Back to Journals » Nature and Science of Sleep » Volume 17
Dreams and Nightmares in Early Pregnancy: A Comparative Study with a Control Group
Authors Scarpelli S , Spinoni M, Gorgoni M , Lasaponara S, Ciolli P, Rech F, Di Muzio M, Med C, Di Pasquale Benedetti I, Grano C , De Gennaro L
Received 10 February 2025
Accepted for publication 22 April 2025
Published 9 May 2025 Volume 2025:17 Pages 851—864
DOI https://doi.org/10.2147/NSS.S520737
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Serena Scarpelli,1,2 Marta Spinoni,1 Maurizio Gorgoni,1,2 Stefano Lasaponara,1 Paola Ciolli,3 Francesco Rech,3 Marco Di Muzio,4 Carlotta Med,1 Ilaria Di Pasquale Benedetti,1 Caterina Grano,1,* Luigi De Gennaro1,*
1Department of Psychology, Sapienza University of Rome, Rome, Italy; 2Body and Action Lab, IRCCS Fondazione Santa Lucia, Rome, Italy; 3Department of Maternal and Child Health and Urological Sciences, Sapienza University of Rome, Rome, Italy; 4Department of Clinical and Molecular Medicine, Sapienza University of Rome, Rome, Italy
*These authors contributed equally to this work
Correspondence: Serena Scarpelli, Department of Psychology, Sapienza University of Rome, Via dei Marsi 78, Rome, 00185, Italy, Email [email protected]
Purpose: This study investigated dream characteristics in women during the first trimester of pregnancy compared to a group of non-pregnant women, aiming to identify variables associated with the observed differences.
Participants and Method: A sample of 100 pregnant women in their first trimester was compared to a control group of 100 age-matched non-pregnant women. Participants completed online questionnaires to assess dream activity, sleep quality, depressive symptoms, and sociodemographic variables.
Results: Controlling for socio-demographic variables, statistical comparisons revealed that pregnant women reported fewer nightmares and showed less interest in their dream activity compared to non-pregnant women. Ordinal logistic regression revealed that being in the control group, greater attention to dreams, the presence of depressive symptoms, and a higher frequency of lucid dreaming were significant predictors of more frequent nightmares. Moderation analysis showed no significant interaction between pregnancy status and dream attitude.
Conclusion: Contrary to expectations, first-trimester pregnant women had fewer nightmares than non-pregnant women. However, the results are coherent with the finding that parasomnia-like events decrease during pregnancy. The rapid hormonal changes and specific sleep and emotional features of this stage of gestation may explain the lower presence of nightmares as compared to our control group. Moreover, we confirmed a crucial role of dream attitude in recalling nightmares, suggesting that some stable, trait-like features may contribute to nightmare experiences independently of pregnancy status. Our results also confirmed, according to the Continuity hypothesis, that depressive symptoms are associated with nightmares. Also, the presence of lucid dreaming in association with nightmares may be interpreted as an attempt to cope with unpleasant emotions. Longitudinal studies are needed to examine how dream activity evolves across pregnancy stages.
Plain Language Summary: Have you ever wondered how particular moments in life, emotional states, and “non-ordinary” physical conditions affect your dreams? This study explores exactly that, focusing on a unique moment in life: pregnancy. Researchers compared the dreaming of 100 women in their first trimester of pregnancy with those of 100 non-pregnant women. The results are quite unexpected: pregnant women in their first trimester reported experiencing fewer nightmares and less interest in their dreams compared to the control group. At first glance, this seems to contrast with other research suggesting that pregnancy often increases dream frequency and nightmares.
However, there is actually very little research specifically focusing on the first trimester of pregnancy —just one prior study— and the findings of this study are consistent with it.
So, what’s behind this reduction in scary dreams? It may be linked to the sleep changes during this period. Pregnancy hormones have sedative effects that can lead to deeper sleep, reducing REM sleep—the stage where the most vivid dreams occur.
Additionally, women reporting higher levels of depressive symptoms and those more engaged with their dreams were more likely to experience nightmares. This suggests that emotional factors and interest in dreams, play a significant role in the occurrence of bad dreams.
This research provides valuable insights into how pregnancy can affect dreams, highlighting the importance of understanding these changes to support the mental health of expectant mothers. Future research should explore how dreams evolve longitudinally across all three trimesters of pregnancy to address the many unanswered questions.
Keywords: oneiric activity, gestation, continuity hypothesis, nightmares, first trimester, depression
Introduction
Pregnancy induces profound physiological, hormonal, and psychological changes, significantly affecting sleep patterns1 and dreaming processes.2 The current literature suggests that the gestational stage is critical in shaping dream frequency and oneiric content. Indeed, dream recall is reported to increase with gestational age,3 alongside a shift in thematic content that reflects concerns about the maternal role, fetal well-being, and childbirth.2,4 This progression in dream themes may serve as a psychological mechanism to process anxieties and adapt to impending motherhood, aligning with the Continuity hypothesis, which posits that dreams reflect ongoing waking-life concerns.5 Furthermore, some evidence supports the Threat simulation theory, which suggests that nightmares may function as a mechanism to simulate and rehearse responses to perceived threats, potentially preparing expectant mothers to cope with dangers related to childbirth and infant care.6 Interestingly, younger and nulliparous women are more likely to report baby-related dream content, suggesting that these dreams may serve as an emotional outlet for processing the uncertainties and anticipations surrounding a first pregnancy.7
A notable pattern observed in the literature is the increase in nightmare frequency during late pregnancy.4,7 Nightmares often reflect heightened emotional arousal and anxiety, frequently revolving around themes of childbirth and infant safety.3 Specifically, poor sleep quality and insomnia, which are prevalent during late gestation, have been shown to correlate with increased nightmare frequency, further complicating maternal emotional regulation.4,8
While the relationship between late pregnancy and dream characteristics has been documented, the early stages of pregnancy remain partly underexplored. Some evidence suggests a decrease in parasomnias, such as nightmares, during the first trimester, potentially due to different hormonal dynamics compared to later stages.9 Moreover, recent findings highlight the potential influence of emotional factors, such as depressive symptoms and alexithymia, on dreaming even during early gestation.10 Specifically, depressive symptoms and alexithymia have been linked to increased nightmare frequency and distress, suggesting a key role of emotional dysregulation on dream characteristics during the first trimester.10
It should be noted that no other studies selectively investigated dream activity during early pregnancy. Furthermore, dream research on the first trimester lacks systematic comparisons with non-pregnant women,9,10 making it challenging to determine whether observed dream features are specific to pregnancy or reflect broader psychological factors.
Firstly, this study aims to fill these gaps by investigating and comparing the dream characteristics of women in their first trimester of pregnancy with those of a matched control group. Secondly, it seeks to identify variables most strongly associated with the observed differences, providing a deeper understanding of the psychological and emotional processes underpinning dream activity in early pregnancy. Considering that the majority of studies revealed higher dream activity in pregnant than non-pregnant women,2 we hypothesized that pregnant women may experience more dreams and nightmares than non-pregnant women. Also, according to the literature, we expected that oneiric activity, and especially nightmare frequency, may be predicted by poor sleep quality,11 depressive symptoms, and lucid dreaming.12
Materials and Methods
Participants and Study Design
The study was part of a research project promoted by the Sapienza University of Rome. Recruitment of pregnant women took place between April 2023 and December 2023 at the Obstetric Outpatient Service of the Policlinico Umberto I University Hospital in Rome, as well as through various social media platforms. Questionnaires were filled in via the Qualtrics platform (Qualtrics@, Provo, UT). Inclusion criteria for the pregnant group were being aged 18 years or older, the ability to understand and complete questionnaires in Italian, and being in a state of pregnancy in the first trimester at the time of questionnaire completion. Exclusion criteria included having any pregnancy-related pathologies or complications or other relevant medical issues.
Additionally, an age-matched control group was recruited through social media platforms. Control group women filled out a parallel online survey during a timeframe comparable to that of the pregnant participants (between March 2023 and May 2024). Inclusion criteria for the control group were being 18 years or older, the ability to understand and complete questionnaires in Italian, and not being pregnant.
All participants voluntarily took part in the study without any form of compensation. After online informed consent was obtained, participants were invited to complete an online questionnaire.
This study is part of a larger project on the psychological and emotional characteristics of pregnant women. Part of the data collected concerning pregnant women was previously used in a published study, which aimed to investigate the relationship between pregnancy-related variables, alexithymia, and depressive symptoms in influencing dream characteristics.10 Ethical approval for the study was granted by the Institutional Review Board of the Psychology Department at Sapienza University of Rome (Prot N. 0002518). The protocol was carried out following the Declaration of Helsinki and adhered to the Code of Ethics of the Italian Psychological Association and the American Psychological Association.
A total of 221 pregnant women completed the questionnaires. Of these, 91 participants were excluded due to missing data on key variables (dreaming, depression, and sleep), and 30 women were excluded because they had a pathological pregnancy. Regarding the control group, 422 women completed the survey, but 5 were excluded as they were pregnant. Of the remaining 417 women, 100 were matched by age to form the control group. The total sample consisted of 200 participants: 100 pregnant women and 100 age-matched non-pregnant women.
Instruments
For data collection, various questionnaires were administered. Both groups filled out online surveys that gathered sociodemographic information, including age, nationality, education, and employment status). Additionally, some information was collected specifically for pregnant women, including parity status, months of gestation, and the presence of any pregnancy-related pathologies.
The following measures were considered for this study:
The Italian version of the Mannheim Dream Questionnaire (MADRE)13 was used to assess dream activity. The questionnaire consists of 20 items, each addressing a specific dimension of the dream experience. For the purposes of this study, five key items examining state-like dream features were analyzed: (a) dream-recall frequency (item 1, DRF) rated by a 7-point scale (0 = never and 6 = almost every morning); (b) emotional intensity of dream contents (item 2, EI) rated by a 5-point scale (0 = not at all intense and 4 = very intense); (c) nightmare frequency (item 4, NMF) rated by an 8-point scale (0 = never and 8 = several times a week); (d) nightmare distress (item 5, ND) rated by a 5-point scale (0 = not at all distressing and 4 = very distressing); and (e) lucid-dream frequency (item 10, LDF) rated by an 8-point scale (0 = never and 8 = several times a week). The frequency was asked with reference to the previous month. Additionally, a composite score related to attitude towards dreams, a trait-like dream-related feature, was included in the analysis to control for “dream attitude”, which is typically related to dream recall frequency.14 Attitude towards dreams (ATD) includes 8 items rated by a 5-point scale (0 = not at all, and 4 = totally).
Sleep quality was assessed using a single item from the Italian version of the Pittsburgh Sleep Quality Index15 (PSQI), which asks: “During the past month, how would you rate your overall sleep quality?” Responses are recorded on a 4-point scale ranging from 1 (very good) to 4 (very bad). As it consists of a single item, this variable was dichotomized [0= good sleepers (score <2); 1= poor sleepers (score ≥2)]. The choice to use a single item stems from the need to keep the battery short to avoid overburdening pregnant women in the hospital setting and to minimize the risk of protocol dropout.
Depressive symptoms among pregnant women were assessed using the Italian version of the Edinburgh Postnatal Depression Scale (EPDS),16,17 a widely validated tool that effectively detects depressive symptoms during pregnancy and the perinatal period. The scale comprises 10 items aimed at evaluating symptoms of depression over the previous week. Responses are scored on a 4-point scale ranging from 0 (not at all) to 3 (most of the time). Based on existing literature, the total EPDS score was dichotomized as follows: 0 = absence of significant depressive symptoms (score <10), and 1 = presence of depressive symptoms (score ≥10).
The Beck Depression Inventory-II (BDI-II)18 was administered to assess depressive symptoms among non-pregnant women. It is a self-reported questionnaire consisting of 21 multiple-choice questions. Each answer provides scores from 0 to 3, which positively correlate with the severity of depressive symptoms. Total scores >13 are indicative of the presence of depressive disorder. BDI score was dichotomized as follows: 0 = absence of significant depressive symptoms (score ≤13), and 1 = presence of depressive symptoms (score ≥14).
Again, the choice of using two different instruments to assess depressive symptoms in pregnant and non-pregnant women was due to the need to minimize participant burden and avoid dropout of pregnant women. As mentioned, they participated in a wider project, and we prioritized the use of a tool that was both brief and specifically validated for pregnancy as the EPDS.
The PSQI, MADRE, and EPDS are publicly available in their respective Italian validation articles (MADRE, https://doi.org/10.11588/ijodr.2019.1.59328; PSQI, https://doi.org/10.1007/s10072-012-1085-y; EPDS, https://doi.org/10.1016/S0165-0327(98)00102-5).
The BDI-II is not in the public domain. However, its use for research and non-commercial educational purposes is regulated under Law No. 633 of April 22, 1941.
Statistical Analysis
All the data were analyzed using the Statistical Package for Social Sciences (SPSS) version 28.0.
Descriptive analyses were conducted to outline the sociodemographic characteristics of the samples, as well as sleep quality and depressive symptoms. The independent t-test was used to confirm the absence of age differences between the two groups. The chi-squared test (or Fisher’s exact test for frequency <5) was used to assess possible differences in sociodemographic characteristics (nationality, education, work status), sleep quality (poor sleeper, good sleeper), and depressive symptoms (presence, absence).
We applied the Z-score method to screen all continuous or ordinal variables used. None of the Z-scores for our variables exceeded the conventional threshold of ±3, indicating that no extreme outliers were present.
To assess the differences concerning dream activity, a one-way MANCOVA was carried out between the pregnant and non-pregnant groups, considering the following dependent variables: DRF, EL, NMF, ND, LDF, and ATD. Also, age, education and work status have been included as covariates. In the case of a significant group effect, a one-way ANOVA was carried out for each measure. Eta squared values (η2) have been also calculated as a measure of effect size. Before carrying out the MANCOVA, we examined skewness and kurtosis, which ranged between −1 and 1, indicating the approximate normality of each dependent variable. Furthermore, we selected Pillai’s Trace as the test statistic, ensuring more cautious and reliable results given the characteristics of our data (ie, ordinal variables).
According to the second aim of the study, ordinal logistic regression analysis was performed only on state-related dream variables that showed statistically significant differences between pregnant and non-pregnant individuals. The independent variables were age, group (pregnant, non-pregnant women), sleep quality (continuous variable), depressive symptoms (absence, presence), ATD (continuous variable), and LDF (ordinal variable). We entered the variables simultaneously into a single model. Multicollinearity between the independent variables was assessed before running the logistic regression by calculating Variable Inflation Factors (VIF). The VIF statistics for all variables included in the regression model were ≤3, indicating only moderate correlation.
Results
Characteristics of Participants
The characteristics of the samples and the differences in sociodemographic features, sleep quality, and depressive symptoms between the groups are detailed in Table 1. The mean age across both groups (N = 200) was approximately 31 years, with more than 90% of participants being Italian.
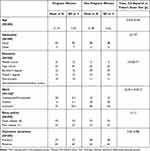 |
Table 1 Characteristics of the Sample, N=100 Pregnant Women and N=100 Non-Pregnant Women |
Significant differences emerged in terms of education level (Fisher’s Exact Test, p < 0.001). Most pregnant women had a high school education (47%), whereas non-pregnant women predominantly held a bachelor’s degree (29%) or a master’s degree (33%). Additionally, there were notable differences in work status (χ² = 32.30, p < 0.001). Among pregnant women, 76.5% were employed, and only 3.1% were students. In contrast, 30% of the control group were students, while 60% were employed.
Both groups included a high percentage of good sleepers, with 73% in each group. Depressive symptoms were present in fewer than 40% of participants, with no significant differences between groups (37% of pregnant women and 38% of non-pregnant women).
Pregnancy-related characteristics are outlined in Table 2. All pregnant participants were in their first trimester, with the majority (66%) in their third month. Most were primiparous (74.7%). Fewer than 30% of the pregnant women had experienced a miscarriage, and over 70% had planned their pregnancy.
 |
Table 2 Pregnancy-Related Features in the Pregnant Group (N=100) |
Dream Activity Differences Between Early Pregnancy and Non-Pregnant Women
The one-way MANCOVA performed on dream variables showed statistically significant differences between pregnant and non-pregnant women (Pillai’s Trace = 0.109, F6,188= 3.823, p = 0.001, η² = 0.109). Since for the “work status” covariate we have two missing data among pregnant women, the analyses were conducted on 98 pregnant women vs 100 non-pregnant women. The covariates (age, education, profession) were not significant (Age: Pillai’s Trace = 0.050, F6,188= 1.645, p = 0.137; Education: Pillai’s Trace = 0.007, F6,188= 0.221, p = 0.970; Work: Pillai’s Trace = 0.037, F6,188= 1.192, p = 0.312).
Specifically, post hoc ANCOVAs showed that the pregnant group has lower NMF (F6,193 = 6.696, p = 0.010, η2 = 0.034), and ATD (F1,193 = 8.145, p =0.005, η2 = 0.040) than the non-pregnant group. Figure 1 shows the means and standard errors for each dream variable in the two groups. All results from univariate ANOVAs are reported in Table 3.
 |
Table 3 Univariate ANCOVAs (Pregnant Women N = 98 vs Non-Pregnant Women N = 100) |
Predictors of Nightmare Frequency
The ordinal logistic regression analysis in Table 4 identified key predictors of NMF (R²N= 0.112, χ² = 0.73, p < 0.001). Women with higher dream attitude (ATD) (β = 0.51, p = 0.004), depressive symptoms (β = 0.43, p = 0.012), and frequent lucid dreams (β = 0.58, p = 0.001) were more likely to experience nightmares. Additionally, being part of the non-pregnant control group (β = 0.47, p = 0.005) was associated with a higher NMF, confirming findings from the previous analysis.
Additional Analysis: Moderation by Dream Attitude?
To investigate whether the higher dream attitude in non-pregnant women could independently account for the observed differences in nightmare frequency, we conducted a moderation analysis. Specifically, we examined how the degree of dream attitude changes the relation between pregnancy (group) and nightmare frequency.
Results confirmed a significant main effect of group (Z = 2.563, p = 0.010) and of dream attitude (Z = 3.090, p = 0.002) on nightmare frequency. However, the interaction between group and dream attitude was not statistically significant (Z = 0.894, p = 0.372), indicating that the moderating effect of dream attitude on the relationship between group status and nightmare frequency did not reach statistical significance.
Discussion
The present study unexpectedly found that women in their first trimester of pregnancy experience fewer nightmares and dreams attitude compared to non-pregnant women, controlling for sociodemographic variables (ie, age, education level, work status). It should be noted that existing studies generally report an increase in dreams and nightmares among pregnant women compared to non-pregnant women. However, these studies predominantly focus on women in the later stages of pregnancy, particularly in the third trimester.4,7,19,20 Nevertheless, only a few studies have conducted statistical comparisons with a non-pregnant group.2 Hence, it is not entirely inconsistent that we did not find differences in DRF during the first trimester between pregnant and non-pregnant women. Indeed, our result aligns with the findings of Blake and Reimann,3 who observed an increase in dream recall as pregnancy progresses. Moreover, Nielsen and Paquette19 found no differences in DRF between pregnant women (without distinguishing between trimesters) and non-pregnant individuals.
Although our findings seem to contrast the current literature on dreaming in pregnancy, they partially align with those of Hedman et al9 who reported a reduction in parasomnias during pregnancy. Their longitudinal study examined various parasomnias from pre-pregnancy through the third trimester, noting a significant decrease in nightmares from 55% pre-pregnancy to 47%, particularly during the first trimester. It could be hypothesized that the rapid hormonal changes during the first trimester of pregnancy might play a role in reducing the nightmare frequency.9,21 Specifically, some evidence from animal studies suggested that administering progesterone has sedative effects, including reduced wakefulness, shorter latency to NREM sleep, and decreased overall amount of REM sleep.22 Also, estrogen, like progesterone, has been found to selectively inhibit REM sleep.23–25 While further research is needed to confirm the potential impact of hormones on dreaming, one speculative explanation for decreasing REM sleep parasomnia, such as nightmares, could stem from evidence of a reduction in REM sleep during pregnancy.26 Furthermore, considering the role of the amygdala, which is particularly active during REM sleep and plays a key role in emotional processing,27 a decrease in REM sleep during the first trimester might contribute to reducing the intensity of negative emotions experienced in dreams.
Additionally, our findings may be interpreted in light of the Activation Hypothesis posits that greater cortical arousal may promote oneiric activity.28–30 In this regard, some evidence found that the first trimester of gestation is often associated with better sleep quality compared to the later trimesters.31 Research has shown that the first trimester is characterized by increased daytime sleepiness, longer total sleep time,26,32 and more delta and theta waves during nighttime sleep compared to later trimesters.33 In other words, the first trimester appears to be characterized by a less deep sleep than later pregnancy and these sleep patterns could contribute to a lower occurrence of nightmares.34
More broadly, there is evidence of a higher perceived quality of life during this trimester.35 Partially coherent, a recent review revealed a decrease in physical quality of life throughout pregnancy, particularly related to decreased physical activity and functional limitations, while the quality of mental life of the pregnant women increased or remained stable throughout the trimesters.36 However, the literature about emotional processing and psychological changes during pregnancy is quite heterogeneous. Pregnant women during early pregnancy may experience pleasant feelings as well as sharp emotional changes and negative physical sensations, ambiguity, and concerns about pregnancy, childbirth, motherhood, and family changes,37 which may provoke different levels of perceived stress. Some evidence showed that during pregnancy and the early postpartum period, a percentage of women reported an increase in negative emotions. Between the middle and the end of pregnancy, their depression ratings had risen considerably, which may reflect the increased physical stress of changing body form and weight as well as a sense that the pregnancy has gone on “forever”.38 Other findings highlighted that the odds of being unhappy among pregnant women were higher in their third trimesters than among women in their first trimesters, indicating that specific factors, such as unplanned pregnancy and intimate relationship violence strongly impact a lower degree of happiness.39 Interestingly, Taubman–Ben-Ari et al40 emphasized that the cognitive strategies to manage emotions may mediate the relationship between stress levels and positive feelings during early pregnancy. Indeed, they found that – during the first trimester - cognitive reappraisal fully mediated the relationship between perceived stress and personal growth, namely the positive psychological transformation that arises from navigating adverse life experiences, such as the development of a stronger sense of personal resilience, deeper and more meaningful relationships with others, and an enhanced appreciation for life.40
An additional possible explanation for the reduced frequency of nightmares in the first trimester could be rooted in an evolutionary perspective. This early stage of pregnancy is a crucial period in which women begin to develop a psychological space for the unborn child and construct their maternal identity, particularly in first-time mothers.41 From this point of view, a lower occurrence of disturbing dreams might serve as an adaptive function by protecting the fetus from maternal stress during a critical phase of development. As pregnancy progresses, the increase in emotionally charged dreams and nightmares could reflect the growing psychological and physiological demands on the mother, ultimately aiding in the preparation for the transition to parenthood.
Considering this literature, which seems to outline a first trimester that is not excessively negative in terms of emotional well-being, the presence of a reduced rate of nightmares as compared to non-pregnant women could be consistent with the idea that there is a continuity between mental activity during sleep and wakefulness (ie, the Continuity Hypothesis).5 Indeed, it could be speculated that women in the first trimester seem to be more focused on daytime physiological changes (eg, nausea, vomiting, dizziness), whereas, in the third trimester, anxiety and emotional distress become more prominent,42 likely contributing to an increase in nightmares. As noted by Hedman et al,9 parasomnia-like events can be associated with anxiety,43,44 such as panic attacks which tend to decrease during pregnancy.45 In other words, the relatively lower occurrence of nightmares in early pregnancy may align with a period characterized by fewer emotional stressors, while the increase in nightmares in later trimesters – found in other studies2_ may correspond to heightened anxiety and psychological distress as childbirth approaches. Notably, the measure of anxiety is absent in our study, and therefore these reflections and hypotheses are purely speculative and should be interpreted with caution.
Among the variables associated with a higher frequency of nightmares, ordinal logistic regression confirms that being in the non-pregnant group is significantly associated with more frequent nightmares. Interestingly, attitude towards dreams explains the higher frequency of nightmares. The relationship between dream attitude and DRF is well-known,46 while the association with nightmares is quite unexplored. It could be hypothesized that individuals with a higher dream attitude tend to be more attuned to emotionally intense mental sleep activity. In other words, this suggests that those who pay more attention to their dreams may inadvertently increase the likelihood of remembering distressing dream content. Notably, Schredl & Goritz47 showed that dream attitude is related to some other trait-like feature such as neuroticism (ie, a personality trait often linked to emotional sensitivity) that, in turn, had a strong correlation with nightmares. The authors hypothesized that since the ATD scale includes items such as “A person who reflects on their dreams is certainly able to learn more about themselves”48 individuals may be interested not only in coping with nightmares but also in gaining deeper self-awareness, for instance as part of a psychotherapeutic treatment.47
However, these considerations remained speculative since we did not collect information about trait-like/personality characteristics of pregnant and non-pregnant women. Additionally, it could not be ruled out that a strong dream attitude, characterizing our non-pregnant women, may be both a cause and consequence of nightmare frequency. Namely, frequent nightmares can increase curiosity and concern about one’s dream life, reinforcing a strong dream attitude. To further investigate this issue, we have conducted an additional analysis to explore the potential independent role of the control group’s higher dream attitude. The results suggest that while both pregnancy status and attitude towards dreams are independently associated with nightmare frequency, there is no evidence that dream attitude moderates the relationship between group and nightmares. In other words, although non-pregnant women reported more frequent nightmares and tended to have higher dream attitude scores, the interaction between these variables was not significant. This means that pregnancy status may influence nightmare rate through mechanisms other than dream attitude, and that the effect of this parameter is additive rather than interactive. Since dream attitude is considered a relatively stable, trait-like characteristic that can amplify nightmare frequency, future studies should consider matching or controlling for dream attitude across groups to better isolate the specific contribution of pregnancy-related changes.
Furthermore, consistent with the literature, we found that depressive symptoms and a higher frequency of lucid dreams are predictors of more frequent nightmares. The association between depressive symptoms and dreaming is complex, with studies reporting mixed and sometimes conflicting results. Some studies indicate that individuals with depression tend to have dreams that are shorter, less vivid, and emotionally subdued.49 While depressive symptoms are often linked to reduced dream recall, they are also associated with a higher incidence of nightmares.50,51 Notably, nightmares have emerged as a critical factor in the relationship between depression and suicidal ideation,52 supporting the Continuity hypothesis.5 More directly, the relationship between depressive symptoms and the frequency of nightmares can be explained through the entrapment perspective proposed by the Integrated Motivational-Volitional Theory of Suicidal Behavior.53 According to this theory, depression is often associated with a sense of emotional and psychological entrapment, where the individual perceives their situation as insurmountable Nightmares, as a form of sleep disturbance, may intensify this feeling of entrapment, as they no longer provide temporary relief from waking life problems but instead amplify them, creating further distress. Consequently, a higher frequency of nightmares could reflect an exacerbation of depressive symptoms and a sense of helplessness, where the individual may begin to view sleep, instead of being an escape, as a continuation of their emotional pain. This reinforces the association between depressive symptoms and nightmare frequency, probably with the risk that the person might consider suicidality as another, more permanent, form of escape from their suffering.53,54 Along this vein, multiple studies show that individuals with depression who frequently experience nightmares face an elevated risk of suicidal thoughts and behaviors.54,55 Furthermore, psychological factors such as rumination and difficulties with emotion regulation intensify this relationship, suggesting that nightmares may serve as a mediator between these traits and suicidal tendencies.56,57
Finally, studies suggest that nightmares and lucid dreams often coexist, with lucid dreaming potentially serving as a coping mechanism for distressing dreams.58 The parallel increase of nightmares and dream attitude may enhance the likelihood of becoming aware within the dream state, a key feature of lucid dreaming.59 Lucid dreaming has been proposed as a therapeutic strategy to mitigate the emotional impact of nightmares.58 By gaining conscious control over the dream narrative, individuals can reduce the severity or frequency of nightmares, particularly in populations suffering from trauma-related sleep disturbances.58 It should be noted that given our cross-sectional design, we cannot determine whether lucid dreaming precedes or follows nightmares. For this reason, our considerations about the relationship between lucid dreaming and nightmares remain speculative and should be taken with caution. Only future research could clarify the temporal dynamics between lucid dreaming and nightmares to better understand their interplay and therapeutic potential.
We are aware that this study has several limitations:
Sociodemographic differences and control of confounding variables: the two groups differed significantly in terms of education level and work status and these differences may be attributed to the contexts of recruitment, with pregnant women being primarily recruited at a public hospital (Policlinico Umberto I, Rome), whereas non-pregnant women were recruited online. This difference in recruitment settings likely influenced the results, and the potential presence of self-selection bias in the non-pregnant group (as individuals with an interest in research, sleep, and dreams were more likely to participate) further complicates the comparison. Although we have tried to control these sociodemographic variables in our analysis (ie, MANCOVA), several other confounding variables, such as body mass index (BMI) and relationship status, have not been collected. Given the importance of social support and the partner’s role during pregnancy,60 these variables could have a significant impact on dreaming3 and should be considered in future research.
Use of different depression tools: we used two different tools to assess depression (EPDS and BDI-II) by considering “depressive symptoms” as dichotomous variables. Although some evidence highlighted that the EPDS has satisfactory sensitivity and specificity and better validity than the BDI-II for detecting major depressive disorder during pregnancy,61 we acknowledged that this may have introduced variability in the results. Future studies should consider standardizing the assessment by using the same tools in both groups to enhance comparability.
Lack of collection of the anxiety measures: as previously mentioned, anxiety, a well-known factor associated with nightmares62,63 was not assessed in this study. The absence of these measures does not allow us to control for this important variable that can have modulated the frequency of nightmares and the emotional intensity of dreams.
Single item for sleep quality assessment: only an item was used to assess sleep quality, primarily to reduce the risk of dropout among pregnant participants. While this approach was necessary, it restricted our ability to fully analyze the relationship between sleep patterns and dreaming. Bearing in mind the relationship between cortical arousal during sleep and dream activity,32 a more comprehensive sleep assessment, possibly using a sleep diary or objective tools like actigraphy or -even better- polysomnography, would provide a deeper understanding of the sleep-dreaming relationship and could be beneficial for future studies.
Limitations of dream content data: we did not collect dream reports, which prevented us from evaluating the presence of specific pregnancy-related content during the first trimester of pregnancy. Indeed, even though the frequency of nightmares appeared to be reduced compared to non-pregnant women, it is possible that certain negative pregnancy-related themes could still impact pregnant women’s well-being. Additionally, we did not gather information on sleep and dreams before pregnancy, which may be relevant to understanding how pregnancy changes dream patterns. Future research should include these aspects for a more complete picture. Also, albeit we included additional analyses to better understand the relationship between ATD and the group membership, it would also be valuable to account for individual differences in dream attitude -which could be considered a trait-like characteristic related to dream salience- by matching participants based on their dream attitudes in future studies.
Different timeframes for questionnaires: the varying timeframes for the EPDS, MADRE, single PSQI item, and BDI-II could have led to inconsistencies in the data. While these instruments are often used together in sleep research, the differing reference periods may have partially influenced the results and should be addressed in future studies.
Conclusion
This study provides new insights into the relationship between early pregnancy and dream activity, specifically contrasting the dream characteristics of women in their first trimester with those of non-pregnant women. It is important to note that this finding is the first in the literature to specifically examine the dream activity of women in their first trimester of pregnancy compared to a group of non-pregnant women. Contrary to the hypothesis, pregnant women reported fewer nightmares and exhibited less interest in their dreams compared to non-pregnant women. These findings challenge the common assumption that pregnancy universally increases dream recall and nightmare frequency. However, our results appear consistent with some previous research suggesting a decrease in parasomnias, including nightmares, across pregnancy’s trimesters.9 Our findings also confirm that individuals who engage more deeply with their dreams are more likely to recall nightmares. Moreover, depressive symptoms emerged as a significant predictor of nightmares, consistent with studies linking depression to increased nightmare frequency and emotional distress.55
Interestingly, lucid dreaming was also identified as a predictor of nightmare frequency. This supports the idea that nightmares and lucid dreams often coexist, with lucid dreaming potentially functioning as a coping mechanism for distressing dream experiences.58
Overall, these findings suggest that dream activity in early pregnancy may be less emotionally charged than in later stages, with significant implications for clinical interventions. Addressing depressive symptoms, enhancing emotion regulation, and exploring lucid dreaming techniques could help manage nightmares and improve psychological well-being in both pregnant and non-pregnant individuals. Future research should further explore early pregnancy’s unique impact on dream patterns and compare these findings across different gestational stages. In particular, we believe that a better understanding of the development of nightmares during pregnancy may help in providing timely and effective interventions when necessary. This would involve the application of various evidence-based treatments for nightmares, such as Imagery Rehearsal Therapy (IRT)64 Exposure, Relaxation, and Rescripting Therapy (ERRT),65 and Cognitive Behavioral Therapy for Nightmares (CBT-N).66
Based on our findings and the several methodological limitations outlined, we believe that further effort should be made in the research on pregnancy to better understand the relationship between dreaming and women’s health. First, a longitudinal design would be highly beneficial: by tracking women from the beginning of pregnancy through the postpartum period, researchers could examine how dream activity evolves across different stages of pregnancy and how it interacts with physiological, hormonal, and psychological changes. Moreover, the inclusion of sleep pattern measures may provide a more comprehensive picture of the factors influencing dream activity. Additionally, future studies should control for a broader range of sociodemographic and psychological variables.
Understanding dream activity during pregnancy, especially during the first trimester, holds significant promise for improving maternal mental health and well-being. Insights gained from such research could inform the development of targeted interventions designed to support women during this sensitive period, ultimately contributing to better outcomes for both mothers and their children.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, SS, upon reasonable request.
Ethics Approval
Ethical approval for the study was granted by the Institutional Review Board of the Psychology Department at Sapienza University of Rome, Protocol Num. 0002518. The protocol was carried out following the Declaration of Helsinki.
Author Contributions
Conceptualization: SS, LDG, CG; Data Curation: MS, MG, SL, PC, FR, CM, IDB, CG; Formal analysis: SS, MS, MDM, LDG, CG; Investigations: SS, MS, MG, SL, PC, FR, MDM, CM, IDB, CG, LDG: Methodology: SS, MS, MG, SL, PC, FR, MDM, CM, IDB, CG, LDG; Project administration: LDG, CG; Resources: MG, SL, PC, FR, MDM, CM, IDB, CG, LDG; Software and Visualization: SS, MS, MG, SL, MDM; Writing original manuscript: SS, MS; Writing review and editing: MG, SL, PC, FR, MDM, CM, IDB, CG, LDG; Validation and Supervision: SS, S, MG, SL, PC, FR, MDM, CM, IDB, CG, LDG.
Caterina Grano and Luigi De Gennaro shared senior authorship. All authors critically reviewed the article, agreed on the journal to which the article will be submitted, reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage, agreed to take responsibility and be accountable for the contents of the article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nowakowski S, Meers J, Heimbach E. Sleep and women’s health. Sleep Med Res. 2013;4(1):1. doi:10.17241/smr.2013.4.1.1
2. Scarpelli S, Alfonsi V, De Gennaro L, Gorgoni M. Dreaming for two: a systematic review of mental sleep activity during pregnancy. Neurosci Biobehav Rev. 2024;163:105763. doi:10.1016/j.neubiorev.2024.105763
3. Blake RL, Reimann J. The pregnancy-related dreams of pregnant women. J Am Board Fam Pract. 1993;6(2):117–122.
4. Lara-Carrasco J, Simard V, Saint-Onge K, Lamoureux-Tremblay V, Nielsen T. Disturbed dreaming during the third trimester of pregnancy. Sleep Med. 2014;15(6):694–700. doi:10.1016/j.sleep.2014.01.026
5. Domhoff GW. The invasion of the concept snatchers: the origins, distortions, and future of the continuity hypothesis. Dreaming. 2017;27(1):14. doi:10.1037/drm0000047
6. Valli K, Revonsuo A. The threat simulation theory in light of recent empirical evidence: a review. Am J Psychol. 2009;122(1):17–38. doi:10.2307/27784372
7. Schredl M, Gilles M, Wolf I, et al. Nightmare frequency in the last trimester of pregnancy. BMC Pregnancy Childbirth. 2016;16(1):1–7. doi:10.1186/s12884-016-1147-x
8. Wołyńczyk-Gmaj D, Różańska-Walędziak A, Ziemka S, et al. Insomnia in pregnancy is associated with depressive symptoms and eating at night. J Clin Sleep Med. 2017;13(10):1171–1176. doi:10.5664/jcsm.6764
9. Hedman C, Pohjasvaara T, Tolonen U, Salmivaara A, Myllylä VV. Parasomnias decline during pregnancy. Acta Neurol Scand. 2002;105(3):209–214. doi:10.1034/j.1600-0404.2002.1o060.x
10. Spinoni M, Scarpelli S, Benedetti IDP, et al. The association between dream activity and alexithymia during pregnancy: a cross-sectional study in a sample of pregnant women. J Sleep Res. 2024. e14423. doi:10.1111/jsr.14423
11. Scarpelli S, Alfonsi V, Mangiaruga A, et al. Pandemic nightmares: effects on dream activity of the COVID‐19 lockdown in Italy. J Sleep Res. 2021;30(5):e13300. doi:10.1111/jsr.13300
12. Carr M, Youngren W, Seehuus M, et al. The effects of lucid dreaming and nightmares on sleep quality and mental health outcomes. Behav Sleep Med. 2024;23:1–8.
13. Settineri S, Frisone F, Alibrandi A, Merlo EM. Italian adaptation of the Mannheim Dream Questionnaire (MADRE): age, gender and dream recall effects. Int J Dream Res. 2019;119–129.
14. Cohen DB, MacNeilage PF. A test of the salience hypothesis of dream recall. J Consult Clin Psychol. 1974;42(5):699. doi:10.1037/h0036948
15. Curcio G, Tempesta D, Scarlata S, et al. Validity of the Italian version of the Pittsburgh sleep quality index (PSQI). Neurol Sci. 2013;34(4):511–519. doi:10.1007/s10072-012-1085-y
16. Benvenuti P, Ferrara M, Niccolai C, et al. The Edinburgh postnatal depression scale: validation for an Italian sample. J Affect Disord. 1999;53(2):137–141. doi:10.1016/S0165-0327(98)00102-5
17. Levis B, Negeri Z, Sun Y, et al. Accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for screening to detect major depression among pregnant and postpartum women: systematic review and meta-analysis of individual participant data. BMJ. 2020;371. doi:10.1136/bmj.m4022
18. Beck AT, Steer RA, Brown GK. Beck depression inventory–II (BDI-II) [Database record]. Psychol Corp. 1996.
19. Nielsen T, Paquette T. Dream-associated behaviors affecting pregnant and postpartum women. Sleep. 2007;30(9):1162–1169. doi:10.1093/sleep/30.9.1162
20. Lara-Carrasco J, Simard V, Saint-Onge K, et al. Maternal representations in the dreams of pregnant women: a prospective comparative study. Front Psychol. 2013;4:551. doi:10.3389/fpsyg.2013.00551
21. Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27(7):1405–1417. doi:10.1093/sleep/27.7.1405
22. Lancel M, Faulhaber J, Holsboer F, Rupprecht R. Progesterone induces changes in sleep comparable to those of agonistic GABAA receptor modulators. Am J Physiol Endocrinol Metab. 1996;271(4):E763–E772. doi:10.1152/ajpendo.1996.271.4.E763
23. Kleinlogel H. The female rat’s sleep during oestrous cycle. Neuropsychobiology. 1983;10(4):228–237. doi:10.1159/000118016
24. Yamaoka S. Participation of limbic-hypothalamic structures in circadian rhythm of slow wave sleep and paradoxical sleep in the rat. Brain Res. 1978;151(2):255–268. doi:10.1016/0006-8993(78)90883-1
25. Colvin GB, Whitmoyer DI, Lisk RD, et al. Changes in sleep-wakefulness in female rats during circadian and estrous cycles. Brain Res. 1968;7(2):173–181. doi:10.1016/0006-8993(68)90095-4
26. Hertz G, Fast A, Feinsilver SH, et al. Sleep in normal late pregnancy. Sleep. 1992;15(3):246–251. doi:10.1093/sleep/15.3.246
27. Maquet P, Péters JM, Aerts J, et al. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383(6596):163–166. doi:10.1038/383163a0
28. Antrobus J. Dreaming: cognitive processes during cortical activation and high afferent thresholds. Psychol Rev. 1991;98(1):96. doi:10.1037/0033-295X.98.1.96
29. Scarpelli S, D’Atri A, Mangiaruga A, et al. Predicting dream recall: EEG activation during NREM sleep or shared mechanisms with wakefulness? Brain Topogr. 2017;30(5):629–638. doi:10.1007/s10548-017-0563-1
30. Siclari F, Baird B, Perogamvros L, et al. The neural correlates of dreaming. Nat Neurosci. 2017;20(6):872–878. doi:10.1038/nn.4545
31. Lopes EA, Carvalho LBC, Seguro PBC, et al. Sleep disorders in pregnancy. Arq Neuropsiquiatr. 2004;62(2A):217–221. doi:10.1590/s0004-282x2004000200005
32. Senaratna CV, Priyadarshanie N, Fernando S, et al. Longitudinal sleep study in pregnancy: cohort profile and prevalence and risk factors for sleep symptoms in the first trimester. Int J Environ Res Public Health. 2023;20(3):2070. doi:10.3390/ijerph20032070
33. Izci-Balserak B, Keenan BT, Corbitt C, et al. Changes in sleep characteristics and breathing parameters during sleep in early and late pregnancy. J Clin Sleep Med. 2018;14(7):1161–1168. doi:10.5664/jcsm.7216
34. Simor P, Horváth K, Ujma PP, et al. Fluctuations between sleep and wakefulness: wake-like features indicated by increased EEG alpha power during different sleep stages in nightmare disorder. Biol Psychol. 2013;94(3):592–600. doi:10.1016/j.biopsycho.2013.05.022
35. Wu H, Sun W, Chen H, et al. Health-related quality of life in different trimesters during pregnancy. Health Qual Life Outcomes. 2021;19(1):1–11. doi:10.1186/s12955-021-01811-y
36. Lagadec N, Steinecker M, Kapassi A, et al. Factors influencing the quality of life of pregnant women: a systematic review. BMC Pregnancy Childbirth. 2018;18(1):455. doi:10.1186/s12884-018-2087-4
37. Bergbom I, Modh C, Lundgren I, Lindwall L. First-time pregnant women’s experiences of their body in early pregnancy. Scand J Caring Sci. 2017;31(3):579–586. doi:10.1111/scs.12372
38. Lips HM. A longitudinal study of the reporting of emotional and somatic symptoms during and after pregnancy. Soc Sci Med. 1985;21(6):631–640. doi:10.1016/0277-9536(85)90202-3
39. Tesfa D, Aleminew W, Tadege M, et al. Level of happiness and its associated factors among pregnant women in South Gondar Zone Hospitals, North Central Ethiopia. Int J Womens Health. 2020;12:983–991. doi:10.2147/IJWH.S275709
40. Taubman-Ben-Ari O, Chasson M, Horowitz E, Azuri J, Davidi O. Personal growth in early pregnancy: the role of perceived stress and emotion regulation. J Reprod Infant Psychol. 2022;40(6):550–562. doi:10.1080/02646838.2021.1925096
41. Darvill R, Skirton H, Farrand P. Psychological factors that impact on women’s experiences of first-time motherhood: a qualitative study of the transition. Midwifery. 2010;26(3):357–366. doi:10.1016/j.midw.2008.07.006
42. Rofé Y, Littner MB, Lewin I. Emotional experiences during the three trimesters of pregnancy. J Clin Psychol. 1993;49(1):3–12. doi:10.1002/1097-4679(199301)49:1<3::AID-JCLP2270490102>3.0.CO;2-A
43. Crisp AH, Matthews BM, Oakey M, Crutchfield M. Sleepwalking, night terrors, and consciousness. BMJ. 1990;300(6721):360–362. doi:10.1136/bmj.300.6721.360
44. Garland EJ, Smith DH. Case study: simultaneous prepubertal onset of panic disorder, night terrors, and somnambulism. J Am Acad Child Adolesc Psychiatry. 1991;30(4):553–555. doi:10.1097/00004583-199107000-00004
45. Hertzberg T, Wahlbeck K. The impact of pregnancy and puerperium on panic disorder: a review. J Psychosom Obstet Gynaecol. 1999;20(2):59–64. doi:10.3109/01674829909075578
46. Beaulieu-Prévost D, Zadra A. Dream recall frequency and attitude towards dreams: a reinterpretation of the relation. Pers Individ Dif. 2005;38(4):919–927. doi:10.1016/j.paid.2004.06.017
47. Schredl M, Göritz AS. Dream recall frequency, attitude toward dreams, and the Big Five personality factors. Dreaming. 2017;27(1):49. doi:10.1037/drm0000046
48. Schredl M, Berres S, Klingauf A, et al. The Mannheim dream questionnaire (MADRE): retest reliability, age and gender effects. Int J Dream Res. 2014;7(2):141–147.
49. Kron T, Brosh A. Can dreams during pregnancy predict postpartum depression? Dreaming. 2003;13(2):67–81. doi:10.1023/A:1023397908194
50. Cartwright R. Affect and dream work from an information processing point of view. J Mind Behav. 1986;7(2/3):411–427.
51. Barrett D, Loeffler M. Comparison of dream content of depressed vs nondepressed dreamers. Psychol Rep. 1992;70(2):403–406. doi:10.2466/pr0.1992.70.2.403
52. Faccini J, Del-Monte J. Bad dream, nightmares and psychopathology: a systematic review. Front Psychiatry. 2024;15:1461495. doi:10.3389/fpsyt.2024.1461495
53. O’Connor RC, Kirtley OJ. The integrated motivational-volitional model of suicidal behaviour. Philos Trans R Soc Lond B Biol Sci. 2018;373(1754):20170268. doi:10.1098/rstb.2017.0268
54. Bolstad CJ, Holzinger B, Scarpelli S, et al. Nightmare frequency is a risk factor for suicidal ideation during the COVID-19 pandemic. J Sleep Res. 2024;33(5):e14165. doi:10.1111/jsr.14165
55. Marinova P, Koychev I, Laleva L, et al. Nightmares and suicide: predicting risk in depression. Psychiatry Danub. 2014;26(2):0–164.
56. Rogers ML, Joiner TE. Rumination, suicidal ideation, and suicide attempts: a meta-analytic review. Rev Gen Psychol. 2017;21(2):132–142. doi:10.1037/gpr0000101
57. Rufino KA, Ward-Ciesielski EF, Webb CA, Nadorff MR. Emotion regulation difficulties are associated with nightmares and suicide attempts in an adult psychiatric inpatient sample. Psychiatry Res. 2020;293:113437. doi:10.1016/j.psychres.2020.113437
58. Holzinger B, Saletu B, Klösch G. Cognitions in sleep: lucid dreaming as an intervention for nightmares in patients with posttraumatic stress disorder. Front Psychol. 2020;11:1826. doi:10.3389/fpsyg.2020.01826
59. Blagrove M, Hartnell SJ. Lucid dreaming: associations with internal locus of control, need for cognition, and creativity. Pers Individ Dif. 2000;28(1):41–47. doi:10.1016/S0191-8869(99)00078-1
60. Rini C, Schetter CD, Hobel CJ, Glynn LM, Sandman CA. Effective social support: antecedents and consequences of partner support during pregnancy. Person Relash. 2006;13(2):207–229.
61. Su KP, Chiu TH, Huang CL, et al. Different cutoff points for different trimesters? The use of Edinburgh Postnatal Depression Scale and Beck Depression Inventory to screen for depression in pregnant Taiwanese women. Gen Hosp Psychiatry. 2007;29(5):436–441. doi:10.1016/j.genhosppsych.2007.05.005
62. Nguyen TT, Madrid S, Marquez H, Hicks RA. Nightmare frequency, nightmare distress, and anxiety. Percept Mot Ski. 2002;95(1):219–225. doi:10.2466/pms.2002.95.1.219
63. Javadi SHS. Evaluation of depression and anxiety, and their relationship with insomnia, nightmare and demographic variables in medical students. Eur Psychiatry. 2017;41(S1):s853. doi:10.1016/j.eurpsy.2017.01.1695
64. Krakow B, Zadra A. Imagery rehearsal therapy: principles and practice. Sleep Med Clin. 2010;5(2):289–298. doi:10.1016/j.jsmc.2010.01.004
65. Pruiksma KE, Cranston CC, Rhudy JL, Micol RL, Davis JL. Randomized controlled trial to dismantle exposure, relaxation, and rescripting therapy (ERRT) for trauma-related nightmares. Psychol Trauma. 2018;10(1):67–75. doi:10.1037/tra0000238
66. Pruiksma KE, Miller KE, Davis JL, et al. An expert consensus statement for implementing cognitive behavioral therapy for nightmares in adults. Behav Sleep Med. 2025;15:1–19.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



