduces the adverse effects of pain and inflammation on walking distance, gait parameters, and body structural metrics.41 Regarding the duration of therapeutic effects, a regimen involving low-energy laser therapy applied exclusively during the initial three weeks followed by a combination of exercise and other treatments over an additional eight weeks can sustain reductions in pain severity and dysfunction, as well as decrease medication usage for up to six months.42 Han et al43 reported that PBMT produced transient analgesic effects when administered to muscles at a dose of 8 J/cm² through nonthermal mechanisms involving neuropeptides, and sustained analgesia was observed following repeated applications. Furthermore, treatment with 670 nm red light significantly diminished neuronal damage and neuroinflammatory fatigue. Overall, PBMT facilitates healing and recovery of injured tissues while assisting patients in alleviating symptoms and improving joint functionality.44 Tim et al45 and Chen et al46 independently confirmed that PBMT promoted chondrocyte and fibroblast proliferation.
Numerous studies have demonstrated the efficacy of PBMT. In an animal trial comparing PBMT and cryotherapy after exercise, DaCosta et al47 found that PBMT was more effective in reducing muscle damage and inflammation. These results were consistent with those reported by Camargo et al48 in a similar investigation. An initial clinical trial assessing cryotherapy versus post-exercise PBMT indicated that administering PBMT five minutes after exercise inhibited increases in the creatine kinase (CK) activity, establishing its superiority over cryotherapy for post-exercise recovery.49 Furthermore, recent investigations50–54 have shown that employing a combination of multiple wavelengths and light sources before exercise can enhance performance outcomes. Compelling evidence supports a paradigm shift from utilizing PBMT solely for rehabilitation after injury to considering it as a prophylactic measure for skeletal muscle protection. With minimal contraindications, PBMT is a unique, noninvasive, and nonpharmacological approach to expedite recovery from muscle fatigue and exercise-induced injuries.
However, Nampo et al55 concluded that phototherapy is not been proven effective in treating DOMS. Variations in light wavelength, dosage, and intensity across different studies may account for these conflicting findings. Thus, further clinical trials are warranted to validate this assertion. A search on the clinical trial registry platform (https://beta.clinicaltrials.gov) revealed numerous RCTs investigating the effects of PBMT on muscle soreness symptoms (eg, NCT06145867, NCT05926752, and NCT05627141), indicating a growing interest in this therapeutic modality.
The strengths of this study include its pioneering effort in elucidating the efficacy heterogeneity across physical therapy modalities based on temporal factors, an essential aspect in clinical applications. Nonetheless, several limitations exist should be noted. First, only English-language studies were included, which may reduce the comprehensiveness of the evidence. Second, variations in methods used to induce DOMS among the included studies could affect the reliability of the results. Additionally, the study focused solely on pain indicators, which may limit the overall evaluation scope. Significant disparities exist regarding the participants’ age and exercise habits, and previous research suggests potential divergent effects among individuals with differing training backgrounds receiving identical treatments. However, owing to the limited literature availability, subgroup analyses concerning physical therapy modalities applications across varying exercise habits were not conducted within this study framework. Finally, the overall quality of the included studies was suboptimal, indicating a certain risk of bias.
Conclusion
Based on the findings of this study, photobiomodulation therapy(PBMT) and sauna treatment demonstrated significant effects 48 hour post-intervention. Beyond this timeframe, the efficacy of photobiomodulation therapy(PBMT) was not statistically significant.Other physical therapy modalities, such as cryotherapy, electrical stimulation, ultrasound therapy, etc., did not demonstrate statistically significant advantages in this study.Overall, physical therapy modalities were more effective within the initial 48-hour period. Notably, photobiomodulation therapy(PBMT) yielded the most favorable outcomes and provided valuable clinical evidence. However, owing to inconsistencies in methodological quality and small sample sizes among the included studies, high-quality multicenter randomized double-blind controlled trials with larger sample sizes are needed to validate these conclusions.
Highlights
1.This study is the first to research the efficacy and safety of different physical therapy modalities(PTMs) in the treatment of delayed-onset muscle soreness(DOMS).Used network meta-analysis to comprehensively compare the efficacy and safety of different PTMs for DOMS, in order to screen the best treatment method and provide more evidence for the treatment of DOMS.
2.We used the STATA16 and R v.4.2.2 tools in this study.
3.Based on existing research results, PTMs in the treatment of DOMS exhibit good efficacy and safety.
Data Sharing Statement
Data are available from the corresponding author (LinyuYin) upon request.
Acknowledgments
We thank the researchers and the study participants for their contributions. We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This work was supported by the Hunan Provincial clinical medical technology demonstration base of chronic musculoskeletal pain rehabilitation (grant number:2023SK4074), the Chronic musculoskeletal pain rehabilitation technology research and development center of Chenzhou City (grant number:2021-02-31).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Hazar Kanik Z, Citaker S, Yilmaz Demirtas C. et al. Effects of Kinesio Taping on the Relief of Delayed Onset Muscle Soreness: a Randomized, Placebo-Controlled Trial. J Sport Rehab. 2019;28(8):781–786. doi:10.1123/jsr.2018-0040
2. Valle X, Til L, Drobnic F, et al. Compression garments to prevent delayed onset muscle soreness in soccer players. Muscle Ligaments Tendons J. 2013;3(4):295–302. doi:10.32098/mltj.04.2013.10
3. Lin J, Guo M, Wang H, et al. Effects of Kinesio Tape on Delayed Onset Muscle Soreness: a Systematic Review and Meta-analysis. Biomed Res Int. 2021;2021(1):6692828. doi:10.1155/2021/6692828
4. Doma K, Deakin GB, Schumann M, et al. Training Considerations for OPBMTimising Endurance Development: an Alternate Concurrent Training Perspective. Sports Med. 2019;49(5):669–682. doi:10.1007/s40279-019-01072-2
5. Fletcher BD, Twist C, Haigh JD, Brewer C, Morton JP, Close GL. Season-long increases in perceived muscle soreness in professional rugby league players: role of player position, match characteristics and playing surface. J Sports Sci. 2016;34(11):1067–1072. doi:10.1080/02640414.2015.1088166
6. Cavaleri R, Imam J, Rio E, et al. Investigating interindividual variability in corticomotor reorganization during sustained hamstring pain: a randomized experimental study. Brain Behav. 2023;13(5):e2996. doi:10.1002/brb3.2996
7. Xiaohong L, Yue G, Qiang L, et al. Research progress in the mechanism, quantitative evaluation and treatment of exercise-induced muscle injury. Chin J Tissue Eng Res. 2021;25(14):2280–2286.
8. Schoenfeld BJ. The use of nonsteroidal anti-inflammatory drugs for exercise-induced muscle damage. Sports Med. 2012;42(12):1017–1028. doi:10.1007/BF03262309
9. Minett GM, Duffield RI. Recovery driven by central or peripheral factors? A role for the brain in recovery following intermittent sprint exercise. Front Physiol. 2014;05:24. doi:10.3389/fphys.2014.00024
10. Dupuy O, Douzi W, Theurot D, et al. An Evidence-Based Approach for Choosing Post-exercise Recovery Techniques to Reduce Markers of Muscle Damage, Soreness, Fatigue, and Inflammation: a Systematic Review With Meta-Analysis. Front Physiol. 2018;9:403. doi:10.3389/fphys.2018.00403
11. Ferlito JV, Ferlito MV, Leal-Junior ECP, et al. Comparison between cryotherapy and photobiomodulation in muscle recovery: a systematic review and meta-analysis. Lasers Med Sci. 2021;37(3):1375–1388. doi:10.1007/s10103-021-03442-7
12. Buoite Stella A, Dragonetti AM, Fontanot S, et al. The Acute Effects of Cold Water Immersion and Percussive Massage Therapy on Neuromuscular Properties and Muscle Soreness after Exercise in Young Male Soccer Players. Sports. 2024;12(6):167. doi:10.3390/sports12060167
13. Heinke L, Javanmardi S, Rappelt L, et al. Comparison of the effects of cold water immersion and percussive massage on the recovery after exhausting eccentric exercise: a three-armed randomized controlled trial. Front Physiol. 2024;15:1432009. doi:10.3389/fphys.2024.1432009
14. Torres R, Ribeiro F, Duarte A, Cabri J. Evidence of the Physiotherapeutic Interventions Used Currently after Exercise-Induced Muscle Damage. Syst Rev Meta-Analysis Phys Ther Sport. 2012;13:101–114.
15. Tan J, Shi X, Witchalls J, Waddington G, Fu L. Wu,A.C.Effects of Pre-Exercise Acute Vibration Training on SymPBMToms of Exercise-Induced Muscle Damage: a Systematic Review andMeta - Analysis. J Strength Cond Res. 2022;36(8):2339–2348. doi:10.1519/JSC.0000000000003789
16. Afonso J, Clemente FM, Nakamura FY, Morouco P, Sarmento H. Inman,R.A.; Ramirez-Campillo,R.The Effectiveness of Post-Exercise Stretching in Short-Term and Delayed Recovery of Strength,Range of Motion and Delayed Onset Muscle Soreness: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Physiol. 2021;12:677581. doi:10.3389/fphys.2021.677581
17. Cheung K, Hume P, Maxwell L. Delayed Onset Muscle Soreness: treatment Strategies and Performance Factors. Sports Med. 2003;33(2):145–164. doi:10.2165/00007256-200333020-00005
18. Nahon RL, Silva Lopes JS, Monteiro De Magalhaes Neto A. Physical therapy Interventions for the Treatment of Delayed Onset Muscle Soreness (DOMS. Syst Rev Meta-Analysis Phys Ther Sport. 2021;52:1–12. doi:10.1016/j.ptsp.2021.07.005
19. Herbert RD, Gabriel M. Effects of Stretching before and after Exercising on Muscle Soreness and Risk of Injury: systematic Review. BMJ. 2002;325(7362):468. doi:10.1136/bmj.325.7362.468
20. Faiz KW. VAS--visuell analog skala [VAS--visual analog scale]. Tidsskr nor Laegeforen. 2014;134(3):323. doi:10.4045/tidsskr.13.1145
21. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928
22. Denegar CR, Perrin DH. Effect of transcutaneous electrical nerve stimulation, cold, and a combination treatment on pain, decreased range of motion, and strength loss associated with delayed onset muscle soreness. J Athl Train. 1992;27(3):200–206.
23. Butterfield DL, Draper DO, Ricard MD, Myrer JW, Schulthies SS, Durrant E. The effects of high-volt pulsed current electrical stimulation on delayed-onset muscle soreness. J Athl Train. 1997;32(1):15–20.
24. Vinck E, Cagnie B, Coorevits P, Vanderstraeten G, Cambier D. Pain reduction by infrared light-emitting diode irradiation: a pilot study on experimentally induced delayed-onset muscle soreness in humans. Lasers Med Sci. 2006;21(1):11–18. doi:10.1007/s10103-005-0366-6
25. Montgomery PG, Pyne DB, Hopkins WG, Dorman JC, Cook K, Minahan CL. The effect of recovery strategies on physical performance and cumulative fatigue in competitive basketball. J Sports Sci. 2008;26(11):1135–1145. doi:10.1080/02640410802104912
26. Glasgow PD, Ferris R, Bleakley CM. Cold water immersion in the management of delayed-onset muscle soreness: is dose important? A randomised controlled trial. Phys Ther Sport. 2014;15(4):228–233. doi:10.1016/j.ptsp.2014.01.002
27. Parker R. Ultrasound v. sham ultrasound for experimentally induced delayed onset muscle soreness: a double-blind, randomised controlled trial. South Afr J Sports Med. 2015;26(4):99–103. doi:10.7196/SAJSM.535
28. Khamwong P, Paungmali A, Pirunsan U, Joseph L. Prophylactic Effects of Sauna on Delayed-Onset Muscle Soreness of the Wrist Extensors. Asian J Sports Med. 2015;6(2):e25549. doi:10.5812/asjsm.6(2)2015.25549
29. de Paiva PR, Tomazoni SS, Johnson DS, et al. Photobiomodulation therapy (PBMT) and/or cryotherapy in skeletal muscle restitution, what is better? A randomized, double-blinded, placebo-controlled clinical trial. Lasers Med Sci. 2016;31(9):1925–1933. doi:10.1007/s10103-016-2071-z
30. Kakaraparthi VN, Alahmari KA, Ahmed I. Effectiveness of pulsed ultrasound and cryotherapy on delayed onset muscle soreness. Saudi J Sports Med. 2016;16(2):133–138. doi:10.4103/1319-6308.180180
31. Machado AF, Almeida AC, Micheletti JK, et al. Dosages of cold-water immersion post exercise on functional and clinical responses: a randomized controlled trial. Scand J Med Sci Sports. 2017;27(11):1356–1363. doi:10.1111/sms.12734
32. Fleckenstein J, Friton M, Himmelreich H, Banzer W. Effect of a Single Administration of Focused Extracorporeal Shock Wave in the Relief of Delayed-Onset Muscle Soreness: results of a Partially Blinded Randomized Controlled Trial. Arch Phys Med Rehabil. 2017;98(5):923–930. doi:10.1016/j.apmr.2016.11.013
33. Guilhem G, Hug F, Couturier A, et al. Effects of air-pulsed cryotherapy on neuromuscular recovery subsequent to exercise-induced muscle damage. Am J Sports Med. 2013;41(8):1942–1951. doi:10.1177/0363546513490648
34. Wiewelhove T, Schneider C, Döweling A, et al. Effects of different recovery strategies following a half-marathon on fatigue markers in recreational runners. PLoS One. 2018;13(11):e0207313. doi:10.1371/journal.pone.0207313
35. Govus AD, Andersson EP, Shannon OM, Provis H, Karlsson M, McGawley K. Commercially available compression garments or electrical stimulation do not enhance recovery following a sprint competition in elite cross-country skiers. Eur J Sport Sci. 2018;18(10):1299–1308. doi:10.1080/17461391.2018.1484521
36. Elkeblawy MA, Mahmoud MS, Ibrahim HH, et al. Influence Of Continuous Airflow Cryotherapy On Serum Creatine Kinase Level In Induced Muscle Soreness In Healthy Subjects: a Randomized Control Trial. J Pharm Negat Res. 2023;14:1.
37. Wang X, Liu Q, Peng J, et al. The effects and mechanisms of PBM therapy in accelerating orthodontic tooth movement. Biomolecules. 2023;13(7):1140. doi:10.3390/biom13071140
38. Ryu HS, Lim NK, Padalhin AR. Improved healing and macrophage polarization in oral ulcers treated with photobio modulation (PBM). Lasers Surg Med. 2022;54(4):600–610. doi:10.1002/lsm.23510
39. Rech CA, Pansani TN, Cardoso LM, et al. Photobiomod ulation using LLLT and LED of cells involved in osseointegration and peri-implant soft tissue healing. Lasers Med Sci. 2022;37(1):573–580. doi:10.1007/s10103-021-03299-w
40. Vassao PG, De Souza MC, Silva BA, et al. Photobio modulation via a cluster device associated with a physical exercise program in the level of pain and muscle strength in middle-aged and older women with knee osteoarthritis: a randomized placebo controlled trial. Lasers Med Sci. 2020;35(1):139–148. doi:10.1007/s10103-019-02807-3
41. Soleimanpour H, Gahramani K, Taheri R, et al. The effect of low-level laser therapy on knee osteoarthritis: prospec tive, descriPBMTive study. Lasers Med Sci. 2014;29(5):1695–1700. doi:10.1007/s10103-014-1576-6
42. Alfredo PP, Bjordal JM, Lopes-Martins RAB, et al. Efficacy of prolonged application of low-level laser therapy com bined with exercise in knee osteoarthritis: a randomized controlled double blind study. Clin rehabilitat. 2022;36(10):1281–1291. doi:10.1177/02692155221111922
43. Han DS, Lee CH, Shieh YD, et al. Involvement of substance P in the analgesic effect of low-level laser therapy in a mouse model of chronic widespread muscle pain. Pain Med. 2019;20(10):1963–1970. doi:10.1093/pm/pnz056
44. Hu D, Moalem-Taylor G, Potas JR. Red-light (670 nm) therapy reduces mechanical sensitivity and neuronal cell death, and alters glial responses after spinal cord injury in rats. J Neurotrauma. 2020;37(21):2144–2260.
45. Tim CR, Martignago CCS, Assis L, et al. Effects of photobiomodulation therapy in chondrocyte response by in vitro experiments and experimental model of osteoarthritis in the knee of rats. Lasers Med Sci. 2022;37(3):1677–1686. doi:10.1007/s10103-021-03417-8
46. Chen HL, Sun SJ, Pan ZH, et al. Effects of photobiomodula tion on high glucose induced oxidative stress in human embryonic skin fibroblasts. IEEE J Sel Top Quantum Electron. 2021;27(4):9. doi:10.1109/JSTQE.2021.3054050
47. da Costa Santos VB, de Paula RS, Milanez VF, et al. LED therapy or cryotherapy between exercise intervals in Wistar rats: antiinflammatory and ergogenic effects. Lasers Med Sci. 2014;29(2):599–605. doi:10.1007/s10103-013-1371-9
48. Camargo MZ, Siqueira CP, Preti MC, et al. Effects of light emitting diode (LED) therapy and cold water immersion therapy on exercise-induced muscle damage in rats. Lasers Med Sci. 2012;27(5):1051–1058. doi:10.1007/s10103-011-1039-2
49. Leal Junior EC, de Godoi V, Mancalossi JL, et al. Comparison between cold water immersion therapy(CWIT) and light emitting diode therapy (LEDT) in short term skeletal muscle recovery after high-intensity exercise in athletes—preliminary results. Lasers Med Sci. 2011;26(4):493–501. doi:10.1007/s10103-010-0866-x
50. Miranda EF, de Oliveira LV, Antonialli FC, Vanin AA, de Carvalho PBMT, Leal-Junior EC. Phototherapy with combination of super-pulsed laser and light-emitting diodes is beneficial in improvement of muscular performance (strength and muscular endurance), dyspnea, and fatigue sensation in patients with chronic obstructive pulmonary disease. Lasers Med Sci. 2015;30(1):437–443. doi:10.1007/s10103-014-1690-5
51. Antonialli FC, De Marchi T, Tomazoni SS, et al. Phototherapy in skeletal muscle performance and recovery after exercise: effect of combination of super-pulsed laser and light-emitting diodes. Lasers Med Sci. 2014;29(6):1967–1976. doi:10.1007/s10103-014-1611-7
52. Miranda EF, Vanin AA, Tomazoni SS, et al. Using pre-exercise photobiomodulation therapy combining super-pulsed lasers and light-emitting diodes to improve performance in progressive cardiopulmonary exercise tests. J Athl Train. 2016;51(2):129–135. doi:10.4085/1062-6050-51.3.10
53. Pinto HD, Vanin AA, Miranda EF, et al. Photobiomodulation therapy (PBMT) improves performance and accelerates recovery of high-level rugby players in field test: a randomized, crossover, double-blind, placebo-controlled clinical study. J Strength Cond Res. 2016;30(12):3329–3338. doi:10.1519/JSC.0000000000001439
54. Vanin AA, Miranda EF, Machado CS, et al. What is the best moment to apply phototherapy when associated to a strength training program? A randomized, double-blinded, placebo-controlled trial: phototherapy in association to strength training. Lasers Med Sci. 2016;31(8):1555–1564. doi:10.1007/s10103-016-2015-7
55. Nampo FK, Cavalheri V, Ramos SP, et al. Effect of low level phototherapy on delayed onset muscle soreness: a systematic review and meta-analysis. Lasers Med Sci. 2016;31(1):165–177. doi:10.1007/s10103-015-1832-4
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
A Systematic Review and Meta-Analysis Protocol on How Best to Use Non-Pharmacologic Therapies to Manage Chronic Low Back Pain and Associated Depression
Guo Y, Ma Q, Zhou X, Yang J, He K, Shen L, Zhao C, Chen Z, Tan CIC, Chen J
Journal of Pain Research 2022, 15:3509-3521
Published Date: 4 November 2022
Clinical Efficacy of Tuina Therapy for Acute Lumbar Sprain: A Bayesian Network Meta-Analysis Based on Randomized Controlled Trials
Chen J, Liu S, Gong Z, Feng X, Li W, Li J
Journal of Pain Research 2024, 17:4365-4375
Published Date: 17 December 2024

