Back to Journals » Journal of Inflammation Research » Volume 17
Dose-Volume Constraints Parameters for Lung Tissue in Thoracic Radiotherapy Following Immune Checkpoint Inhibitor Treatment
Authors Wang K , Yang F, Feng C, Xu F, Li L, Duan J, Yuan S
Received 26 June 2024
Accepted for publication 4 October 2024
Published 9 October 2024 Volume 2024:17 Pages 7141—7154
DOI https://doi.org/10.2147/JIR.S484489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Kang Wang,1 Fengchang Yang,1 Changxing Feng,1 Fuhao Xu,1 Li Li,1 Jinghao Duan,1 Shuanghu Yuan1,2
1Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China; 2Department of Radiation Oncology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, People’s Republic of China
Correspondence: Shuanghu Yuan, Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, No. 440 Jiyan Road, Jinan, Shandong, 250117, People’s Republic of China, Tel +86-531-67626931, Fax +86-531-87984079, Email [email protected]
Purpose: This study aims to identify risk factors associated with symptomatic radiation pneumonitis (RP, Grade ≥ 2) following immunotherapy preceding thoracic radiotherapy (ICI-TRT) and establish safe dose constraints.
Patients and Methods: This retrospective study enrolled patients diagnosed with non-small-cell lung cancer (NSCLC) who underwent thoracic radiotherapy (TRT) following immune checkpoint inhibitors (ICIs) treatment. The primary endpoint was the occurrence of symptomatic RP (Grade ≥ 2), as defined by the Common Terminology Criteria for Adverse Events version 5.0. Clinical and lung dosimetric parameters were analyzed to determine their associations with symptomatic RP. Dosimetric parameters included mean lung dose (MLD) and the percentage of lung volume receiving ≥ 10 Gy (V10), ≥ 20 Gy (V20), ≥ 30 Gy (V30), and ≥ 40 Gy (V40). Receiver operating characteristic curves were used to predict the risk of developing symptomatic RP to establish optimal threshold values for each dosimetric predictor.
Results: Among the 118 patients included, the incidence of symptomatic RP was 25.4%. Tumor locations, intervals between immunotherapy and radiotherapy, and MLD, V10, V20, V30, and V40 were identified as independent risk factors for symptomatic RP. The area under the curve (AUC) values for MLD, V10, V20, V30, and V40 were 0.788 (95% confidence interval [CI] 0.704– 0.873), 0.789 (95% CI 0.705– 0.874), 0.791 (95% CI 0.706– 0.876), 0.784 (95% CI 0.697– 0.871), and 0.749 (95% CI 0.656– 0.842), respectively. The optimal threshold values for MLD, V10, V20, V30, and V40 were 9.7 Gy, 26.3%, 15.9%, 13.3%, and 8.6%, respectively. These thresholds are lower than current guideline recommendations, and maintaining dosimetric parameters below these values resulted in a cumulative symptomatic RP incidence of < 12%.
Conclusion: The recommended dose thresholds for MLD, V10, V20, V30, and V40 are lower than the current guidelines, underscoring the importance of radiotherapy planning to minimize symptomatic RP occurrence in patients receiving ICI-TRT.
Keywords: radiation therapy, immunotherapy, non-small-cell lung cancer, radiation pneumonitis, risk factors
Introduction
The development of immune checkpoint inhibitors (ICIs) has revolutionized the treatment of non-small-cell lung cancer (NSCLC), offering new therapeutic options that significantly enhance tumor control, prolong overall survival, and enhance the quality of life of affected patients.1,2 Thoracic radiotherapy (TRT) has traditionally been a cornerstone of NSCLC treatment.3,4 Combining ICIs with TRT synergistically improves NSCLC treatment outcomes by enhancing the cytotoxic effects of effector T-cells and extending the distant impact of radiotherapy. Radiotherapy induces immunogenic cell death and leads to the production of damage-associated molecular patterns and cytokines or chemokines in the tumor microenvironment that promotes the recruitment of immune cells with antitumor effects, such as dendritic cells, effector T cells, Treg cells, and tumor-associated macrophages. Radiotherapy kills cancer cells while releasing large amounts of tumor-specific antigens, which are then presented to cytotoxic CD8+ T cells by antigen-presenting cells. The cytotoxic CD8+ T cells attack tumors far from the radiation field, thus generating a systemic anti-tumor immune response to local radiation therapy.5–7
However, combining ICIs with TRT can elevate the risk of radiation pneumonitis (RP).8,9 Immunotherapy stimulates activated T-cells to release inflammatory cytokines and recruit additional immune cells, thereby amplifying the inflammatory response in irradiated normal tissues. This excessive immune cell infiltration, coupled with the release of inflammatory cytokines, contributes to increased lung toxicity.10 Furthermore, the combined toxicity of immune medications and radiotherapy can exacerbate tissue damage and augment the incidence of RP.11 Prior research indicates that TRT toxicity increases in patients with autoimmune diseases due to a heightened pro-inflammatory state, triggering an inflammatory cascade response.12 The association between RP development and clinical and dosimetric factors has been demonstrated. Clinical factors, including age, presence of chronic obstructive pulmonary disease (COPD), smoking status, tumor location, concurrent chemotherapy, and chemotherapy type, are correlated with RP.13 Dosimetric parameters, such as the mean lung dose (MLD), the percentage of total lung volume receiving ≥20 Gy dose (V20), and the percentage receiving ≥5 Gy dose (V5), are crucial predictors of RP.14–16 Yorke et al17 suggested that the percentage of total lung volume receiving ≥10 Gy (V10) is a more effective predictor of severe acute RP than V20. To mitigate the risk of RP, the 2022 National Comprehensive Cancer Network Clinical Practice Guidelines recommended specific lung dosimetric thresholds for patients undergoing concurrent chemoradiotherapy: V20 should be ≤ 35% and the MLD should not exceed 20 Gy.18 Additionally, limiting the percentage of total lung volume receiving ≥30 Gy (V30) to approximately 18% significantly decreases the risk of RP.19 However, research on lung dosimetric factors for predicting symptomatic RP in immunotherapy remains limited.
Thus, previously recommended dosimetric thresholds may be inadequate for patients receiving combined immunotherapy and thoracic radiotherapy (ICI-TRT), potentially increasing the risk of RP. This study investigates the correlation between symptomatic RP occurrence and lung dosimetric factors in patients with NSCLC undergoing combined ICI-TRT to establish safe dose limits.
Materials and Methods
Patients
We retrospectively analyzed patients with NSCLC who underwent ICI-TRT between 2021 and September 2023. The requirement for obtaining informed consent was waived given the retrospective nature of the study. This study was approved by the Ethics Committee of Shandong Cancer Hospital and Institute (approval number: SDTHEC2022009020) and was conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
The inclusion criteria comprised the following: (i) histopathologically confirmed primary diagnosis of NSCLC, including squamous cell carcinoma, adenocarcinoma, and other subtypes like large cell carcinomas; (ii) clinical stages II–IV, deemed inoperable; and (iii) patients who underwent immunotherapy followed by chest radiotherapy (concurrent treatment was permitted and not a basis for exclusion to prevent selection bias). The exclusion criteria were as follows: (i) prior history of chest radiotherapy or lung tumor resection; and (ii) absence of chest computed tomography (CT) evaluation either during or within 3 months post-radiotherapy. A total of 118 patients were ultimately eligible.
Treatment Planning and Collection of Dosimetric Parameters
All patients underwent 3-mm layer-thick CT scans using a Philips 16-slice Brilliance large-aperture CT scanner (Philips Medical Systems, Amsterdam, Netherlands). CT images were imported into the Eclipse 16.1 treatment planning system (Varian, USA) to delineate targets and organs at risk. The gross tumor volume (GTV), comprising the primary tumor and metastatic lymph nodes identified on CT images, was determined. The clinical target volume (CTV) included microscopically visible tumor microfoci beyond the GTV boundaries. Adenocarcinomas extended 0.8 cm beyond the GTV, whereas non-adenocarcinomas extended 0.6 cm. Due to various errors, the planning target volume extended 0.5–1 cm beyond the CTV. A junior physician outlined all target areas, which were subsequently reviewed by a senior physician.
All patients received intensity-modulated radiotherapy (IMRT). Lung dosimetric parameters were obtained and assessed using the Eclipse 16.1 treatment planning system. The parameters MLD, V10, V20, V30, and V40 were extracted from dose-volume histograms (total lung volume minus GTV). Vn was defined as the lung volume irradiated with doses exceeding n Gy as a percentage of the total lung volume.
Toxicity Assessments
Following radiotherapy, the patients underwent chest or whole-body CT scans every 2–3 months for up to 6 months. The study endpoint was the occurrence of symptomatic RP within 6 months of radiotherapy. RP diagnosis relies on chest CT findings, hematological analysis, and clinical presentation. Typical imaging changes included patchy hyper-density, ventilatory bronchiolar signs, striated shadows, solid lung shadows, and honeycomb-like changes in the radiological field. These lesions did not conform to the anatomical distribution of the lung lobes or segments, while other potential causes, such as infections, tumor progression, or immune-associated pneumonia, were excluded. The severity of RP was graded according to the Common Terminology Criteria for Adverse version 5.0, which were as follows: grade 1 RP was asymptomatic pneumonitis or minimal symptoms that did not require intervention; grade 2 RP was accompanied by coughing, chest distress or other symptoms that did not interfere with daily activities and needed symptomatic treatment; grade 3 RP exhibited severe symptoms and required corticosteroids or the administration of oxygen; grade 4 RP required urgent intervention, such as ventilation, for life-threatening respiratory symptoms; and grade 5 RP was fatal.
Statistical Analysis
Descriptive statistics characterized the patients’ baseline and treatment features. Univariate and multivariate analyses, employing binary logistic regression, were used to evaluate the association between clinical and dosimetric factors and the risk of symptomatic RP. Due to the strong linear correlations between the dosimetric factors, each of them was individually incorporated into multifactor logistic regression analyses. The area under the receiver operating characteristic (ROC) curve (AUC) was used to assess the predictive capacity of identified factors for symptomatic RP and establish the optimal threshold values for the dosimetric predictors. Independent-sample t-tests were used to compare the cumulative symptomatic RP incidence between the groups. The Kaplan-Meier method was used to assess patient survival, and the Log rank test was used to evaluate the heterogeneity of the survival analysis. Progression-free survival (PFS) was defined as the interval from the start of treatment to the first occurrence of local-regional progression, distant metastasis, death, or last follow-up, inclusive of disease progression during therapy and post-treatment recurrence. Overall survival (OS) was defined as the interval from the start of treatment to death or the last follow-up. Statistical analyses were conducted using IBM SPSS Statistics for Windows (version 25.0; IBM Corp., Armonk, NY). All tests were two-sided, and statistical significance was defined as p < 0.05.
Results
Patient Characteristics
This study included a cohort of 118 patients, with their demographic details outlined in Table 1. The median age of the participants was 62 years. Among them, 65 patients (55.1%) had a history of smoking (current or former), while 23 (19.5%) had pre-existing lung conditions, such as interstitial lung disease (ILD), COPD, and acute and chronic bronchitis, among others. Most tumors were located in the upper or middle lung lobes, with 86 patients (72.9%) falling into this category, while 32 (27.1%) had tumors in the lower lobes.
 |
Table 1 Patient Characteristics |
Table 2 lists the pertinent chest radiotherapy and immunotherapy characteristics of all included patients. Before chest radiotherapy, all patients received programmed cell death 1 (94.9%) or programmed death-ligand 1 (5.1%) inhibitors. The median time interval between the initiation of immunotherapy and chest radiotherapy was 12.5 days (interquartile range [IQR], 5–28 days). Conventional fractionated radiotherapy was administered to all patients. The median (IQR) values for MLD, V10, V20, V30, and V40 were 9.9 Gy (7.3–12.2), 26.0% (18.4–32.2), 16.9% (13.1–21.4), 12.3% (8.8–16.9), and 8.3% (5.3–11.4), respectively.
 |
Table 2 Characteristics of Radioimmunotherapy |
RP Incidence and Characteristics
At the final follow-up, 61 patients (51.7%) did not develop RP, while 57 (48.3%) experienced RP. Among those who developed RP, 27 (22.9%) had Grade 1 (asymptomatic) RP, while the prevalence of symptomatic RP (Grade ≥ 2) was 25.4%. Within this subset, 28 patients (23.7%) had Grade 2 RP, two (1.7%) had Grade 3 RP, and no instances of Grades 4–5 RP were observed. The median duration from the conclusion of radiotherapy to symptomatic RP onset was 83.5 days (IQR, 39.8–111.8 days). Most symptomatic RP cases occurred post-radiotherapy, with one patient experiencing symptoms during treatment. In this specific instance, the planned total radiotherapy dose was 60 Gy/30F; however, only 50 Gy/25F was delivered, leading to premature treatment cessation due to symptomatic RP (Grade 2 in this patient). Figure 1 provides a visual representation of a patient diagnosed with Grade 2 RP. Before radiotherapy, the patient was diagnosed with squamous cell carcinoma of the left lung and underwent three cycles of immunotherapy (tislelizumab) combined with chemotherapy. The patient received 30 fractions, totaling 60 Gy of radiotherapy, with an MLD of 10.2 Gy, V10 of 29.3%, and V20 of 13.9%. Symptomatic RP developed in the patient 6.9 weeks post-radiotherapy. Symptoms included cough, production of white mucoid sputum, regular inflammatory markers, negative sputum culture for pathogenic bacteria, and CT findings showing multiple patchy hyperdense shadows with blurred margins and a partially visible bronchial insufflation sign. Their symptoms resolved following the administration of intravenous methylprednisolone sodium succinate.
Risk Factors of Symptomatic RP
Tables 3 and 4 present the correlations between baseline clinical characteristics, dosimetric factors, and symptomatic RP. Patient-related factors, including age, gender, smoking status, and history of lung disease, showed no significant associations with symptomatic RP (p > 0.05). However, tumor- and treatment-related factors (Table 3), such as tumor location (p = 0.023) and the time interval between radiotherapy and immunotherapy (p = 0.003), showed significant associations with symptomatic RP. There were no significant differences observed in tumor pathology, TNM stage, or radiotherapy dose (p > 0.05).
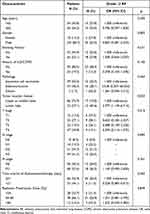 |
Table 3 Patient and Tumor Characteristics Correlate with Grade ≥2 Radiation Pneumonitis |
 |
Table 4 Dosimetric Characteristics Correlate with Grade ≥2 Radiation Pneumonitis |
In the analysis of dosimetric factors (Table 4), univariate logistic regression revealed significant associations between whole-lung MLD, V10, V20, V30, and V40 and symptomatic RP (p < 0.001). In the multivariate logistic regression model, significant dosimetric parameters were adjusted for significant clinical factors, such as tumor location and the time interval between radiotherapy and immunotherapy. Due to the strong linear correlations among the MLD, V10, V20, V30, and V40, each parameter was subsequently incorporated individually in the multivariate logistic regression analysis in order to ensure the accuracy of the predicted parameters. In the multifactorial logistic regression analysis (Table 4), MLD (odds ratio [OR] 1.385, 95% confidence interval [CI] 1.190–1.612, p < 0.001), V10 (OR 1.130, 95% CI 1.069–1.194, p < 0.001), V20 (OR 1.188, 95% CI 1.097–1.286, p < 0.001), V30 (OR 1.232, 95% CI 1.118–1.357, p < 0.001), and V40 (OR 1.230, 95% CI 1.106–1.369, p < 0.001) all showed statistically significant associations. Figure 2 illustrates MLD, V10, V20, V30, and V40 levels in both the symptomatic and non-symptomatic RP groups.
ROC curves were constructed using significant risk factors to predict the development of symptomatic RP (Figure 3). Figure 3A shows the AUC values for the following dosimetric parameters: MLD, 0.788 (95% CI 0.704–0.873); V10, 0.789 (95% CI 0.705–0.874); V20, 0.791 (95% CI 0.706–0.876); V30, 0.784 (95% CI 0.697–0.871); and V40, 0.749 (95% CI 0.656–0.842).
When considering only significant clinical factors (tumor location and time interval between radiotherapy and immunotherapy), the AUC value was 0.702 (95% CI 0.604–0.800). However, combining clinical and dosimetric factors significantly improved the predictive accuracy. The ROC curves in Figure 3B demonstrated that the AUC values for clinical factors combined with dosimetric parameters yielded a superior predictive performance: clinical factors combined with MLD = 0.828 (95% CI 0.752–0.905), V10 = 0.822 (95% CI 0.743–0.901), V20 = 0.831 (95% CI 0.754–0.907), V30 = 0.826 (95% CI 0.747–0.904), and V40 = 0.798 (95% CI 0.713–0.884). The ROC curve showed an AUC value of 0.832 (95% CI 0.753–0.909) for clinical factors combined with all dosimetric parameters. These combined factors outperformed individual dosimetric or clinical factors.
Using the Youden index derived from ROC analysis, we established the optimal threshold values for each dosimetric parameter: MLD = 9.7 Gy, V10 = 26.3%, V20 = 15.9%, V30 = 13.3%, and V40 = 8.6%. Patients whose dosimetric parameters were below these thresholds had a cumulative incidence of symptomatic RP < 12%. Notably, for patients with MLD < 9.7 Gy (n = 4) and MLD ≥ 9.7 Gy (n = 26) (Figure 4A), the rates at 24 weeks were 7.1% and 41.9%, respectively (p < 0.001). In Figure 4B, for the V10 < 26.3% (n = 5) and V10 ≥ 26.3% (n = 25) groups, the rates at 24 weeks were 8.1% and 44.6%, respectively (p < 0.001). In Figure 4C, for the V20 < 15.9% (n = 2) and V20 ≥ 15.9% (n = 28) groups, the rates at 24 weeks were 4.2% and 40.0%, respectively (p < 0.001). In Figure 4D, for the V30 < 13.3% (n = 8) and V30 ≥ 13.3% (n = 22) groups, the rates at 24 weeks were 11.4% and 45.8%, respectively (p < 0.001). Finally, in Figure 4E, for the V40 < 8.6% (n = 7) and V40 ≥ 8.6% (n = 23) groups, the rates at 24 weeks were 7.1% and 41.9%, respectively (p < 0.001). The calculated values of sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for these five dosimetric parameters are shown in Table 5.
 |
Table 5 Sensitivity, Specificity, PPV, NPV, and Accuracy of Dosimetric Characteristics |
Up to September 2024, 63 patients exhibited disease progression and 53 patients died. The median PFS for all patients was 19.9 months (95% CI 14.3–25.5) and the median OS was 30.0 months (95% CI 26.4–33.6). Kaplan-Meier analysis showed that after grouping patients according to the determined optimal threshold values, no significant difference in OS was observed between the upper and lower threshold groups (p > 0.05) (Figure 5). Patients in the MLD ≥ 9.7 Gy group had a better PFS compared with those in the MLD < 9.7 Gy group (Hazard ratio 1.738, 95% CI 1.058–2.854, p = 0.03). There was no significant difference in PFS in the remaining subgroups (p > 0.05) (Supplementary Figure 1).
Discussion
Based on the PACIFIC criteria, approximately 50% of patients with unresectable Stage III NSCLC who undergo concurrent chemoradiotherapy are ineligible for durvalumab treatment.20,21 An alternative treatment approach for patients with unresectable locally advanced NSCLC involves immunochemotherapy induction followed by radiotherapy.22 However, combining ICIs with radiotherapy increases the risk of RP. Therefore, analyzing the clinical and dosimetric factors associated with symptomatic RP in this context and exploring their safe dosimetric thresholds is crucial. In our study, data on various clinical and dosimetric parameters were collected from 118 patients with NSCLC undergoing ICI-TRT. We observed a symptomatic RP incidence of 25.4%, which is within the expected range.23,24 No Grade 4–5 RP cases were observed, possibly attributable to approximately 89.9% of patients receiving an irradiation dose of ≤60 Gy.
This study identified the MLD, as well as the V10, V20, V30, and V40, as independent risk factors for symptomatic RP. Furthermore, the optimal thresholds for the MLD, V10, V20, V30, and V40 in combined ICI-TRT were determined to be 9.7 Gy, 26.3%, 15.9%, 13.3%, and 8.6%, respectively. These findings are consistent with those of previous studies. For example, Tsujino et al15 concluded in their analysis of predicting severe RP after concurrent chemoradiotherapy for locally advanced NSCLC that all DVH metrics significantly correlated with Grade ≥3 RP occurrence. The comparison highlights that various lung dosimetric parameters symptomatic RP in patients undergoing combined ICI-TRT. Nevertheless, the previously recommended thresholds for dosimetric parameters have been revised. Traditional thresholds for lung dosimetric parameters were established based on the direct effects of RT on tumors and normal tissues.
However, the addition of ICIs strengthened the antitumor immune response by inducing lymphocyte differentiation and upregulating cytokine and autoantibody levels. This resulted in excessive cytokine release and increased immune cell infiltration, amplifying the inflammatory response in irradiated normal tissues.10 Additionally, radiation exposure to lung tissue induces oxidative damage to DNA and proteins, leading to the release of tumor antigens and inflammatory factors. This, in turn, stimulates cytokine and inflammatory cell accumulation in the alveolar cavities, triggering an inflammatory response.25 Moreover, the antitumor response increases following ICIs treatment. The accumulation of self-DNA released from deceased tumor cells can activate the cGAS-STING signaling pathway, resulting in the production of interferons and inflammatory cytokines, ultimately inciting inflammatory reactions and fibrosis.26
In the current study, the cumulative occurrence of symptomatic RP was below 12% in the cohorts with MLD, V10, V20, V30, and V40 values below the respective optimal thresholds. The absence of a definitive threshold for pulmonary radiation dosage linked to RP following the integration of immunotherapy remains to be addressed. Shintani et al27 emphasized V20 as a significant risk factor for Grade ≥ 2 RP, reporting a 12-month incidence of Grade ≥ 2 RP reaching 50% when V20 ≥ 26%. Bi et al28 established a progressive risk of RP based on MLD as a continuous variable, identifying an MLD value of 14.1 Gy predictive of a 50% risk of grade 2 or higher RP.
Bi et al29 also examined the dosimetric risk factors for acute RP in patients undergoing ICI treatment, focusing solely on MLD, V5, and V20. Their results suggested that MLD and V20 affect acute RP in this patient cohort. However, their analyses included limited dosimetric parameters. Therefore, we expanded the study to encompass MLD, V10, V20, V30, and V40. To mitigate the instability of predictive parameters and inaccuracies, we conducted multicollinearity tests for each dosimetric parameter. For dosimetric factors exhibiting strong linear correlations, they were individually incorporated into multifactorial logistic regression analyses one by one, enhancing the interpretive and predictive capabilities of the parameters.
Our investigation revealed a correlation between tumor location and the occurrence of symptomatic RP (p = 0.023). Consistent with previous studies, individuals undergoing radiotherapy for lung cancer with tumors located in the lower lung lobes exhibited heightened vulnerability to RP.30–32 Seppenwoolde et al31 reported that patients with lower lung lobe tumors faced a RP risk of up to 40% post-radiotherapy, significantly higher than that of patients with middle (16%) and upper lobe tumors (11%). This difference can potentially be attributed to the respiratory motion characteristic of the lungs, where increased mobility of the lower lung lobes causes further irradiation of normal lung tissues or lung tissues that should have received low-dose irradiation. In a rat model, Khan et al33 illustrated that lower lung irradiation induced more substantial DNA damage than upper lung irradiation. Additionally, tumor involvement, tissue inflammation, and gravitational influence may impact lung ventilation and enhance perfusion and ventilation in the lower lungs, thereby exacerbating RP severity.34 These insights will help to facilitate managing patients with lower lung lobe tumors undergoing chest RT combined with ICIs.
Chronic lung diseases, such as ILD, COPD, and acute and chronic bronchitis, are associated with the development of RP. Prior retrospective studies have identified ILD as an independent risk factor for grade 2 or higher RP,35,36 and the extent of ILD involvement correlates with the incidence of RP.37 Herein, we found no significant difference between prior ILD and symptomatic RP. Liu et al38 showed a significant correlation between a history of respiratory disease and the risk of RP. Notably, their study population had a median age of 73 years, which was higher than that of our study. This age difference may be related to the fact that older patients often have other underlying diseases and poor lung function. Additionally, since combination immunotherapy-radiotherapy is not recommended for patients with ILD exhibiting significant clinical symptoms, and because some patients could not tolerate immunotherapy and therefore discontinued it, the limited number of patients with prior lung disease included in this study may have contributed to discrepancies in the findings compared with conventional radiotherapy. We suggest that the dosimetric threshold be individually considered when planning radiotherapy, mainly to control lung receptivity in patients with ILD.
Our study was based on patients with locally advanced or metastatic NSCLC, for whom all treatments have involved first-line ICIs followed by TRT to the primary chest lesions and metastatic areas. These data from this study will become more applicable as the use of ICIs and TRT in combination gradually expands,39–41 as well as multiple ongoing clinical trials (eg the NRG LU002, SARON, and SABR-COMET-10 trials). Different treatment modes and sequences may result in different RP-related risk factors and incidence rates. For example, the QUANTEC model, which was proposed long before the widespread use of ICIs, showed that the risk of symptomatic RP was less than 20% when MLD did not exceed 20 Gy.42 This is quite different from the MLD not exceeding 10 Gy proposed for the ICI-TRT population in this study. We encourage clinicians to use stricter dose limits than QUANTEC in patients receiving combination therapy with ICIs and TRT.
The current investigation is subject to some limitations. Primarily, this was a retrospective analysis with a limited sample size and a relatively short monitoring duration, which may have potentially underestimated the total occurrence of RP. Additionally, we did not evaluate other factors linked to symptomatic RP, such as initial lung function, primarily due to the availability of baseline lung function data for only 33 enrolled patients. Lastly, our conclusions were drawn from a single institution, emphasizing the need for supplementary multicenter investigations to validate our findings. We also recognize that these data may not necessarily apply to lung cancer patients who did not receive ICIs prior to TRT (eg patients with stage III NSCLC treated using the PACIFIC trial model) or to patients with other types of cancer.
Conclusion
In the era of immunotherapy, tumor location, the interval between radiotherapy and immunization, the MLD, and radiation doses at V10, V20, V30, and V40 have emerged as independent risk factors for symptomatic RP (Grade ≥ 2). For patients receiving ICI-TRT, maintaining MLD, V10, V20, V30, and V40 below 9.7 Gy, 26.3%, 15.9%, 13.3%, and 8.6%, respectively, will reduce symptomatic RP risk to 12%. These values are lower than the dose thresholds recommended by current guidelines and can potentially prompt updates in lung dose thresholds to enhance the monitoring and management of patients undergoing ICI-TRT.
Abbreviations
AUC, area under the ROC curve; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CT, computed tomography; CTV, clinical target volume; GTV, gross tumor volume; Gy, gray; ILD, interstitial lung disease; ICIs, immune checkpoint inhibitors; IQR, interquartile range; IMRT, intensity-modulated radiotherapy; MLD, mean lung dose; NSCLC, non-small-cell lung cancer; OR, odds ratio; OS, overall survival; PFS, progression-free survival; ROC, receiver operating characteristic; RP radiation pneumonitis; TRT, thoracic radiotherapy; Vn, lung volume irradiated with doses exceeding n Gy as a percentage of the total lung volume.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the Shandong Cancer Hospital and Institute (ethics approval number: SDTHEC2022009020), which waived informed consent from participants given the retrospective nature of the study. We declare that patient information will be kept confidential and that we adhere to the principles of the Declaration of Helsinki.
Acknowledgments
We would like to thank the patients and their families who participated in this study as well as the investigators and research staff involved.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC82073345), Natural Science Innovation and Development Joint Foundation of Shandong Province (ZR202209010002), Jinan Clinical Medicine Science and Technology Innovation Plan (202019060), and Taishan Scholars Program to Shuanghu Yuan.
Disclosure
The authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
1. Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol. 2017;8:1–20. doi:10.5306/wjco.v8.i1.1
2. Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small cell lung cancer. J Clin Oncol. 2022;40:1301–1311. doi:10.1200/JCO.21.01308
3. Spencer K, Morris E, Dugdale E, et al. 30-day mortality in adult palliative radiotherapy—a retrospective population-based study of 14,972 treatment episodes. Radiother Oncol. 2015;115:264–271. doi:10.1016/j.radonc.2015.03.023
4. Jumeau R, Vilotte F, Durham AD, Ozsahin EM. The current landscape of palliative radiotherapy for non-small cell lung cancer. Transl Lung Cancer Res. 2019;8:S192–S201.
5. Theelen WSME, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomized trials. Lancet Respir Med. 2021;9:467–475. doi:10.1016/S2213-2600(20)30391-X
6. Girard N, Bar J, Garrido P, et al. Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: findings from the PACIFIC-R study. J Thorac Oncol. 2023;18:181–193. doi:10.1016/j.jtho.2022.10.003
7. Demaria S, Formenti SC. Role of T lymphocytes in tumor response to radiotherapy. Front Oncol. 2012;2:95. doi:10.3389/fonc.2012.00095
8. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi:10.1056/NEJMoa1709937
9. Aiad M, Fresco K, Prenatt Z, et al. Comparison of pneumonitis rates and severity in patients with lung cancer treated by immunotherapy, radiotherapy, and immunoradiotherapy. Cureus. 2022;14:e25665.
10. Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic radiation therapy augments antigen-specific pd-1-mediated antitumor immune responses via cross-presentation of tumor antigens. Cancer Immunol Res. 2015;3:345–355. doi:10.1158/2326-6066.CIR-14-0196
11. Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi:10.1172/JCI67313
12. Lin D, Lehrer EJ, Rosenberg J, et al. Toxicity after radiotherapy in patients with historically accepted contraindications to treatment (CONTRAD): an international systematic review and meta-analysis. Radiother Oncol. 2019;135:147–152. doi:10.1016/j.radonc.2019.03.006
13. Robnett TJ, Machtay M, Vines EF, et al. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2000;48:89–94. doi:10.1016/S0360-3016(00)00648-9
14. Wang S, Liao Z, Wei X, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys. 2006;66:1399–1407. doi:10.1016/j.ijrobp.2006.07.1337
15. Tsujino K, Hashimoto T, Shimada T, et al. Combined analysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves the prediction of severe radiation pneumonitis after concurrent chemoradiotherapy for locally advanced non-small cell lung cancer. J Thorac Oncol. 2014;9:983–990. doi:10.1097/JTO.0000000000000187
16. Kwa SL, Lebesque JV, Theuws JC, et al. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998;42:1–9. doi:10.1016/S0360-3016(98)00196-5
17. Yorke ED, Jackson A, Rosenzweig KE, et al. Correlation of dosimetric factors and radiation pneumonitis for non-small-cell lung cancer patients in a recently completed dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:672–682. doi:10.1016/j.ijrobp.2005.03.026
18. Ettinger DS, Wood DE, Aisner DL, et al. Non-small Cell Lung Cancer. version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Nunk. 2022;20:497–530.
19. Hernando ML, Marks LB, Bentel GC, et al. Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:650–659. doi:10.1016/S0360-3016(01)01685-6
20. Eichkorn T, Bozorgmehr F, Regnery S, et al. Consolidation immunotherapy after platinum-based chemoradiotherapy in patients with unresectable stage III non-small cell lung cancer-cross-sectional study of eligibility and administration rates. Front Oncol. 2020;10:586449. doi:10.3389/fonc.2020.586449
21. Cotarla I, Boron ML, Cullen SL, et al. Treatment decision drivers in stage III non-small-cell lung cancer: outcomes of a web-based survey of Oncologists in the United States. JCO Oncol Pract. 2020;16:e1232–e1242. doi:10.1200/JOP.19.00781
22. Wu L, Cheng B, Sun X, et al. Induction immunochemotherapy followed by definitive chemoradiotherapy for unresectable locally advanced non-small cell lung cancer: a multi-institutional retrospective cohort study. Medicine. 2024;5:e501.
23. Jabbour SK, Lee KH, Frost N, et al. Pembrolizumab plus concurrent chemoradiotherapy in patients with unresectable, locally advanced, stage III non-small cell lung cancer: the Phase 2 KEYNOTE-799 nonrandomized Trial. JAMA Oncol. 2021;7:1–9. doi:10.1001/jamaoncol.2021.2301
24. Gray JE, Villegas A, Daniel D, et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-Update from PACIFIC. J Thorac Oncol. 2020;15:288–293. doi:10.1016/j.jtho.2019.10.002
25. Hwang WL, Pike LRG, Royce TJ, et al. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol. 2018;15:477–494. doi:10.1038/s41571-018-0046-7
26. Corrales L, Gajewski TF. Endogenous and pharmacologic targeting of the STING pathway in cancer immunotherapy. Cytokine. 2016;77:
27. Shintani T, Kishi N, Matsuo Y, et al. Incidence and risk factors of symptomatic radiation pneumonitis in non-small cell lung cancer patients treated with concurrent chemoradiotherapy and consolidation durvalumab. Clin Lung Cancer. 2021;22:401–410. doi:10.1016/j.cllc.2021.01.017
28. Bi J, Meng R, Yang D, et al. Dosimetric predictors of radiation pneumonitis in patients with prior immunotherapy exposure: a multi-institutional analysis. Radiother Oncol. 2024;190:110040. doi:10.1016/j.radonc.2023.110040
29. Bi J, Qian J, Yang D, et al. Dosimetric risk factors for acute radiation pneumonitis in patients with prior receipt of immune checkpoint inhibitors. Front Immunol. 2021;12:828858. doi:10.3389/fimmu.2021.828858
30. Yamada M, Kudoh S, Hirata K, et al. Risk factors of pneumonitis following chemoradiotherapy for lung cancer. Eur J Cancer. 1998;34:71–75. doi:10.1016/S0959-8049(97)00377-8
31. Seppenwoolde Y, De Jaeger K, Boersma LJ, et al. Regional differences in lung radiosensitivity after radiotherapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;60:748–758. doi:10.1016/j.ijrobp.2004.04.037
32. Bradley JD, Hope A, El Naqa I, et al. A nomogram to predict radiation pneumonitis, derived from a combined analysis of RTOG 9311 and institutional data. Int J Radiat Oncol Biol Phys. 2007;69:985–992. doi:10.1016/j.ijrobp.2007.04.077
33. Khan MA, Van Dyk J, Yeung IW, et al. Partial volume rat lung irradiation; assessment of early DNA damage in different lung regions and effect of radical scavengers. Radiother Oncol. 2003;66:95–102. doi:10.1016/S0167-8140(02)00325-0
34. Kong FM, Wang S. Nondosimetric risk factors for radiation-induced lung toxicity. Semin Radiat Oncol. 2015;25:100–109. doi:10.1016/j.semradonc.2014.12.003
35. Ueki N, Matsuo Y, Togashi Y, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J Thorac Oncol. 2015;10:116–125. doi:10.1097/JTO.0000000000000359
36. Glick D, Lyen S, Kandel S, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival in patients treated with lung stereotactic body radiation therapy (SBRT). Clin Lung Cancer. 2018;19:e219–e226. doi:10.1016/j.cllc.2017.06.021
37. Liu Y, Zhu Y, Wu R, et al. Stereotactic body radiotherapy for early-stage non-small cell lung cancer in patients with subclinical interstitial lung disease. Transl Lung Cancer Res. 2020;9:2328–2336. doi:10.21037/tlcr-20-1050
38. Liu Y, Wang W, Shiue K, et al. Risk factors for symptomatic radiation pneumonitis after stereotactic body radiation therapy (SBRT) in patients with non-small cell lung cancer. Radiother Oncol. 2021;156:231–238. doi:10.1016/j.radonc.2020.10.015
39. Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(14):1505–1517. doi:10.1200/JCO.19.03136
40. Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year survival with durvalumab after chemoradiotherapy in stage III NSCLC-an update from the PACIFIC trial. J Thorac Oncol. 2021;16(5):860–867. doi:10.1016/j.jtho.2020.12.015
41. Hsieh K, Dickstein DR, Runnels J, et al. Radiotherapy and immunotherapy in lung cancer. Biomedicines. 2023;11(6):1642. doi:10.3390/biomedicines11061642
42. Bentzen SM, Constine LS, Deasy JO, et al. Quantitative analyses of normal tissue effects in the clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S3–S9. doi:10.1016/j.ijrobp.2009.09.040
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Spotlight on Cemiplimab-rwlc in the Treatment of Non-Small Cell Lung Cancer (NSCLC): Focus on Patient Selection and Considerations
Ahn J, Nagasaka M
Cancer Management and Research 2023, 15:627-634
Published Date: 11 July 2023
Radiotherapy with Targeted Therapy or Immune Checkpoint Inhibitors for Hepatocellular Carcinoma with Hepatic Vein and/or Inferior Vena Cava Tumor Thrombi
Li Z, Zhai Y, Wu F, Cao D, Ye F, Song Y, Wang S, Liu Y, Song Y, Tang Y, Jing H, Fang H, Qi S, Lu N, Li YX, Wu J, Chen B
Journal of Hepatocellular Carcinoma 2024, 11:1481-1493
Published Date: 5 August 2024






