Back to Journals » Drug Design, Development and Therapy » Volume 19
Drug Interactions of Imperatorin and Curcumin on Macitentan in vitro and in vivo
Authors Wu H, Liu Q, Shen Y , Qi H, Zhao J, Li Q, Xia H, Xu RA, Shi L
Received 25 November 2024
Accepted for publication 9 April 2025
Published 29 April 2025 Volume 2025:19 Pages 3459—3475
DOI https://doi.org/10.2147/DDDT.S505960
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Muzammal Hussain
Hualu Wu,1 Qian Liu,2 Yuxin Shen,1 He Qi,3 Jiade Zhao,3 Qiaoying Li,4 Hailun Xia,1 Ren-Ai Xu,1 Lu Shi1
1Department of Pharmacy, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China; 2Department of Clinical Laboratory, Aerospace Center Hospital, Beijing, People’s Republic of China; 3College of Basic Medicine and Forensic Medicine, Henan University of Science and Technology, Luoyang, Henan, People’s Republic of China; 4Lishui Liandu District Adverse Drug Reaction Monitoring Station, Lishui, Zhejiang, People’s Republic of China
Correspondence: Lu Shi; Ren-Ai Xu, The First Affiliated Hospital of Wenzhou Medical University, Nanbaixiang Street, Wenzhou, 325000, People’s Republic of China, Tel/Fax +86 105 557 9706, Email [email protected]; [email protected]
Purpose: The purpose of this study was to establish an in vitro incubation system and an in vivo model to investigate the potential kinetic interactions of macitentan with imperatorin and curcumin, and to validate the potential inhibitory mechanisms using molecular docking.
Methods: In vitro, the enzyme kinetic profile of macitentan was explored in rat liver microsomes (RLM) and human liver microsomes (HLM). Furthermore, molecular docking technique was used to study the sites of action of macitentan, imperatorin, and curcumin with CYP 3A4. In vivo, the pharmacokinetic parameters of macitentan were investigated in Sprague-Dawley (SD) rats administered the drug orally, both as a single agent and in combination with imperatorin and curcumin.
Results: In vitro, the results indicated that imperatorin and curcumin could inhibit the metabolism of macitentan, with IC50 values of 6.58 μM and 10.86 μM in RLM and 6.97 μM and 5.71 μM in HLM, respectively. And in the study of inhibition type, in RLM, the inhibition types of imperatorin and curcumin on macitentan were mixed and non-competitive, respectively; in HLM, the inhibition types of imperatorin and curcumin on macitentan were both mixed. Furthermore, additional molecular docking studies demonstrated that both imperatorin and curcumin occupied the CYP3A4 site. In vivo, the result showed significant increases in AUC(0-t), AUC(0-∞), Tmax, t1/2, and Cmax for macitentan while a decrease in CLz/F when combined with imperatorin. The metabolite ACT-132577 exhibited substantial increases in t1/2, Tmax, and Cmax. Combined with curcumin, the AUC(0-∞) and Tmax of macitentan were significantly increased, while CLz/F was significantly decreased. Conversely, the metabolite ACT-132577 exhibited a substantial decrease in CLz/F, accompanied by notable increases in AUC(0-∞) and Tmax.
Conclusion: In vitro and in vivo studies revealed that imperatorin and curcumin exhibited inhibitory effects on the metabolism of macitentan. Furthermore, molecular docking revealed that the metabolic inhibition of macitentan by imperatorin and curcumin occurred through binding to the site on CYP3A4. However, further investigation is necessary to ascertain whether this phenomenon will occur in humans.
Keywords: pharmacokinetics, molecular docking, UPLC-MS/MS, inhibition mechanism
Introduction
Pulmonary arterial hypertension (PAH) is a rare cardiovascular disease characterized by a pathology of high pulmonary arteries and increased pulmonary vascular resistance, which ultimately leads to increased right ventricular afterload, right heart failure and functional decline.1,2 This condition eventually progresses to cardiac arterial hypertension.3 The endothelin (ET) pathway plays a pivotal role in the pathogenesis of PAH.4 In the context of PAH pathophysiology, the activation of endothelin A (ETA) receptors has been shown to promote vasoconstriction, smooth muscle proliferation, and fibrosis.5,6 In contrast, endothelin B (ETB) receptors have been observed to mediate endothelium-dependent vasodilatation, involving the release of nitric oxide, and pro-fibrotic responses.7,8 This intricate receptor complexity underscores the necessity for targeted therapeutic modulation. For the treatment, ET receptor antagonists (ERAs) have become important therapeutic agents for PAH.9
Macitentan is a novel dual (ETA and ETB) ERA that has been approved for the treatment of PAH.10 By blocking the endothelin system, it improves hemodynamics, right ventricular hypertrophy and survival.11 First, macitentan has been shown to inhibit vasoconstriction and remodeling by inhibiting ET-1-mediated vascular smooth muscle contraction through the inhibition of the ETA receptor and the reduction of smooth muscle cell proliferation and migration.12 Furthermore, the simultaneous inhibition of ETA and ETB receptors has been shown to reduce the expression of inflammatory and pro-fibrotic factors, thereby delaying the progression of PAH.13 Macitentan is based on the structure of bosentan, with the sulphonamide portion replaced by an aminosulfonyl portion that is more efficacious, better tolerated and safer, and reduces the risk of liver injury.14
Studies have demonstrated that macitentan is primarily metabolized by CYP3A4 to the pharmacologically active metabolite ACT-132577 (also known as N-despropyl macitentan) and the inactive metabolite ACT-373898.15 The active metabolite, ACT-132577, is of particular importance. Some factors affecting enzyme activity, such as drug combinations, may inhibit drug metabolism and increase plasma exposure, which may result in increased adverse drug reactions.
Imperatorin is a naturally occurring furanocoumarin that has been demonstrated to reduce cardiac hypertrophy, attenuate myocardial fibrosis and thereby improve pulmonary oedema.16–18 It is used clinically in a wide range of cardiovascular patients, especially those suffering from hypertension, hyperlipidaemia and diabetes mellitus.19,20 Curcumin is a natural polyphenolic compound extracted from turmeric,21 which has the potential to reverse the development of PAH and pulmonary vascular remodeling by preserving mitochondrial function in VSMCs and delaying pulmonary fibrosis.22 A frequent adverse effect of macitentan is hepatic injury.23 Prior research has indicated that imperatorin exerts a protective effect against acetaminophen-induced hepatic injury, and curcumin has been demonstrated to have a protective effect against liver ischemia/reperfusion (I/R) injury.24,25 Consequently, it may be hypothesized that the combination of macitentan with imperatorin or curcumin could potentially reduce the risk of liver injury adverse effects and hepatotoxicity. Therefore, the combination of imperatorin and curcumin with PAH drugs has potential possibilities. However, imperatorin and curcumin share the same metabolic enzyme, CYP3A4, with macitentan. This suggests the possibility of CYP3A4-mediated drug-drug interactions between macitentan and imperatorin or curcumin.
The aim of this study was to investigate how imperatorin and curcumin interact with macitentan and the potential mechanisms of inhibition. The present study established two in vitro incubation systems, including rat liver microsomes (RLM) and human liver microsomes (HLM) incubation systems to assess the inhibitory effects and potential mechanisms of imperatorin and curcumin on macitentan metabolism. The results of molecular docking also predict the underlying mechanisms. Furthermore, an in vivo pharmacokinetic study in rats was performed to evaluate the possible pharmacokinetic interactions of imperatorin and curcumin with macitentan.
Materials and Methods
Chemicals and Reagents
Macitentan (Figure 1A),26 its main metabolite ACT-132577 (Figure 1B),26 imperatorin (Figure 1C),27 curcumin (Figure 1D),28 and bosentan (used as internal standard, IS) were bought from Shanghai Canspec Scientific Instruments Co., Ltd. (Shanghai, China). The HLM used in the experiment was purchased from iPhase Pharmaceutical Services Co., Ltd. (Jiangsu, China). For reagents, we bought nicotinamide adenine dinucleotide phosphate (NADPH) from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China), and methanol and acetonitrile in LC grade from Merck (Darmstadt, Germany). Formic acid was purchased from Anaqua Chemicals Supply (ACS, USA). Ultrapure water was prepared using a Milli-Q water purification system from Millipore (Bedford, MA, USA). All other chemicals and reagents used were also of LC grade.
 |
Figure 1 Schematic chemical structures of macitentan (A), ACT-132577 (B), imperatorin (C), and curcumin (D). |
Instruments and Analytical Conditions
Chromatographic separations were conducted on an UPLC-MS/MS system comprising a Waters Xevo TQ-S triple quadrupole tandem mass spectrometer (Milford, MA, USA) with an electrospray ionization source and a Waters Acquity UPLC I-Class system. An Acquity BEH C18 column (2.1 mm × 50 mm, 1.7 μm) was utilized to facilitate the separation of the compounds of interest and potential interferences. The mobile phase was consisted of a 0.1% formic acid solution (solution A) and acetonitrile (solution B). The gradient elution procedure was as follows: 0–0.5 min (90% solution A), 0.5–1.0 min (90%-10% solution A), 1.0–1.4 min (10% solution A), 1.4–1.5 min (10%-90% solution A), 1.5–2.0 min (90% solution A). A single sample was injected into the UPLC-MS/MS at a volume of 2.0 μL and eluted at a flow rate of 0.40 mL/min for 2.0 min. The temperature of the autosampler was maintained at 10°C, while the temperature of the column was maintained at 40°C.
The samples were quantified by a Waters Xevo TQ-S triple quadrupole tandem mass spectrometer. The levels of macitentan, ACT-132577 and IS were detected in positive mode using multiple reaction monitoring (MRM). The data were collected and analyzed using MassLynx 4.1 software (Waters Corp, Milford, MA, USA). Typical UPLC-MS/MS chromatograms in rat plasma samples were presented in Figure 2.
Rat Liver Microsomes (RLM)
Six rat livers were weighed and rinsed three times with phosphate-buffered saline (PBS). They were then cut into small pieces. The samples were homogenized in a homogenizer using PBS-0.25 mM sucrose buffer and subsequently subjected to ultracentrifugation at 11,000 rpm for 15 min at 4°C, this process was repeated twice. Subsequently, the precipitate was discarded, and the supernatant was collected. The supernatant was then removed by centrifugation at 75,600 × g for 2 h at 4°C. The supernatant was then discarded, and the precipitate was collected and diluted with a volume of cold PBS that was between two and three times the original volume. This solution was then pipetted repeatedly with a pipette gun. The diluted solution was then poured back into the same centrifuge tube to homogenize it. Finally, the solution was dispensed and stored in a container at −80°C. The Bradford Protein Assay Kit (Thermo Scientific, Waltham, MA, USA) was used to measure the protein concentration.29
In vitro Experiment
The experimental design was informed by extant published literature.29 The 190 μL primary incubation system was consisted of PBS (pH 7.4, 100 mM), different concentrations of macitentan (1, 5, 10, 20, 50, 100, 150, 200 μM), and RLM or HLM (0.3 mg/mL). The enzyme reaction was initiated by the addition of 10 μL of NADPH (1 mM), following a 5 min preincubation at 37°C. The samples were incubated for 30 min and stored in a refrigerator at −80°C. Following the termination of the reaction, 400 μL acetonitrile and 20 μL of the IS working solution were added to the system. The samples were then vortexed for 2 min and subsequently centrifuged in a pre-cooled centrifuge for 10 min. Thereafter, 100 μL of the supernatant was aspirated for the quantification of the metabolite of macitentan by UPLC-MS/MS. The objective was to determine the Michaelis constant (Km) of macitentan.
The 190 μL primary incubation system was consisted of PBS (pH 7.4, 100 mM), macitentan (concentration in RLM: 8.81 μM, concentration in HLM: 68.30 μM), imperatorin/curcumin (0.01, 0.1, 1, 10, 25, 50, 100 μM), and RLM or HLM (0.3 mg/mL). The samples were prepared in triplicate. Subsequent samples were also subjected to the aforementioned method in order to determine the half-maximum inhibitory concentration (IC50) of imperatorin/curcumin on macitentan.
In addition, IC50 shift experiments were performed to verify whether the inhibition of macitentan by imperatorin and curcumin was time-dependent. The pre-incubation system was contained of PBS, macitentan (8.81 μM), imperatorin/curcumin (0.01, 0.1, 1, 10, 25, 50, 100 μM), and RLM (0.3 mg/mL). The samples were prepared in triplicate. The samples were divided into two groups: one group was pre-incubated without NADPH, and the other group was pre-incubated with NADPH. Following a 30-min pre-incubation period, macitentan (8.81 μM) was added to the samples, and the samples were then incubated for an additional 30 min. Thereafter, the samples were transferred to −80°C. Subsequent samples were treated in an identical manner to the aforementioned samples.
In order to illustrate the inhibitory effects of imperatorin and curcumin on macitentan in vitro, a series of concentration gradients were established based on the IC50 and Km values. In RLM, the concentration of macitentan ranged from 2.20 to 17.61 μM, the concentration of imperatorin ranged from 0 to 6.53 μM, and the concentration of curcumin ranged from 0 to 21.72 μM. In HLM, the concentration of macitentan ranged from 17.08 to 136.60 μM, the concentration of imperatorin ranged from 0 to 6.97 μM, and the concentration of curcumin ranged from 0 to 5.71 μM. The samples were prepared in triplicate. The incubation mixtures were prepared as previously described, and the samples were handled in accordance with the aforementioned methodology.
In vivo Pharmacokinetic Experiments
All male Sprague-Dawley (SD) rats (200–220 g) were sourced from the Animal Experimental Center of the First Affiliated Hospital of Wenzhou Medical University (Zhejiang, China). The entirety of the experiment was overseen by Institutional Animal Care and Use Committee of the First Affiliated Hospital of Wenzhou Medical University, and was granted ethical approval with the ethical review number: WYYY-IACUC-AEC-2024-042. This experiment was conducted according to the Regulations and Rules of “Guidelines for Ethical Review of the Welfare in Laboratory Animals” (2018). The rats were maintained in the SPF environment, provided with standard feed and water ad libitum, and fasted for 12 h prior to the pharmacokinetic experiments. The SD rats were housed under controlled temperature conditions, maintained within a range of 18–29°C, with a maximum daily temperature fluctuation of 3°C. The relative humidity was kept at a range of 40%–70%, and the light exposure was alternated between 12 h of light and 12 h of darkness. A total of fifteen SD rats were randomly divided into three groups (n = 5). In the report by Patricia N. Sidharta et al, plasma concentrations were found to be intermediate when 100 mg of macitentan was administered to humans at doses ranging from 0.2 to 600 mg.30 Therefore, the conversion of human to animal doses was performed by means of body surface area normalization. In this regard, rats were administered 10 mg/kg of macitentan orally. The doses of imperatorin and curcumin referenced by previous authors have been documented in the extant literature.31,32 Group A was served as the control group and was received 10 mg/kg of macitentan. Groups B and C were the experimental groups and were received 30 mg/kg of imperatorin and 20 mg/kg of curcumin, respectively. Drug administration was conducted via gavage. At the commencement of the experiment, 30 mg/kg of imperatorin and 20 mg/kg of curcumin were administered to Group B and C, respectively. 30 min later, 10 mg/kg of macitentan was administered to all three groups. Macitentan, imperatorin, and curcumin were all dissolved in corn oil to create homogeneous solutions and their concentrations were 2, 6 and 4 mg/mL, respectively. The precise volume of the administered drug for rats was 5.0 mL/kg. Blood samples were collected from the tail vein at 0.5, 0.75, 1, 1.5, 2, 4, 6, 8, 12, 24, and 36 h, and were centrifuged (13,000 rpm, 5 min, 4°C). Finally, 100 μL of the supernatant was aspirated and stored temporarily in a −80°C refrigerator. Prior to analysis, the samples were thawed and 10 μL of IS working solution and 300 μL of acetonitrile were added. The samples were vortexed for 2 min and centrifuged (13,000 rpm, 10 min, 4°C). The 100 μL aliquot of the supernatants were aspirated for quantitative analysis by UPLC-MS/MS. After the experiment, all rats were euthanized by intravenous injection of 150 mg/kg phenobarbital.
Molecular Docking
The protein structures were obtained from the Protein Data Bank (PDB ID: 5TE8; https://www.rcsb.org/), and the three-dimensional sdf-format files for macitentan, imperatorin, curcumin, midazolam (probe substrate of CYP3A4) and ketoconazole (strong CYP3A4 inhibitor) were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/). The three-dimensional protein conformation was optimized using PyMOL, with the removal of chain B, chain C, the original ligand and water, and the addition of hydrogen atoms using AutoDock Tools 1.5.6. Semi-flexible docking was performed using AutoDock 4.2.6, with a maximum output of 50 gestures. The best scoring conformations were visualized using PyMOL.
Data Analysis
In vitro enzyme kinetic parameters, including Km, IC50, and inhibition constant (Ki), were calculated using GraphPad Prism 8.3 software. All relevant curves, including plasma concentration-time graphs, were plotted. Individual pharmacokinetic parameters were calculated in vivo in rats using Drug and Statistics (DAS) 2.0 software. Statistical analyses between groups were performed using independent samples t-test in SPSS 25.0 software, with a significance level of P < 0.05.
Results
Optimization of Analytical Conditions
As illustrated in Figure 2, the chromatographic and mass spectrometric conditions were effectively optimised for the quantitative analysis of macitentan and ACT-132577 utilising a dependable and efficient UPLC-MS/MS analytical methodology. The peaks of macitentan, IS and ACT-132577 were observed at 1.52, 1.43 and 1.42 min, respectively, and the three analytes were successfully separated without any interference from other peaks. The chromatographic conditions were optimised in the course of the experiment. The separation was conducted on an Acquity BEH C18 column (2.1 mm × 50 mm, 1.7 μm). The mobile phase was comprised of a 0.1% solution of formic acid (solution A) and acetonitrile (solution B) at a flow rate of 0.40 mL/min, with a gradient elution time of 2.0 min. It was established that a solution of 0.1% formic acid and acetonitrile yielded the optimal response and peak shape. The brief elution time and high efficiency met the requirements for a multi-sample assay.
The mass spectrometry conditions were also optimised, and the mass spectral information was obtained from the ESI source in both positive and negative ion modes. The highest peak intensities of the analytes were observed in the positive ion mode. The mass spectrometry analysis of macitentan, IS and ACT-132577 revealed that the most prevalent protolated molecules were observed at m/z 588.64, 551.97, and 546.90, respectively. Additionally, the most abundant fragment ion spectra appeared at m/z 203.05, 201.94, and 200.60, respectively.
Effects of Imperatorin and Curcumin on the Metabolism of Macitentan in vitro
The effects of imperatorin and curcumin on the metabolism of macitentan in vitro were evaluated in two models (RLM and HLM). The results were presented in Figure 3–6, which showed Michaelis-Menten plots, IC50 curves (including IC50 curves for RLM), Lineweaver-Burk plots for RLM and HLM. The Km values of macitentan in RLM and HLM were 8.81 μM (Figure 3A) and 68.30 μM (Figure 3B), respectively. The IC50 values of imperatorin and curcumin in RLM were 6.58 ± 1.14 μM (Figure 4A) and 10.86 ± 0.13 μM (Figure 4B), respectively. In HLM, the IC50 values of imperatorin and curcumin were 6.97 ± 0.07 μM (Figure 4C) and 5.71 ± 0.15 μM (Figure 4D), respectively. The above data illustrated that imperatorin strongly inhibited the metabolism of macitentan in both RLM and HLM, whereas curcumin inhibited the metabolism of macitentan more strongly in HLM than in RLM. IC50 shift experiments demonstrated that the IC50 values in the presence of imperatorin were 2.80 ± 0.04 μM and 1.25 ± 0.12 μM for pre-incubation without and with NADPH, respectively, with an IC50 shift fold of 2.24 (Figure 4E). In the presence of curcumin, the IC50 values in the pre-incubation solution with and without NADPH were 5.69 ± 0.25 μM and 9.00 ± 0.24 μM, respectively, with an IC50 shift fold of 0.63 (Figure 4F). Their IC50 shift folds were less than 10, which could be considered to be a non-time-dependent inhibition.33
 |
Figure 3 Michaelis-Menten plots for macitentan in RLM (A) and HLM (B). V, velocity of enzyme catalysis; Km: Michaelis-Menten constant. |
 |
Figure 4 IC50 curves of imperatorin and curcumin on macitentan metabolism in RLM (A and B) and HLM (C and D). IC50 shift curves of imperatorin and curcumin on macitentan metabolism in RLM (E and F). |
 |
Figure 5 In RLM (A) and HLM (B), Lineweaver-Burk plots, Ki secondary plots and αKi secondary plots of inhibition of macitentan metabolism by different concentrations of imperatorin. |
 |
Figure 6 In RLM (A) and HLM (B), Lineweaver-Burk plots, Ki secondary plots and αKi secondary plots of inhibition of macitentan metabolism by different concentrations of curcumin. |
Therefore, the mechanism of the inhibition of macitentan in different microsome incubation systems was further explored (Figure 5 and 6). In RLM, the Lineweaver-Burk plots of imperatorin and curcumin demonstrated that the type of metabolic inhibition of macitentan by imperatorin and curcumin were mixed and non-competitive, respectively. In HLM, the Lineweaver-Burk plots of imperatorin and curcumin demonstrated that the type of metabolic inhibition of macitentan by imperatorin and curcumin were mixed. In summary, both imperatorin and curcumin could inhibit macitentan metabolism in vitro.
Effects of Imperatorin and Curcumin on the Metabolism of Macitentan in vivo
Figure 7 illustrated the mean plasma concentration versus time curves of macitentan and ACT-132577 in different SD rats, and Table 1 and Table 2 presented the main pharmacokinetic parameters. The AUC(0-t), AUC(0-∞), t1/2, Tmax, CLz/F, and Cmax of macitentan were significantly altered following imperatorin administration in comparison to the control group. In particular, the AUC(0-t), AUC(0-∞), and Tmax were increased by approximately one-fold. The Cmax of macitentan was increased by 0.56-fold, while the t1/2 was also correspondingly prolonged (from 2.64 ± 0.40 h to 3.40 ± 0.53 h). In addition, the CLz/F was significantly reduced by approximately 0.46-fold. For the metabolite ACT-132577, both t1/2, Tmax and Cmax exhibited significant alterations. Its t1/2 was shortened from 6.73 ± 0.63 h to 5.51 ± 0.71 h. Following curcumin administration, macitentan exhibited significant increases in AUC(0-∞) and Tmax by 0.34-fold and 1.8-fold, respectively, and a significant decrease in CLz/F (from 0.41 ± 0.06 L/h/kg to 0.31 ± 0.07 L/h/kg). Although the t1/2 values were not statistically different, there was a tendency for the t1/2 to be prolonged (from 2.64 ± 0.40 h to 4.02 ± 1.92 h). For the metabolite ACT-132577, there were significant increases in AUC(0-∞) and Tmax, and a significant decrease in CLz/F. The t1/2 was found to be prolonged from 6.73 ± 0.63 h to 8.98 ± 3.13 h. These results indicated that both imperatorin and curcumin significantly inhibited the metabolism of macitentan in SD rats, and the inhibitory effect of imperatorin on the plasma exposure of macitentan was more significant in comparison to curcumin.
 |
Table 1 Main Pharmacokinetic Parameters of Macitentan in Different Groups of SD Rats (n = 5, Mean ± SD) |
 |
Table 2 Main Pharmacokinetic Parameters of ACT-132577 in Different Groups of SD Rats (n = 5, Mean ± SD) |
 |
Figure 7 Mean plasma concentration-time curves of macitentan (A and C) and its metabolite ACT-132577 (B and D) in different groups of SD rats (n = 5). |
Molecular Docking
To further validate the inhibitory effects of imperatorin and curcumin on macitentan, molecular docking analyses were performed. As illustrated in Figure 8A and B, imperatorin (cyan), curcumin (slate), and macitentan (salmon) all spontaneously bind to the active site of CYP3A4. Molecular docking analyses demonstrated that macitentan exhibited hydrogen bonding interaction forces with GLU-374 (3.6 Å) and LEU-216 (1.8 Å), while imperatorin formed a hydrogen bond with PHE-304 (3.6 Å). Curcumin was also able to bind to HEM-601 (2.3 Å), ARG-372 (3.0 Å), LEU-216 (2.8 Å) and ALA-305 (2.0 Å) to form one hydrogen bond and ASP-214 (1.8 Å and 3.0 Å) to form two hydrogen bonds, respectively. The binding energies of the macitentan, imperatorin and curcumin to CYP3A4 were −9.24 kcal/mol, −6.60 kcal/mol and −9.13 kcal/mol, respectively. As illustrated in Figure 8C, midazolam could bind to CYP3A4 with a binding energy of −8.44 kcal/mol, whereas ketoconazole could bind to GLU-374 (2.7 Å), ALA-370 (3.4 Å), and PHE-213 (2.5 Å), forming a total of three hydrogen bonds with a binding energy of −9.52 kcal/mol. Furthermore, macitentan, imperatorin, and curcumin were docked with CYP3A4. The docking results demonstrated that the distances from the action groups of macitentan, imperatorin, and curcumin to the ferrous ions in hemoglobin were 12.7, 7.2, and 3.6 Å (Figure 9A–C). It was observed that imperatorin and curcumin exhibited a closer proximity to ferrous ions in comparison to macitentan, indicating that imperatorin and curcumin possess a potentially stronger affinity for CYP3A4 compared to macitentan. This observation may provide a crucial rationale for the observed inhibition of macitentan metabolism by imperatorin and curcumin in HLM.
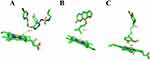 |
Figure 9 Schematic representation of the distances of macitentan (A), imperatorin (B), and curcumin (C) to ferrous ions in the heme moiety in CYP3A4. |
Discussion
Macitentan, the most recent treatment option for PAH, is metabolized in vivo by CYP3A4, with the main metabolite being the pharmacologically active N-despropyl macitentan (ie, ACT-132577).34,35 This metabolite has recently been shown to be efficacious in the treatment of recalcitrant hypertension.36 A previous study demonstrated that the combination of macitentan with ketoconazole, a potent CYP3A4 inhibitor, resulted in a significant increase in macitentan plasma exposure, with a two-fold increase in both AUC(0-t) and AUC(0-∞).37 This enhanced the oral bioavailability of macitentan. In the presence of rifampicin, a CYP3A4 inducer with a strong effect, the plasma exposure of macitentan was reduced by approximately four times, which may affect the overall pharmacological activity of macitentan in vivo.38 Given that macitentan shares the same binding enzyme with imperatorin and curcumin and has the potential to be used in combination in clinical practice, it is of great interest to explore whether there is a drug-drug interaction between macitentan and imperatorin/curcumin.
Firstly, in vitro studies were conducted to assess the enzyme kinetic parameters of macitentan using different microsome models. In this study, two hepatic models, RLM and HLM, were employed to investigate the changes on the metabolism of macitentan in the presence of imperatorin or curcumin. The results of the RLM and HLM experiments demonstrated that imperatorin and curcumin inhibited the metabolism of macitentan in vitro. However, the degree of inhibition differed between the two models. Imperatorin demonstrated a robust inhibitory effect in both RLM and HLM, with comparable inhibitory potency. In contrast, curcumin exhibited a more pronounced inhibitory effect on macitentan in HLM, with an IC50 value half of that observed in RLM. Additionally, the inhibitory mechanism was investigated, revealing distinct inhibition patterns of macitentan in RLM and HLM. In RLM, imperatorin exhibited mixed-type inhibition, while curcumin exhibited non-competitive inhibition. In HLM, both imperatorin and curcumin exhibited mixed-type inhibition. In one report, imperatorin demonstrated robust inhibition of CYP3A4 (IC50 = 0.5 μM) and a mixed-type inhibition pattern,39 which aligned with our findings. The IC50 value for human CYP3A4 inhibition by curcumin was 16.30 μM, indicating a markedly weaker inhibitory effect than that of imperatorin.40 This differed from the results of our study in HLM, where the IC50 values of curcumin and imperatorin were found to be similar. This discrepancy may be attributed to individual differences in CYP enzymes involved in metabolism. Furthermore, IC50 shift experiments revealed that the inhibition of macitentan by imperatorin and curcumin would not be time-dependent.
Subsequently, in vivo pharmacokinetic studies were conducted in rats to further validate the inhibitory effects of imperatorin and curcumin on macitentan. A previous study reported that following oral administration of 3 mg/kg containing 60 μCi/kg 14C-macitentan to rats, the peak concentration of macitentan was found to be lower than that of the metabolite M6 (ie, ACT-132577), with Cmax of 0.34 nmol/mL and 0.69 nmol/mL, respectively.41 It was observed that following oral administration of 10 mg/kg of macitentan to rats, the Cmax of macitentan was lower than the prototype concentration of ACT-132577, which was consistent with the trend reported in the aforementioned literature. In the presence of imperatorin, the AUC(0-t) and AUC(0-∞) of macitentan were significantly increased, with a corresponding prolongation of the t1/2 to 3.40 ± 0.53 h. The Tmax was observed to extend to 7.60 ± 0.89 h. The results demonstrated that the Cmax was significantly increased, while the CLz/F was significantly decreased. The increase in macitentan exposure may be due to the fact that imperatorin inhibited macitentan metabolism. Additionally, the t1/2, Tmax and Cmax of ACT-132577 exhibited significant alterations. In the presence of curcumin, the AUC(0-∞) of macitentan was significantly increased, the Tmax was significantly prolonged, while the CLz/F was significantly decreased. The AUC(0-∞) of ACT-132577 was significantly increased, and the Tmax was also prolonged to 12.00 ± 0.00 h, which was almost the same as that of macitentan. Prolongation of the t1/2 of macitentan was observed when administered concomitantly with either imperatorin or curcumin. This phenomenon can be attributed to the inhibition of the activity of metabolic enzymes, leading to a reduced rate of macitentan metabolism. Consequently, an increased residence time was attained in vivo, resulting in an augmented plasma exposure and, thereby, a lengthened t1/2. Although macitentan is primarily metabolized to ACT-132577 by CYP3A4, it can also be metabolized to ACT-132577 via CYP2C8, CYP2C9, and CYP2C19 (though with a lesser contribution compared to CYP3A4).42 In the presence of imperatorin or curcumin, CYP3A4 activity mainly be inhibited, allowing macitentan to be metabolized via the CYP2C8, CYP2C9, and CYP2C19 pathways. This phenomenon may have contributed to the increased concentration of ACT-132577. The metabolism of macitentan was significantly inhibited in the presence of imperatorin/curcumin, resulting in increased bioavailability, indicating that a larger proportion of macitentan may be converted to ACT-132577. The reduced clearance of macitentan may be attributed to two primary factors. First, in terms of metabolism, imperatorin/curcumin had been shown to inhibit the CYP3A4 enzyme. Second, in regard to transport, the hepatic uptake of macitentan was dependent on OATP1B1/1B3,43 and curcumin exhibited an inhibitory effect on the OATP1B1/OATP1B3 activity,44 while imperatorin demonstrated no significant inhibitory effect on OATP1B1/OATP1B3 activity, according to the available reports.45 Therefore, it can be hypothesized that curcumin may cause a decrease in the clearance of macitentan by partly inhibiting OATP1B1/OATP1B3. The significant multi-fold increase in the Tmax of ACT-132577 may be attributed to several potential mechanisms. First, it is hypothesized that imperatorin and curcumin themselves may potentially inhibit the CYP3A4 enzyme,39,46 and that this inhibition may result in a delay in the conversion of macitentan to ACT-132577. Second, it is hypothesized that changes in gastrointestinal motility, pH, or efflux transporter protein interactions may slow the absorption of macitentan, thereby delaying ACT-132577 formation and resulting in a delayed time to peak.47 Furthermore, ACT-132577 may undergo enterohepatic recycling, whereby ACT-132577 is reabsorbed from the intestine after biliary excretion,41,48 a process that may also result in a prolonged Tmax. Further studies are warranted to elucidate the precise mechanisms underlying this observation.
Since our results indicated that imperatorin and curcumin inhibited the metabolism of macitentan in rats, therefore, elevated plasma concentrations of macitentan may result in an augmented probability of adverse reactions, such as headache, nasopharyngitis, and anemia. Nonetheless, the present study is not without limitations, and the results observed in rats must be extrapolated with caution to humans, as interspecies differences in drug metabolism (particularly those related to CYP3A4 activity) and the absence of clinical validation at present constitute significant study limitations.49 Consequently, the necessity for further clinical pharmacokinetic studies is evident.
A study found that when imperatorin was co-administered with diazepam, which is mainly metabolized by CYP2C19 and CYP3A4, the AUC(0–12h) and Cmax were significantly increased, while the apparent clearance CLz/F was significantly decreased.50 A further in vivo pharmacokinetic study in rats revealed that the presence of curcumin (at doses of 2 and 8 mg/kg) resulted in a 35.1% and 50.8% increase in the AUC of etoposide (a main liver metabolic enzyme that is CYP3A4), respectively, accompanied by a notable rise in its plasma exposure.51 These findings suggested that imperatorin and curcumin significantly inhibited the metabolism of drugs by CYP3A4, which was in line with our findings.
Furthermore, imperatorin docking was achieved at a higher binding energy (−6.60 kcal/mol), while curcumin docking was achieved at a lower binding energy (−9.13 kcal/mol), which was associated with its relative in vitro inhibition ability (in HLM, imperatorin: IC50 = 6.97 μM, Ki = 8.27 μM, curcumin: IC50 = 5.71 μM, Ki = 4.64 μM). Moreover, both imperatorin and curcumin formed hydrogen bonds with different amino acid residues on CYP3A4. In conclusion, imperatorin and curcumin binded to distinct sites on CYP3A4, thereby inhibiting the metabolic action of this enzyme on macitentan. In addition, our findings revealed a closer proximity and stronger affinity of imperatorin and curcumin to ferrous ions in hemoglobin, which may serve as a significant factor contributing to the inhibition of macitentan metabolism by imperatorin and curcumin in HLM.
Conclusion
In conclusion, imperatorin and curcumin were found to inhibit the metabolism of macitentan in vitro, although the degree of metabolism varied in different systems. In SD rats, a significant increase in plasma exposure of macitentan in vivo further confirmed the inhibitory potency of imperatorin and curcumin. Furthermore, molecular docking demonstrated that the metabolic inhibition of macitentan by imperatorin and curcumin was achieved by binding to the site on CYP3A4. Nevertheless, the results of experiments conducted on rats cannot be accurately extrapolated to humans due to inherent differences between species. Consequently, further validation of this inhibitory effect through the study of human pharmacokinetics may be necessary.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Luna-López R, Ruiz Martín A, Escribano Subías P. Pulmonary arterial hypertension. Med Clin. 2022;158(12):622–629. doi:10.1016/j.medcli.2022.01.003
2. Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53(1):1801887. doi:10.1183/13993003.01887-2018
3. Zhang MQ, Wang CC, Pang XB, et al. Role of macrophages in pulmonary arterial hypertension. Front Immunol. 2023;14:1152881. doi:10.3389/fimmu.2023.1152881
4. Dupuis J, Hoeper MM. Endothelin receptor antagonists in pulmonary arterial hypertension. Eur Respir J. 2008;31(2):407–415. doi:10.1183/09031936.00078207
5. Böhm F, Pernow J, Lindström J, Ahlborg G. ETA receptors mediate vasoconstriction, whereas ETB receptors clear endothelin-1 in the splanchnic and renal circulation of healthy men. Clin Sci. 2003;104(2):143–151. doi:10.1042/cs20020192
6. Gallelli L, Pelaia G, D’Agostino B, et al. Endothelin-1 induces proliferation of human lung fibroblasts and IL-11 secretion through an ET(A) receptor-dependent activation of MAP kinases. J Cell Biochem. 2005;96(4):858–868. doi:10.1002/jcb.20608
7. Dupuis J, Goresky CA, Fournier A. Pulmonary clearance of circulating endothelin-1 in dogs in vivo: exclusive role of ETB receptors. J Appl Physiol. 1996;81(4):1510–1515. doi:10.1152/jappl.1996.81.4.1510
8. Rubens C, Ewert R, Halank M, et al. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest. 2001;120(5):1562–1569. doi:10.1378/chest.120.5.1562
9. Aversa M, Porter S, Granton J. Comparative safety and tolerability of endothelin receptor antagonists in pulmonary arterial hypertension. Drug Saf. 2015;38(5):419–435. doi:10.1007/s40264-015-0275-y
10. Belge C, Delcroix M. Treatment of pulmonary arterial hypertension with the dual endothelin receptor antagonist macitentan: clinical evidence and experience. Ther Adv Respir Dis. 2019;13:1753466618823440. doi:10.1177/1753466618823440
11. Steriade A, Seferian A, Jaïs X, et al. The potential for macitentan, a new dual endothelin receptor antagonist, in the treatment of pulmonary arterial hypertension. Ther Adv Respir Dis. 2014;8(3):84–92. doi:10.1177/1753465814530182
12. Nadeau V, Potus F, Boucherat O, et al. Dual ET(A)/ET(B) blockade with macitentan improves both vascular remodeling and angiogenesis in pulmonary arterial hypertension. Pulm Circ. 2018;8(1):2045893217741429. doi:10.1177/2045893217741429
13. Knobloch J, Yanik SD, Körber S, Stoelben E, Jungck D, Koch A. TNFα-induced airway smooth muscle cell proliferation depends on endothelin receptor signaling, GM-CSF and IL-6. Biochem Pharmacol. 2016;116:188–199. doi:10.1016/j.bcp.2016.07.008
14. Bolli MH, Boss C, Binkert C, et al. The discovery of N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N’-propylsulfamide (Macitentan), an orally active, potent dual endothelin receptor antagonist. J Med Chem. 2012;55(17):7849–7861. doi:10.1021/jm3009103
15. Sidharta PN, Lindegger N, Ulč I, Dingemanse J. Pharmacokinetics of the novel dual endothelin receptor antagonist macitentan in subjects with hepatic or renal impairment. J Clin Pharmacol. 2014;54(3):291–300. doi:10.1002/jcph.193
16. Pae HO, Oh H, Yun YG, et al. Imperatorin, a furanocoumarin from Angelica dahurica (Umbelliferae), induces cytochrome c-dependent apoptosis in human promyelocytic leukaemia, HL-60 Cells. Pharmacol Toxicol. 2002;91(1):40–48. doi:10.1034/j.1600-0773.2002.910107.x
17. Zhang Y, Cao Y, Zhan Y, Duan H, He L. Furanocoumarins-imperatorin inhibits myocardial hypertrophy both in vitro and in vivo. Fitoterapia. 2010;81(8):1188–1195. doi:10.1016/j.fitote.2010.07.023
18. Zhang Y, Cao Y, Duan H, Wang H, He L. Imperatorin prevents cardiac hypertrophy and the transition to heart failure via NO-dependent mechanisms in mice. Fitoterapia. 2012;83(1):60–66. doi:10.1016/j.fitote.2011.09.011
19. Deng M, Xie L, Zhong L, Liao Y, Liu L, Li X. Imperatorin: a review of its pharmacology, toxicity and pharmacokinetics. Eur J Pharmacol. 2020;879:173124. doi:10.1016/j.ejphar.2020.173124
20. Nasser MI, Zhu S, Hu H, Huang H, Guo M, Zhu P. Effects of imperatorin in the cardiovascular system and cancer. Biomed Pharmacother. 2019;120:109401. doi:10.1016/j.biopha.2019.109401
21. Mhillaj E, Tarozzi A, Pruccoli L, Cuomo V, Trabace L, Mancuso C. Curcumin and Heme Oxygenase: neuroprotection and Beyond. Int J mol Sci. 2019;20(10):2419. doi:10.3390/ijms20102419
22. Li KX, Wang ZC, Machuki JO, et al. Benefits of Curcumin in the Vasculature: a Therapeutic Candidate for Vascular Remodeling in Arterial Hypertension and Pulmonary Arterial Hypertension? Front Physiol. 2022;13:848867. doi:10.3389/fphys.2022.848867
23. Dong S, Guo X, Wang H, Sun C. Liver injury due to endothelin receptor antagonists: a real-world study based on post-marketing drug monitoring data. Ther Adv Respir Dis. 2024;18:17534666231223606. doi:10.1177/17534666231223606
24. Gao Z, Zhang J, Wei L, et al. The Protective Effects of Imperatorin on Acetaminophen Overdose-Induced Acute Liver Injury. Oxid Med Cell Longev. 2020;2020:8026838. doi:10.1155/2020/8026838
25. Bavarsad K, Riahi MM, Saadat S, Barreto G, Atkin SL, Sahebkar A. Protective effects of curcumin against ischemia-reperfusion injury in the liver. Pharmacol Res. 2019;141:53–62. doi:10.1016/j.phrs.2018.12.014
26. Sidharta PN, Treiber A, Dingemanse J. Clinical pharmacokinetics and pharmacodynamics of the endothelin receptor antagonist macitentan. Clin Pharmacokinet. 2015;54(5):457–471. doi:10.1007/s40262-015-0255-5
27. Wang L, Lu W, Shen Q, et al. Simultaneous determination of imperatorin and its 2 metabolites in dog plasma by using liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2012;70:640–646. doi:10.1016/j.jpba.2012.06.034
28. Sahu PK, Sahu PK, Sahu PL, Agarwal DD. Structure activity relationship, cytotoxicity and evaluation of antioxidant activity of curcumin derivatives. Bioorg Med Chem Lett. 2016;26(4):1342–1347. doi:10.1016/j.bmcl.2015.12.013
29. He J, Fang P, Zheng X, et al. Inhibitory effect of celecoxib on agomelatine metabolism in vitro and in vivo. Drug Des Devel Ther. 2018;12:513–519. doi:10.2147/dddt.S160316
30. Sidharta PN, Krähenbühl S, Dingemanse J. Pharmacokinetic and pharmacodynamic evaluation of macitentan, a novel endothelin receptor antagonist for the treatment of pulmonary arterial hypertension. Expert Opin Drug Metab Toxicol. 2015;11(3):437–449. doi:10.1517/17425255.2015.1000859
31. Bunbupha S, Prasarttong P, Poasakate A, Maneesai P, Pakdeechote P. Imperatorin alleviates metabolic and vascular alterations in high-fat/high-fructose diet-fed rats by modulating adiponectin receptor 1, eNOS, and p47(phox) expression. Eur J Pharmacol. 2021;899:174010. doi:10.1016/j.ejphar.2021.174010
32. Liu H, Wang C, Qiao Z, Xu Y. Protective effect of curcumin against myocardium injury in ischemia reperfusion rats. Pharm Biol. 2017;55(1):1144–1148. doi:10.1080/13880209.2016.1214741
33. Jin C, He X, Zhang F, et al. Inhibitory mechanisms of celastrol on human liver cytochrome P450 1A2, 2C19, 2D6, 2E1 and 3A4. Xenobiotica. 2015;45(7):571–577. doi:10.3109/00498254.2014.1003113
34. Hill NS, Badesch D, Benza RL, et al. Perspectives on oral pulmonary hypertension therapies recently approved by the U.S. Food and Drug Administration. Ann Am Thorac Soc. 2015;12(2):269–273. doi:10.1513/AnnalsATS.201501-020AS
35. Iglarz M, Binkert C, Morrison K, et al. Pharmacology of macitentan, an orally active tissue-targeting dual endothelin receptor antagonist. J Pharmacol Exp Ther. 2008;327(3):736–745. doi:10.1124/jpet.108.142976
36. Brussee JM, Sidharta PN, Dingemanse J, Krause A. Population pharmacokinetics of the dual endothelin receptor antagonist aprocitentan in subjects with or without essential or resistant hypertension. J Pharmacokinet Pharmacodyn. 2024;51(3):243–252. doi:10.1007/s10928-024-09902-1
37. Atsmon J, Dingemanse J, Shaikevich D, Volokhov I, Sidharta PN. Investigation of the effects of ketoconazole on the pharmacokinetics of macitentan, a novel dual endothelin receptor antagonist, in healthy subjects. Clin Pharmacokinet. 2013;52(8):685–692. doi:10.1007/s40262-013-0063-8
38. Bruderer S, Aänismaa P, Homery MC, et al. Effect of cyclosporine and rifampin on the pharmacokinetics of macitentan, a tissue-targeting dual endothelin receptor antagonist. Aaps J. 2012;14(1):68–78. doi:10.1208/s12248-011-9316-3
39. Kimura Y, Ito H, Ohnishi R, Hatano T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food Chem Toxicol. 2010;48(1):429–435. doi:10.1016/j.fct.2009.10.041
40. Appiah-Opong R, Commandeur JN, van Vugt-Lussenburg B, Vermeulen NP. Inhibition of human recombinant cytochrome P450s by curcumin and curcumin decomposition products. Toxicology. 2007;235(1–2):83–91. doi:10.1016/j.tox.2007.03.007
41. Treiber A, Miraval T, Bolli MH, Funel JA, Segrestaa J, Seeland S. The metabolism of the dual endothelin receptor antagonist macitentan in rat and dog. Xenobiotica. 2016;46(3):253–267. doi:10.3109/00498254.2015.1070302
42. Patel T, McKeage K. Macitentan: first global approval. Drugs. 2014;74(1):127–133. doi:10.1007/s40265-013-0156-6
43. Lepist EI, Gillies H, Smith W, et al. Evaluation of the endothelin receptor antagonists ambrisentan, bosentan, macitentan, and sitaxsentan as hepatobiliary transporter inhibitors and substrates in sandwich-cultured human hepatocytes. PLoS One. 2014;9(1):e87548. doi:10.1371/journal.pone.0087548
44. Sun X, Li J, Guo C, et al. Pharmacokinetic effects of curcumin on docetaxel mediated by OATP1B1, OATP1B3 and CYP450s. Drug Metab Pharmacokinet. 2016;31(4):269–275. doi:10.1016/j.dmpk.2016.02.005
45. Wang Z, Shang H, Li Y, et al. Transporters (OATs and OATPs) contribute to illustrate the mechanism of medicinal compatibility of ingredients with different properties in yuanhuzhitong prescription. Acta Pharm Sin B. 2020;10(9):1646–1657. doi:10.1016/j.apsb.2020.05.012
46. Pradeepa BR, Vijayakumar TM, Dhivya LS, Manikandan K. In-silico comparison of cytochrome P450 inhibitory and dopaminergic activity of Piperine, Curcumin and Capsaicin. Nat Prod Res. 2023;37(17):2888–2893. doi:10.1080/14786419.2022.2134862
47. Shugarts S, Benet LZ. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm Res. 2009;26(9):2039–2054. doi:10.1007/s11095-009-9924-0
48. Roberts MS, Magnusson BM, Burczynski FJ, Weiss M. Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet. 2002;41(10):751–790. doi:10.2165/00003088-200241100-00005
49. Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2(6):875–894. doi:10.1517/17425255.2.6.875
50. Zhou Y, Meng D, Chen F, et al. Inhibitory Effect of Imperatorin on the Pharmacokinetics of Diazepam In Vitro and In Vivo. Front Pharmacol. 2020;11:01079. doi:10.3389/fphar.2020.01079
51. Lee CK, Ki SH, Choi JS. Effects of oral curcumin on the pharmacokinetics of intravenous and oral etoposide in rats: possible role of intestinal CYP3A and P-gp inhibition by curcumin. Biopharm Drug Dispos. 2011;32(4):245–251. doi:10.1002/bdd.754
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



