Back to Journals » Drug Design, Development and Therapy » Volume 19
Dose-Response Analysis of Nalbuphine for Alleviating Catheter-Related Bladder Discomfort After Ureteroscopic Lithotripsy in Men: A Retrospective Study
Received 26 January 2025
Accepted for publication 12 June 2025
Published 19 June 2025 Volume 2025:19 Pages 5283—5292
DOI https://doi.org/10.2147/DDDT.S511613
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Jiayi Tang, Huadong Ni, Ming Yao
Department of Anesthesiology and Pain Research Center, The First Hospital of Jiaxing or The Affiliated Hospital of Jiaxing University, Jiaxing, People’s Republic of China
Correspondence: Huadong Ni; Ming Yao, Department of Anesthesiology and Pain Research Center, The First Hospital of Jiaxing or The Affiliated Hospital of Jiaxing University, No. 1882 South Zhonghuan Road, Nanhu District, Jiaxing, Zhejiang, People’s Republic of China, Tel +86 573 18258359815, Email [email protected]; [email protected]
Purpose: This study aimed to determine the median effective dose (ED50) and effective dose 95% (ED95) of nalbuphine for alleviating moderate-severe catheter-related bladder discomfort (CRBD) after ureteroscopic lithotripsy (URL) in male patients using probit regression analysis.
Patients and Methods: This retrospective study analyzed anesthesia records of all male patients who underwent URL and received nalbuphine under general anesthesia at Jiaxing University Affiliated Hospital between August 2023 and August 2024. Patients were categorized into four groups based on nalbuphine dosage: 0.025, 0.05, 0.075, and 0.10 mg/kg. Data on patient demographics, CRBD scores, agitation scores, the Ramsay sedation scale, and urinary catheter-related pain (UCRP) scores were collected 5 min after laryngeal mask removal. Hemodynamic parameters, including mean blood pressure, heart rate (HR), and oxygen saturation (SPO2), were collected at various time points: post-operation (T0), and at immediately (T1), 5 (T2), 10 (T3), 20 (T4), and 30 min (T5) after laryngeal mask removal. The incidence of adverse events was documented. Probit regression analysis was employed to calculate the ED50 and ED95 of nalbuphine for alleviating moderate-severe CRBD.
Results: Data from 76 anesthesia records were retrieved. CRBD, agitation, and UCRP scores decreased with increasing nalbuphine doses, while the Ramsay sedation scores increased. No significant differences were observed in hemodynamic parameters across dose groups at any time point (P > 0.05). Similarly, the incidence of adverse effects did not differ significantly among the groups (P > 0.05). Probit regression analysis revealed that the ED50 of nalbuphine for alleviating moderate-severe CRBD following URL in male patients was 0.03 mg/kg (95% confidence interval [CI]: 0.013– 0.046), while the ED95 was 0.108 mg/kg (95% CI: 0.085– 0.188).
Conclusion: Nalbuphine is a safe and effective agent for mitigating CRBD, with an ED50 of 0.03 mg/kg and ED95 of 0.108 mg/kg.
Keywords: nalbuphine, ureteroscopic lithotripsy, catheter-related bladder discomfort, median effective dose, men
Introduction
Indwelling urinary catheters are essential in surgical care, preventing bladder injury, monitoring fluid balance, and managing urinary retention.1–3 With advancements in patient-centered medical care, surgeons increasingly prefer inserting catheters after general anesthesia, minimizing discomfort during the procedure. However, most patients experience catheter-related bladder discomfort (CRBD), characterized by suprapubic discomfort and an urge to void during the anesthesia recovery phase.4,5 These symptoms primarily manifest as urinary frequency and urgency.6
With advancements in minimally invasive techniques, ureteroscopic lithotripsy (URL) has become a preferred endoscopic procedure for managing urinary stones.7–9 Despite its benefits, routine indwelling urinary catheterization associated with URL significantly increases the incidence of CRBD. Severe cases may lead to postoperative complications, including agitation, delusion, and catheter displacement.10 Studies indicate that male patients are at a higher risk of developing CRBD than females,11,12 which underscores the focus of this study on male patients undergoing URL under general anesthesia.
Nalbuphine is a semi-synthetic opioid analgesic with mixed agonist-antagonist properties. It demonstrates rapid onset, sustained efficacy, low addiction potential, and a favorable safety profile.13,14 Nalbuphine is widely used in anesthesia induction, postoperative analgesia, and chronic pain treatment.15–17 Recent clinical study confirm that combined nalbuphine significantly lowers the scores of CRBD and prolongs the duration of analgesia in patients after urological surgery,18 However, it is associated with dose-dependent adverse reactions, such as delayed extubation, nausea, vomiting, respiratory depression. Optimizing the nalbuphine dose is crucial for minimizing CRBD severity while reducing the likelihood of complications.19 That is to say, there is no consensus on the optimal dose required to prevent moderate-severe CRBD in this population.
This dose-response study aimed to determine the median effective dose (ED50) and effective dose 95% (ED95) of nalbuphine for alleviating moderate-severe CRBD in male patients undergoing URL under general anesthesia.
Patients and Methods
Patients
This retrospective study analyzed anesthesia records of all male patients who underwent ureteroscopic lithotripsy under general anesthesia at Jiaxing University Affiliated Hospital between August 2023 and August 2024.
All patient data were anonymized to ensure confidentiality, and the study adhered to relevant ethical guidelines and regulations.
Study Protocol
Anesthesia records of male patients who received nalbuphine during URL were reviewed for this analysis.
Inclusion criteria: (1) Male patients; (2) Aged from 18 to 85 years old; (3) Patients undergoing ureteroscopic lithotripsy and indwelling urinary catheter placement under general anesthesia; (4) Standardized anesthesia protocol, including induction with sufentanil 0.2 µg/kg, rocuronium 0.6 mg/kg, and propofol 2 mg/kg; placement of a laryngeal mask and ventilator-assisted ventilation; maintenance with remifentanil 0.1–0.2 µg/kg/mL by intravenous infusion and 2% sevoflurane inhalation; postoperative analgesia using 0.025–0.10 mg/kg nalbuphine administered 5 minutes before the end of the operation, along with ondansetron 4 mg for preventing postoperative nausea and vomiting (PONV); reversal of residual muscle relaxants with sugammadex sodium after the operation; uniform application of the above anesthesia protocol for all patients.
Exclusion criteria: (1) Patient refusal to participate in the study; (2) Preoperative diagnoses of neurogenic bladder, overactive bladder, or discomfort in the suprapubic area; (3) Preoperative cognitive dysfunction; (4) Postoperative admission to the intensive care unit (ICU); (5) Incomplete anesthesia records.
Patients were categorized into four groups based on the nalbuphine dose administered: 0.025, 0.05, 0.075, and 0.10 mg/kg. A total of 80 anesthesia records were initially retrieved, and two were excluded due to incomplete data. Furthermore, one male patient from the 0.05 mg/kg group and another from the 0.075 mg/kg group were excluded due to a preoperative diagnosis of overactive bladder. Ultimately, 76 patients were included: 19 in the 0.025 mg/kg group, 18 in the 0.05 mg/kg group, 19 in the 0.075 mg/kg group, and 20 in the 0.10 mg/kg group.
Measurement
Data Collection
- General characteristics: age, body mass index (BMI), operation duration, anesthesia duration (from the start of anesthesia to the end of operation), time to laryngeal mask removal (time from the end of operation to laryngeal mask removal).
- Clinical indicators: Incidence and severity of CRBD, agitation score, Ramsay sedation scale score, and urinary catheter-related pain (UCRP) score, all measured 5 min after laryngeal mask removal.
- Physiological parameters: the mean blood pressure, HR, and SpO2 levels were recorded at the following time points: post-operation (T0), immediately after laryngeal mask removal (T1), and at 5 (T2), 10 (T3), 20 (T4), and 30 min after laryngeal mask removal (T5). The occurrence of adverse events, including PONV, respiratory depression (SpO2 < 95%), vertigo, and headache was documented before leaving the postanesthesia care unit (PACU).
Evaluation Criteria
CRBD Score
0 (none): no urethral or bladder discomfort when queried; 1 (mild): mild urethral discomfort upon questioning; 2 (moderate): self-reported catheter discomfort without accompanying behavioral responses; 3 (severe): self-reported catheter discomfort with significant behavioral responses, such as verbal complaints, body movements, or attempts to remove the catheter.20
A trained observer assessed the occurrence and severity of CRBD at 5 minutes post-laryngeal mask removal. Moderate-severe CRBD (CRBD score ≥2) required clinical intervention.
Agitation Score
0: cooperative patient with no agitation; 1: physical agitation upon stimulation, relieved by verbal reassurance; 2: agitation without stimulation, including attempts to remove the catheter or drainage tube; 3: severe agitation with intense physical struggle, requiring multiple staff to restrain the patient.21
Ramsay Sedation Scale Score
1: the patient is anxious or restless; 2: the patient is awake, calm, cooperative, and oriented; 3: the patient is asleep but responsive to instructions and reacts promptly; 4: drowsy patient who responds quickly to loud verbal stimuli; 5: drowsy patient with a slow response to loud verbal stimuli; 6: unresponsive patient in deep sleep or anesthesia. Sedation was considered satisfactory with a score of 2–4 and excessive if the score was 5–6.22
Urinary Catheter-Related Pain (UCRP) Score
Pain was measured using the numerical rating scale (NRS) ranging from 0 to 10, where 0 indicates no discomfort, and 10 indicates intolerable pain.
Statistical Analyses
The sample size was calculated using Power Analysis and Sample Size (PASS) 15 software (National Software Computing Corporation [NSCC], Lunar Computing Corporation [LCC], Kaysville, Utah) based on the Cochran–Armitage test for proportion trends. The incidence of moderate-severe CRBD was estimated to be 52%, 30%, 21%, and 3% for nalbuphine doses of 0.025, 0.05, 0.075, and 0.1 mg/kg, respectively. Calculations indicated that a sample size of 16 patients per group would achieve 90% power to detect a linear trend using a two-tailed Z test with an α level of 0.05. The total required sample size was set at 80 patients, with 20 patients assigned to each group, to account for a potential 20% attrition rate.
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) statistical software (version 26.0; International Business Machines Corporation, New York, USA). Data normality was assessed using the Shapiro–Wilk test and visual inspection of histograms. Continuous variables with normal distribution are presented as mean ± standard deviation (SD), while non-normally distributed variables are presented as medians with interquartile ranges (IQRs). Categorical variables are summarized as frequencies and percentages. Comparisons of two normally distributed continuous variables were conducted using independent t-tests, while variance analysis with post-hoc Least Significant Difference (LSD) t-tests was applied for group comparisons. Non-normally distributed variables were compared using the Mann–Whitney U-test for two groups and the Kruskal–Wallis H-test for multiple groups. Categorical variables were analyzed using the chi-squared test. Probit regression analysis was employed to calculate the ED50, ED95, and their corresponding 95% confidence intervals for nalbuphine in alleviating moderate-severe CRBD in male patients undergoing URL. GraphPad Prism 8 (GraphPad Software, LLC, San Diego, California, USA) was used for data visualization. Statistical significance was set at P < 0.05.
Results
A total of 80 anesthesia records were retrieved, with two incomplete records excluded from the analysis. In the 0.05 mg/kg and 0.075mg/kg dose groups, one male patient from each group was excluded due to a preoperative diagnosis of overactive bladder. After data screening, 76 patients were included in the final analysis, distributed as follows: 19 in the 0.025 mg/kg group, 18 in the 0.05 mg/kg group, 19 in the 0.075 mg/kg group, and 20 in the 0.10 mg/kg group. A flowchart of the patient selection process is shown in Figure 1.
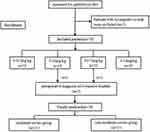 |
Figure 1 A flowchart of the patient selection process. |
Comparison of the General Characteristics Across Dose Groups
No significant differences were observed among the dose groups regarding age, BMI, operation duration, anesthesia duration, or laryngeal mask removal time (P > 0.05). These findings are summarized in Table 1.
 |
Table 1 Comparison of the General Characteristics Across Dose Groups |
Comparison of the General Characteristics by CRBD Severity
Patients were categorized into two groups based on their CRBD scores: the moderate-severe group (CRBD score ≥ 2) and the non-moderate-severe group (CRBD score < 2). No substantial differences were noted in age, BMI, operation duration, anesthesia duration, and laryngeal mask removal time (P > 0.05). Details are presented in Table 2.
 |
Table 2 Comparison of the General Characteristics by CRBD Severity |
Comparison of Scores Across Dose Groups
The CRBD and agitation scores decreased with increasing nalbuphine dosage. Patients in the 0.10mg/kg group had significantly lower scores than those in the 0.025 mg/kg group (F=9.809, P=0.003; F=7.144, P=0.011). Similarly, NRS scores decreased with higher doses, with the 0.10 mg/kg group having substantially lower scores than the 0.025 mg/kg and 0.05 mg/kg groups (F=14.253, P=0.001; F=6.389, P=0.016). Ramsay sedation scores increased with higher doses. The 0.075 mg/kg and 0.10 mg/kg groups had significantly higher scores than the 0.025 mg/kg group (F=11.102, P=0.002; F=13.368, P=0.001). Most Ramsay sedation scale score in the 0.075 mg/kg and 0.10 mg/kg groups were concentrated at 2 points, indicating satisfactory sedation. The results are detailed in Table 3.
 |
Table 3 Comparison of Scores Across Dose Groups |
Comparison of Hemodynamic Parameters Across Dose Groups
No considerable differences were found among the dose groups in mean blood pressure, heart rate, or SpO2 levels (P > 0.05). The data are presented in Tables 4–6.
 |
Table 4 Comparison of Mean Blood Pressure Across Dose Groups |
 |
Table 5 Comparison of Heart Rate Across Dose Groups |
 |
Table 6 Comparison of SpO2 Across Dose Groups |
Postoperative Complications
No significant differences were observed in the incidence of PONV, respiratory depression, vertigo, or headache among the different dose groups (P > 0.05), as shown in Table 7.
 |
Table 7 Comparison of Postoperative Complications Across Dose Groups |
ED50 and ED95 of Nalbuphine
The ED50 for nalbuphine in alleviating moderate-severe CRBD following URL in male patients was 0.03 mg/kg (95% CI: 0.013–0.046), while the ED95 was 0.108 mg/kg (95% CI: 0.085–0.188). The dose-response curves for nalbuphine’s inhibition of moderate-severe CRBD are shown in Figure 2.
 |
Figure 2 The dose-response curves for nalbuphine’s inhibition of moderate-severe CRBD. |
Discussion
Catheter-related bladder discomfort (CRBD) is characterized by a sensation of urgency and discomfort in the suprapubic region during postoperative anesthesia.10,11 It is commonly observed in the post-anesthesia care unit (PACU), where patients undergo early recovery from surgical anesthesia. Despite efforts by anesthesiologists and nurses to comfort patients, those with moderate-severe CRBD often remain agitated and unable to adapt, resulting in significant restlessness. The incidence of CRBD ranges from 47% to 90%, leading to prolonged PACU recovery times, extended hospital stays, and increased safety risks such as surgical wound bleeding, wound dehiscence, and displacement of drainage tubes. These complications further contribute to the patient’s hospitalization burden.11 Therefore, proactive measures are needed to alleviate patient discomfort and reduce associated risks.
Numerous clinical drugs are available for CRBD management, including tramadol, dezocine, and oxycodone.23–25 However, these medications are often associated with adverse effects, such as excessive sedation, respiratory depression, nausea, and vomiting. Traditional opioid receptors are categorized into κ,μ, and δ.26 When activated, κ-receptors, primarily located in the cerebral cortex, produce mild analgesia, pupil constriction, and disorientation. These effects are typically accompanied by minimal inhibition of the respiratory and circulatory systems. The μ-receptors are primarily located in the brainstem, and their activation by morphine produces analgesia, frequently accompanied by euphoria. This effect can alleviate discomfort caused by foreign objects, such as catheters. However, because the brainstem regulates both respiratory and cardiovascular functions, activation of μ-receptors can suppress these systems, leading to adverse reactions such as respiratory depression and hypotension. Moreover, stimulation of μ-receptors may cause nausea, vomiting, and decreased gastrointestinal motility. An ideal agent for preventing or treating CBRD should substantially reduce bladder spasms, minimize adverse nerve stimulation, and alleviate discomfort associated with urinary catheters during recovery from general anesthesia without causing significant adverse reactions.
Nalbuphine interacts with μ, κ, and δ opioid receptors,27 producing analgesic effects by activating κ receptors at the spinal level, which are particularly effective for managing visceral pain; its partial antagonism of μ receptors reduces the likelihood of adverse effects such as respiratory depression and addiction; its weak δ receptor activity minimizes irritability and anxiety.
Recent clinical studies have demonstrated that administration of nalbuphine during general anesthesia induction is more helpful in reducing the incidence and severity of CRBD during postoperative recovery.28 However, in clinical practice, it is associated with dose-dependent adverse reactions,29,30 such as delayed extubation, nausea, vomiting, respiratory depression, dizziness, and headaches. Optimizing the nalbuphine dose is crucial for minimizing CRBD severity while reducing the likelihood of complications. This study’s comparisons across four dose groups revealed that CRBD, agitation, and UCRP scores decreased with increasing doses, while the Ramsay sedation scores increased and stabilized at 2 points. This finding suggests that higher nalbuphine doses more effectively reduce CRBD severity, and improve sedation, analgesia, and agitation prevention. No considerable differences in mean blood pressure, HR, or SPO2 were observed across the dose groups, suggesting that nalbuphine does not disrupt hemodynamic stability. Moreover, the incidence of nausea, vomiting, respiratory depression, vertigo, and headache did not vary significantly with dose, suggesting that increasing the nalbuphine dose does not exacerbate these adverse effects. These findings indicate that nalbuphine is a safe and effective treatment for reducing CRBD severity, preventing its onset, and improving patient outcomes.
Previous studies frequently employed the Dixon sequential method to determine drug ED50.31,32 While this method requires a smaller sample size, its primary limitation is reduced accuracy, as inadequate representation at extreme doses can result in significant bias. Conversely, this study included 76 male patients, ensuring a larger sample size, reduced bias, and more reliable results. The results revealed that the ED50 of nalbuphine for alleviating moderate-severe CRBD following URL in male patients was 0.03 mg/kg (95% CI: 0.013–0.046), and the ED95 was 0.108 mg/kg (95% CI: 0.085–0.188). Using probit regression analysis, the calculated ED50 and ED95 effectively reduced CRBD severity without increasing dose-related adverse effects.
Despite its strengths, this study has some limitations. First, its retrospective nature precluded the direct involvement of researchers in preoperative patient education and anxiety management, which are key factors contributing to CRBD. Second, the retrospective design limited the collection of extended follow-up on CRBD, agitation, sedation and UCRP score, which could have provided a more comprehensive evaluation of nalbuphine’s efficacy. Finally, the study is limited to male patients undergoing a single surgical procedure at one center. Thus, the findings may not yet be generalizable to female patients with other types of surgeries in other medical centers. In future studies, we will include larger, more diverse populations and multicenter cohorts to draw more robust conclusions.
Conclusion
Nalbuphine is a safe and effective option for reducing CRBD severity. The ED50 for alleviating moderate-severe CRBD after URL in male patients was 0.03 mg/kg (95% CI: 0.013–0.046), and the ED95 was 0.108 mg/kg (95% CI: 0.085–0.188).
Data Sharing Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). The study was approved by Ethics Committee of the First Hospital of Jiaxing. Written informed consent to participate in this study was provided by the patient or participants’ legal guardian.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article. We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by funding from Zhejiang Province and Jiaxing City Jointly--Pain Medicine (2019-ss-ttyx), Key Discipline of Anesthesiology of Jiaxing City (2019-zc-06), Science and Technology Project of Jiaxing City (2020AY30011) and Jiaxing Key Laboratory of Neurology and Pain Medicine, Jiaxing Provinces and Cities Jointly Cultivate Discipline--General Surgery (Minimally Invasive) (2023-PYXK-001), Zhejiang Provincial Clinical Key Specialties-Anesthesiology (2023-ZJZK-001) and Scientific Research Fund of National Health Commission-Zhejiang Provincial Health Major Science and Technology Plan Project (WKJ-ZJ-2448).
Disclosure
The authors report no conflicts of interest related to this work.
References
1. Feneley RC, Hopley IB, Wells PN. Urinary catheters: history, current status, adverse events and research agenda. J Med Eng Technol. 2015;39(8):459–470. doi:10.3109/03091902.2015.1085600
2. Lopez C, Trautner BW, Kulkarni PA. Managing External Urinary Catheters. Infectious disease clinics of North America. Infectious Disease Clinics of North America. 2024;38(2):343–360. doi:10.1016/j.idc.2024.03.011
3. Rognoni C, Tarricone R. Intermittent catheterisation with hydrophilic and non-hydrophilic urinary catheters: systematic literature review and meta-analyses. BMC Urology. 2017;17(1):4. doi:10.1186/s12894-016-0191-1
4. Mitobe Y, Yoshioka T, Baba Y, et al. Predictors of Catheter-Related Bladder Discomfort After Surgery: a Literature Review. J Clin Med Res. 2023;15(4):208–215. doi:10.14740/jocmr4873
5. Kim WH, Kim JT. Catheter-related bladder discomfort in gynecologic surgery. J Anesth. 2020;34(3):478. doi:10.1007/s00540-020-02757-z
6. Li S, Li P, Wang R, et al. Different interventions for preventing postoperative catheter-related bladder discomfort: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2022;78(6):897–906. doi:10.1007/s00228-021-03251-5
7. Dasgupta R, Cameron S, Aucott L, et al. Shockwave lithotripsy compared with ureteroscopic stone treatment for adults with ureteric stones: the TISU non-inferiority RCT. Health Technol Assess. 2022;26(19):1–70. doi:10.3310/WUZW9042
8. Zhang J, Li B, Li G, et al. Rigid ureteroscopic lithotripsy in the lateral decubitus position for upper urinary tract stones. BMC Urol. 2022;22(1):24. doi:10.1186/s12894-022-00977-x
9. Wang J, Wang D, Wang Y, et al. Predicting narrow ureters before ureteroscopic lithotripsy with a neural network: a retrospective bicenter study. Urolithiasis. 2022;50(5):599–610. doi:10.1007/s00240-022-01341-2
10. Li SY, Song LP, Ma YS, et al. Predictors of catheter-related bladder discomfort after gynaecological surgery. BMC Anesthesiol. 2020;20(1):97. doi:10.1186/s12871-020-01018-6
11. Lim N, Yoon H. Factors Predicting Catheter-Related Bladder Discomfort in Surgical Patients. J Perianesth Nurs. 2017;32(5):400–408. doi:10.1016/j.jopan.2016.03.012
12. Zhang HW, Fan T, Shen D, et al. Use of disposable painless silicone urethral catheter during urological surgery for male patients: a randomized controlled study. World J Urol. 2025;43(1):199. doi:10.1007/s00345-025-05506-7
13. Narver HL. Nalbuphine, a non-controlled opioid analgesic, and its potential use in research mice. Lab Anim. 2015;44(3):106–110. doi:10.1038/laban.701
14. Schnabel A, Reichl SU, Zahn PK, et al. Nalbuphine for postoperative pain treatment in children. Cochrane Database Syst Rev. 2014;2014(7):CD009583. doi:10.1002/14651858.CD009583.pub2
15. Luo MS. Nalbuphine reduces the incidence of emergence agitation in children undergoing adenotonsillectomy. J Clin Anesth. 2023;87:111115. doi:10.1016/j.jclinane.2023.111115
16. Liu J, Lin L, Li X, et al. Nalbuphine 20 mg combined with sufentanil 2 μg/kg exerts a better postoperative analgesia effect in patients undergoing a second cesarean section: a randomised trial. Ann Palliat Med. 2022;11(10):3213–3223. doi:10.21037/apm-22-1026
17. Yu P, Zhang J, Zou Y, et al. Effect of Preventive Analgesia with Nalbuphine and Dexmedetomidine in Endoscopic Sinus Surgery. Pain Res Manag. 2022;2022:2344733. doi:10.1155/2022/2344733
18. Prajapati DJ, Patel M, Patel P, et al. Effect of caudal bupivacaine alone and with adjuvant fentanyl and nalbuphine to minimize the catheter-related bladder discomfort after tubeless percutaneous nephrolithotomy: a prospective randomized study. J Anaesthesiol Clin Pharmacol. 2020;36(4):524–530. doi:10.4103/joacp.JOACP_285_18
19. Hou C, Zhang S, Zhu Y, et al. Comparative efficacy and safety of nalbuphine and hydromorphone in painless colonoscopy techniques: a randomized controlled trial. BMC Anesthesiol. 2025;25(1):187. doi:10.1186/s12871-025-03038-6
20. Park JY, Yu J, Kim CS, et al. Transcutaneous electrical nerve stimulation and catheter-related bladder discomfort following transurethral resection of bladder tumour: a randomised controlled trial. Eur J Anaesthesiol. 2024;41(11):821–830. doi:10.1097/EJA.0000000000002050
21. Jones E, Aigbogun MS, Pike J, et al. Agitation in Dementia: real-World Impact and Burden on Patients and the Healthcare System. J Alzheimers Dis. 2021;83(1):89–101. doi:10.3233/JAD-210105
22. Rasheed AM, Amirah MF, Abdallah M, et al. Ramsay Sedation Scale and Richmond Agitation Sedation Scale: a Cross-sectional Study. Dimens Crit Care Nurs. 2019;38(2):90–95. doi:10.1097/DCC.0000000000000346
23. Liao X, Xie M, Li S, et al. Comparison of tramadol and lornoxicam for the prevention of postoperative catheter-related bladder discomfort: a randomized controlled trial. Perioper Med. 2023;12(1):27. doi:10.1186/s13741-023-00317-z
24. Zhang GF, Guo J, Qiu LL, et al. Effects of dezocine for the prevention of postoperative catheter-related bladder discomfort: a prospective randomized trial. Drug Des Devel Ther. 2019;13:1281–1288. doi:10.2147/DDDT.S199897
25. Xiong J, Chen X, Weng C, et al. Intra-operative Oxycodone Reduced Postoperative Catheter-Related Bladder Discomfort Undergoing Transurethral Resection Prostate. A Prospective, Double Blind Randomized Study. Urol J. 2019;16(4):392–396. doi:10.22037/uj.v0i0.4267
26. Zhang B, Zhang T, Sromek AW, et al. Synthesis and binding affinity of novel mono- and bivalent morphinan ligands for κ, μ, and δ opioid receptors. Bioorg Med Chem. 2011;19(9):2808–2816. doi:10.1016/j.bmc.2011.03.052
27. Jao SW, Hsiao KH, Lin HC, et al. Safety and Efficacy of Oral Nalbuphine on Postoperative Pain in Hemorrhoidectomy Patients: a Randomized, Double-blind, Placebo-controlled, Pivotal Trial. Clin J Pain. 2023;39(12):686–694. doi:10.1097/AJP.0000000000001160
28. Yang WY, Li L, Ren HQ, et al. Comparison of nalbuphine and sufentanil in preventing of catheter-related bladder discomfort after transurethral ureteroscopic lithotripsy. Basic Clinl Med. 2023;43(10):1567–1571. doi:10.16352/j.issn.1001-6325.2023.10.1567
29. Xue Y, Huang Z, Cheng B, et al. Analgesic efficacy and safety of nalbuphine versus morphine for perioperative tumor ablation: a randomized, controlled, multicenter trial. Trials. 2022;23(1):887. doi:10.1186/s13063-022-06825-5
30. Chen P, Wang HH, Liu DJ, et al. The Optimal Dosage of the Nalbuphine Preemptive Analgesia on Postoperative Pain in Patients Undergoing Laparoscopic Cholecystectomy: a Randomised, Controlled, Double-Blind Study. J Coll Physicians Surg Pak. 2025;35(4):403–407. doi:10.29271/jcpsp.2025.04.403
31. Zeidan A, Mazoit JX, Ali Abdullah M, et al. Median effective dose (ED₅₀) of paracetamol and morphine for postoperative pain: a study of interaction. Br J Anaesth. 2014;112(1):118–123. doi:10.1093/bja/aet306
32. Sheng ZM, Liu X, Lin K, et al. Determining the effective dose of esketamine combined with propofol for painless hysteroscopy: a prospective dose-finding study. Front Pharmacol. 2024;15(1):1419732. doi:10.3389/fphar.2024.1419732
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

