Back to Journals » Cancer Management and Research » Volume 17
Drug Repurposing in Pancreatic Cancer: A Multi-Stakeholder Perspective to Improve Treatment Options for Pancreatic Cancer Patients
Authors Hewitt E, Bouche G, Alencar AC, Bigelsen SJ , Radu R, Stoyanova-Beninska V , Carrato A , Valsecchi F, Soler Cantón A, van der Meer HG , García Bermejo ML, Budillon A, Cardone L, Rooman I, Platteeuw H, Baijet J, Fuchs C
Received 29 August 2024
Accepted for publication 2 February 2025
Published 1 March 2025 Volume 2025:17 Pages 429—440
DOI https://doi.org/10.2147/CMAR.S483151
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Evelyn Hewitt,1 Gauthier Bouche,2 Alexandre Costa Alencar,3 Stephen J Bigelsen,4 Raluca Radu,5 Violeta Stoyanova-Beninska,6 Alfredo Carrato,7,8 Federica Valsecchi,9 Alicia Soler Cantón,10 Helene G van der Meer,11 María Laura García Bermejo,12 Alfredo Budillon,13 Luca Cardone,14,15 Ilse Rooman,2,16 Hans Platteeuw,17 Judit Baijet,18 Claudia Fuchs18
1Beacon: for Rare Disease, Cambridge, UK; 2The Anticancer Fund, Meise, Belgium; 3Rising Tide Foundation for Clinical Cancer Research, Schaffhausen, Switzerland; 4Patient Advocate, Department of Allergy, Asthma and Immunology, Rutgers New Jersey Medical School, Newark, NJ, USA; 5Medicines for Europe, Brussels, Belgium; 6College ter Beoordeling van Geneesmiddelen, Utrecht, the Netherlands; 7Department of Medical Oncology, Alcalá University, Instituto Ramón Y Cajal de Investigación Sanitaria (IRYCIS), Centro de Investigación Biomédica En Red de Cáncer (CIBERONC), Ramon Y Cajal University Hospital, Madrid, Spain; 8Pancreatic Cancer Europe, Brussels, Belgium; 9Fondazione Nadia Valsecchi, Brescia, Italy; 10EATRIS ERIC, European Infrastructure for Translational Medicine, Amsterdam, the Netherlands; 11ZonMw, The Hague, the Netherlands; 12Biomarkers and Therapeutic Targets Group, Instituto Ramón Y Cajal de Investigación Sanitaria (IRYCIS), Ramon Y Cajal University Hospital, Madrid, Spain; 13Scientific Directorate, Istituto Nazionale Tumori – IRCCS - Fondazione G. Pascale, Naples, Italy; 14Institute of BiOChemistry and Cell Biology (IBBC), CNR c/o Campus Internazionale “a.buzzati-Traverso”, Monterotondo Scalo Roma (Rome), Italy; 15Department of Tumour Immunology and Immunotherapy, IRCCS Regina Elena National Cancer Institute, Rome, Italy; 16Translational Oncology Research Center (TORC), Laboratory of Medical and Molecular Oncology, (LMMO) Vrije Universiteit Brussel, Brussels, Belgium; 17Galenicap, Brussels, Belgium; 18EURORDIS - Rare Diseases Europe, Paris, France
Correspondence: Claudia Fuchs, EURORDIS - Rare Diseases Europe, Rare Diseases Platform, 96 rue Didot, Paris, 75014, France, Email [email protected] Evelyn Hewitt, Beacon for Rare Diseases, 66 Devonshire Road, Cambridge, CB1 2BL, United Kingdom, Email [email protected]
Abstract: Pancreatic cancer (PC) remains one of the most challenging malignancies to treat. Current therapeutic options are unsatisfactory, and there is an urgent need for more effective and less toxic drugs to improve the dismal prognosis of PC. In recent years, drug repurposing (DR) has emerged as an attractive strategy to identify novel treatments for PC by leveraging existing drugs approved for other indications. Through the use of electronic medical records, Artificial Intelligence, study of metabolic pathways, signalling pathways, and many other approaches, it has become much easier in recent years to identify potential novel uses for old drugs. Although policy, funding and research attention in this area are steadily growing, major challenges to efficient and effective patient-centric DR in PC need to be addressed. These include but are not limited to regulatory, financial and funding barriers and the lack of coordination and collaboration among several sectors and stakeholders. To explore the opportunities and challenges associated with DR in PC, a one-day multi-stakeholder meeting was held on 14th of November 2023 in Brussels, Belgium as part of the REMEDi4ALL project. This meeting provided a platform for researchers, clinicians, industry representatives, funders, regulatory experts, and patient advocates to discuss and propose actions to optimize and accelerate DR in PC. Insights from this meeting support the potential of DR to enhance PC treatment options while highlighting the importance of systemic and supportive changes in the regulatory, policy and funding landscapes, interdisciplinary collaboration, data sharing, and patient involvement in driving therapeutic innovation. This summary highlights key outcomes and recommendations from the meeting in informing future efforts to advance DR initiatives in the context of PC.
Keywords: drug repurposing, pancreatic cancer, multi-stakeholder discussion, collaboration, patient centricity
Introduction
Pancreatic Cancer
Pancreatic cancer (PC) is one of the leading causes of cancer-related deaths worldwide.1 The disease is increasing in incidence, with Western Europe having been identified as the region carrying the highest lifetime risk of developing PC.2,3
PC’s high mortality rate can be attributed to several factors; particularly its asymptomatic nature in early-stage disease, non-specific gastrointestinal presentations in later-stage disease and consequent delays in diagnosis and treatment initiation. Only one-fifth of patients are eligible for surgical intervention, currently the only curative option. Current treatment options for unresectable PC (a combination of chemotherapy, radiation therapy, and palliative care) are limited and unsatisfactory (eg, high rates of chemotherapeutic resistance and adverse effects).
While there have been significant improvements in 5-year overall survival (OS) rate for the 7 most common types of cancer (breast, skin, colon, prostate, blood, lung, and ovarian cancer) across Europe and the US, PC has been left behind. The 5-year OS rates for PC remain still very low, ranging from 3 to 12%.4,5
Here, we discuss why this is the case – a complex combination of a tricky disease and trickier landscape, and where drug repurposing (DR) may represent an alternative avenue in development of efficacious anti-neoplastic therapeutic regimens for PC.
Drug Repurposing
Drug repurposing (DR) is used routinely in oncology. Both oncology and non-oncology drugs are used as monotherapy or in combination therapy to enhance other therapeutic options across multiple cancer types.6 DR lends itself to the personalised medicine approach commonly seen in oncology, where drugs are matched to the specific biology and genetics of individuals and their tumours.7
Except for liposomal irinotecan, all drugs used to treat PC have been repurposed from use in other tumour groups. Gemcitabine, a fundamental in PC standard of care, was repurposed due to its significant and organ agnostic cytotoxic effects on cancer cells. DR candidates in PC target multiple hallmarks of cancer, including key pathways implicated in tumour progression and survival. The KRAS signalling pathway, which is frequently mutated in PC, is a major focus, with repurposed drugs aiming to inhibit downstream effectors such as MAPK/ERK and PI3K/AKT.8 Additionally, autophagy - a survival mechanism exploited by PC tumour cells – can be exploited through use of repurposed non-oncology drugs like chloroquine, a drug originally developed for use in malaria.9 Other promising pathways include DNA damage repair mechanism, metabolic vulnerabilities such as glutamine dependency, and the tumour microenvironment.9
DR is often viewed as a route to medicines access that is “easier” than de novo drug development. This is not necessarily the case and often DR can give rise to challenges that may not exist in traditional drug development.10 Many new and existing initiatives across the globe are coming together to make repurposing simpler for all stakeholders, including REMEDi4ALL (Repurposing Medicines for All, remedi4all.org/).
REMEDi4ALL, an EU-funded initiative, focuses on optimizing and implementing the process of identifying new medical applications for drugs already recognized as safe and effective, making it more efficient, reliable, and patient-centric. REMEDiALL seeks to show that, in many cases, “repurposed” drugs can be taken all the way into clinic and to market more rapidly and at the fraction of the cost needed to develop a new drug from scratch.
Patient Centricity and Multi-Stakeholder Meetings
REMEDi4ALL is implementing multi-stakeholder meetings (MSM) to discuss disease and repurposing specific challenges, explore collaborative opportunities between stakeholders and thus facilitate accelerated, patient-centric drug repurposing efforts.
A multi-stakeholder approach has been seen in other EU-funded projects:
- ACCELERATE (accelerate-platform.org)
- Connect4children (conect4children.org)
Providing informal, collegial environments for knowledge sharing, dialogue, and engagement means these meetings can contribute to inclusive, effective and collaborative discussions, driving positive change in multiple contexts.11,12 These fora are essential for patient centricity, providing patient communities with a unique platform to voice perspectives, concerns, and needs directly to relevant stakeholders.
In line with REMEDi4ALL’s patient-centric approach, the patient voice was the core narrative throughout the first REMEDi4ALL MSM “Drug Repurposing, an Attractive Strategy in Pancreatic Cancer Treatment?” (Figure 1).
 |
Figure 1 Program outline of the first REMEDi4ALL Multistakeholder Meeting “Drug Repurposing, an Attractive Strategy in Pancreatic Cancer Treatment?” held on the 14th of November 2023 in Brussels. |
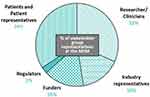
Prior to World Pancreatic Cancer Day 2023, a group spanning key stakeholders (Figure 2) met in Brussels on the 14th of November 2023 to discuss the potential of patient-centric DR in PC. This article summarizes key points discussed and recommendations/action items identified during the meeting.
Materials and Methods
Framework
Representatives of all key stakeholder groups were included in sessions and round tables (Figure 2). As highlighted in Figure 1 the first session explored the topic of DR in PC from all stakeholder perspectives. Session two summarised DR projects in PC (Table 1), while session three tackled current hurdles for efficient DR in oncology.
 |
Table 1 Repurposing Trials Presented at the Workshop |
The meeting ended with two roundtable discussions, highlighting lessons learned from failures in past trials and current limitations of the repurposing ecosystem; culminating in recommendations/actions needed to advance the field to better meet patient needs.
All participants agreed that views expressed during the meeting could be used in this article summarizing the outcomes.
Results
Challenges and Hurdles
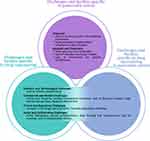
Although DR represents an attractive and valuable therapeutic opportunity for PC, with several studies investigating this potential8,13 (Table 1), major challenges and hurdles (grouped into three main categories as below and in Figure 3) were highlighted during the meeting.
Challenges and Hurdles Specific to PC
Diagnosis
Though not immediately relevant to the discussion of DR, it is important when approaching drug development to understand that PC is often diagnosed at an advanced stage; contributing to poor prognosis. Late diagnosis is associated with:
- Lack of screening regimens (as seen for breast and colorectal)
- Lack of predictive biomarkers
- Lack of symptom awareness.
Research and Treatment
The intrinsic characteristics of the pancreas and PC are challenging; with significant heterogeneity, both between patients and within individual’s tumours (high tumour cell plasticity and complex stroma.14–18 This complex, dynamic disease state affects treatment response and complicates targeted therapy development. Lack of robust non-clinical models that recapitulate the complexity of PC biology limits accessible and effective research.19
Challenges and Hurdles Specific to DR
Although DR holds great promise for accelerating discovery, development and access to treatment, efficacious research must focus on improving patient outcomes, and not be naive in addressing challenges related to rarer cancer types alongside issues and hurdles specific to DR. DR across all diseases sees significant scientific, technological, commercial, clinical, regulatory, social and collaborative challenges. These challenges are exhaustively discussed in existing literature.10,20 During the meeting, several barriers were emphasized, and we have grouped these into 4 major themes (Figure 4).
Scientific and Technological Challenges
In addition to challenges highlighted in Figure 4, perceived lack of interest in DR among researchers was highlighted. The perception that DR is not innovative enough relates to several interconnected factors across the four categories, particularly scientific and technical complexity, commercial considerations, regulatory challenges, limited funding sources and publication bias.
Commercial and Market Challenges
Issues within this category related to lack of funding and additional commercial challenges were raised by all stakeholders (Figure 4). Pharmaceutical companies often prioritize novel drug development over DR due to a more favourable business model and potential for profit. DR raises the unique challenge of posing a high initial investment risk and in the case of on-patent drugs, intellectual properties (IP) queries.10,20
The market for repurposed drugs may be highly competitive, with multiple companies vying for market share with similar products. This can lead to pricing pressures and reduced profitability, particularly if there are already established treatments available for the repurposed indication. The market for repurposed drugs is also often limited to niche indications or subgroups of patients, again resulting in smaller revenue potential for pharmaceutical companies.
Clinical and Regulatory Challenges
Designing and executing clinical trials for repurposed drugs involves unique considerations in selecting appropriate treatment regimens, patient populations, stratification, and recruitment.
Patients may be hesitant to enrol in trials for drugs already approved for other indications, and recruitment faces competition from trials testing novel drugs or regimens.
DR for new indications requires navigating complex regulatory pathways, including obtaining approval for new clinical trials which may have novel designs implemented to appropriately address the need of the population. Regulatory requirements for repurposed drugs are not well known or understood, leading to uncertainty and delays in the approval process.
Social and Collaborative Challenges
DR faces scepticism from different stakeholder groups, including patients who may perceive it as less innovative or effective compared to novel treatments. Overcoming negative perceptions and building confidence in safety and efficacy of DR is essential.
Challenges and Hurdles Specifically Related to DR in PC
During the meeting, it was agreed that DR represents an attractive option for PC and a number of preclinical and clinical studies in the area have been conducted in the past and are currently ongoing (Table 1).8,13 Despite the increasing interest among drug developers for DR in oncology and PC, the number of repurposed medicines reaching market is low.20
While DR in PC might offer several advantages, including reduced costs and faster timelines compared to developing new drugs, there are significant challenges that limit its success as summarised above. One of the primary obstacles is the generally weak potency of repurposed drugs against the highly aggressive and therapy-resistant biology of PC. Repurposed drugs, originally designed for non-cancer indications, often lack the specificity and strength needed to overcome key oncogenic drivers in PC, such as KRAS mutations, enhanced DNA damage repair mechanisms, and metabolic reprogramming. Achieving therapeutic efficacy frequently requires higher doses, which can lead to unacceptable toxicity and poor patient tolerance. For instance, drug regimens exceeding 1–2 grams several times daily may not only be challenging for patients to adhere to but can also amplify systemic side effects, reducing the overall therapeutic benefit.
Moreover, the unique challenges of PC further complicate the application of DR. PC is characterized by its dense stromal microenvironment, which serves as a physical and biochemical barrier, impeding drug delivery and reducing the effectiveness of systemic therapies. This, combined with the tumour’s intrinsic resistance to cell death and its propensity for early metastasis, often diminishes the efficacy of repurposed drugs unless used as part of a well-optimized therapeutic strategy.
Despite these hurdles, DR remains an appealing approach for PC, particularly given the disease’s poor prognosis, limited treatment options, and urgent need for more accessible therapies. Unlike the protracted and costly development of new cancer drugs, DR leverages the established safety and pharmacokinetic profiles of existing medicines, which allows for faster progression to clinical trials and reduced development costs. However, the efficacy of these agents in PC is often limited by their original pharmacological design, as many were not developed with the specific requirements of targeting aggressive cancers in mind. To enhance the success of DR in PC, several strategies must be prioritized. Combination therapies represent a promising avenue, where repurposed drugs are used alongside chemotherapies (eg, FOLFIRINOX or gemcitabine), targeted therapies, or emerging modalities such as immunotherapy.21 For example, repurposed drugs that disrupt metabolic vulnerabilities in PC, such as glutamine dependency, could complement standard treatments to enhance tumour control. Similarly, drugs targeting the tumour microenvironment—such as those that modulate stromal interactions or improve immune recognition—could help overcome key barriers to treatment efficacy.
Beyond scientific innovation, addressing the structural and regulatory challenges, as further discussed below is essential.22 Regulatory pathways for investigator-initiated trials should be streamlined to facilitate faster testing of repurposed drugs in this hard-to-treat cancer. Additionally, policies that encourage off-label use and provide funding for independent clinical trials can help drive DR efforts forward. Given the limited financial incentives for pharmaceutical companies to invest in DR for PC, strong government involvement and public-private partnerships will be vital. Governments could also enact legislation that supports easier access to repurposed drugs and simplifies regulatory approvals for these agents in oncology.
Recommendations and Actions Needed
Improving success in DR for PC requires a multifaceted, collaborative approach that addresses the numerous challenges across the research and development pipeline. Different recommendations were made during the meeting and all participants agreed on three essential, major recommendations. Specific recommendations are summarized in Table 2.
 |
Table 2 Actions Needed to Accelerate and Improve Drug Repurposing (DR) in Pancreatic Cancer (PC) Highlighted by Different Stakeholders |
Need for Economic Investment/Funding
All meeting participants agreed on need for investment/funding from preclinical research through to biomarker discovery, clinical development and implementation into practice. Increased investment is needed for research into diagnosis, prevention and treatment, but also into supporting and encouraging collaborations across the PC community. Aligned with current thinking,23,24 it was agreed that, while cancer mortality in many tumour groups will fall over coming years, PC mortality is likely to remain unchanged, with a grim prognosis in that by 2030, PC will be the second leading cause of cancer-related deaths in Europe and the US. Such a high disease burden to healthcare systems, society and the individual should warrant major focus of public and private investments/funding.
Need for Education and Awareness
The need for education and awareness was highlighted. Enhanced education about the signs and symptoms of PC will increase the number of patients pursuing help and clinicians recognising the disease, improving early diagnosis rate and treatment outcomes. Campaigns initiated by PC organisations (eg, Campaign launched by Pancreatic Cancer UK (pancreaticcancer.org.uk/what-we-do/we-campaign-for-change/earlier-diagnosis/), Campaign launched by the Italian Foundation Nadia Valsecchi (fondazionevalsecchi.org/quanto-pesano-80-grammicampagna-di-sensibilizzazione-sul-tumore-al-pancreas/)) often aim to increase global awareness of PC with the hope of advocating for more research funding, seeking policy changes and importantly, highlighting key disease characteristics.
There is a significant level of scepticism regarding DR in the patient community which may stem from public perceptions of repurposed drugs as “second-best” treatments compared to novel therapies.25 This can impact the patient’s decision on treatment. It is essential for clinical staff to inform and empower patients on all available treatment options, including DR, to ensure they can make informed decisions about their care.
To fully explore the benefits of DR and encourage investment and collaborative efforts, educational tools and training campaigns targeting all stakeholders can be crucial.
While highlighting the value and potential benefits, it is equally important to explain that DR is complex and requires careful consideration of scientific, clinical, regulatory, and commercial factors.
Need of Collaborative Efforts
Collaborative, multidisciplinary efforts are critical in addressing the challenges associated with PC. Bringing together experts across multiple sectors, ie, diagnosis, research, therapeutic approaches, as has been the case in this MSM, helps generate insights on how best to develop therapies and improve patient outcomes. During the meeting the need for resource and data sharing (eg, sharing of patient samples, clinical data, biomaterials, research infrastructures) was emphasized.
Discussion
PC remains a global health challenge with one of the lowest survival rates among all major cancers, underscoring the urgent need for innovative therapeutic strategies. Despite advances in oncology, the development of effective treatments for PC continues to face significant scientific, clinical, and regulatory hurdles. DR represents a promising strategy in this landscape, offering potential advantages such as reduced costs and faster timelines. However, as highlighted in this report, DR’s application in PC is fraught with challenges that require coordinated efforts and unified collaboration.
PC’s unique biology poses significant obstacles to the success of repurposed drugs. Its dense stromal microenvironment and intrinsic therapy resistance reduce the effectiveness of systemic therapies, necessitating the exploration of combination regimens and advanced drug delivery methods. Moreover, the weak potency of repurposed drugs against key oncogenic drivers often necessitates high doses that are difficult to administer and poorly tolerated by patients. Overcoming these hurdles requires scientific innovation, strategic policymaking, and effective collaboration across sectors.
The REMEDi4ALL MSM underscored the importance of coordinated efforts to address these challenges and served as a starting point for driving collaboration and catalysing collective actions in tackling the intricate challenges associated with DR in PC.
Anecdotally, the meeting demonstrated significant value for participants, who identified the need for follow-up meetings with focused agendas addressing specific aspects of the dynamic PC and DR landscapes. These proposed actions, outlined in Table 3, highlight the critical need for ongoing collaboration. The REMEDi4ALL initiative is uniquely positioned to lead this effort, with its outreach and mandate enabling the organization of impactful discussions like the MSM. Moreover, this framework can be expanded to facilitate collaborations across industry and academia, with the ultimate goal of streamlining patient access to effective therapies.
 |
Table 3 Concrete Actions Implemented/Planned Within REMEDi4ALL to Accelerate and Improve Drug Repurposing (DR) in Pancreatic Cancer (PC) |
Beyond its immediate outcomes, the MSM serves as a model for how multi-stakeholder collaboration can catalyse progress in patient-centric drug development. Governments, academic institutions, and industry players should draw inspiration from this approach, fostering partnerships that align research and development efforts with patient needs. Importantly, policies must evolve to simplify regulatory pathways for investigator-initiated trials, support off-label use, and incentivize public-private partnerships in DR.
In conclusion, while DR offers immense potential in PC, its success will depend on overcoming biological, logistical, and systemic barriers. Multi-stakeholder collaboration, such as that exemplified by the REMEDi4ALL MSM, represents a critical first step toward addressing these challenges and advancing innovative solutions. We hope that the momentum generated by this meeting will serve as a catalyst for continued efforts, fostering a unified approach to tackling the intricate challenges associated with DR in PC and ultimately improving outcomes for patients worldwide.
Data Sharing Statement
No new data were generated or analysed in support of this article. All data are derived from sources in the public domain.
Acknowledgments
We thank all the speakers and contributors to the meeting for their time and considered input. We thank Gisela Pairó, Donald Lo and Martin de Kort for their valuable and constructive suggestions.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the REMEDi4ALL consortium, which has received funding from the European Union’s Horizon Europe research and innovation programme under grant agreement No 101057442.
Disclosure
The views expressed in the article reflect the views of the authors and are not intended to convey the views of their employers or affiliations. The authors declare that they have no competing interest in this work.
References
1. Pizot C, Dragomir M, Macacu A, Koechlin A, Bota M, Boyle P. Global burden of pancreas cancer: regional disparities in incidence, mortality and survival. J Health Inequalities. 2019;5:96–112. doi:10.5114/jhi.2019.87844
2. Wang S, Zheng R, Li J, et al. Global, regional, and national lifetime risks of developing and dying from gastrointestinal cancers in 185 countries: a population-based systematic analysis of GLOBOCAN. Lancet Gastroenterol Hepatol. 2024;9:229–237. doi:10.1016/S2468-1253(23)00366-7
3. Pourshams A, Sepanlou SG, Ikuta KS, et al. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2019;4:934–947. doi:10.1016/S2468-1253(19)30347-4
4. American Cancer Society. Cancer Facts & Figures 2023. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.
5. Pancreatic Cancer Across Europe. n.d Available from :https://ueg.eu/files/771/b7ee6f5f9aa5cd17ca1aea43ce84849.
6. Bertolini F, Sukhatme VP, Bouche G. Drug repurposing in oncology—patient and health systems opportunities. Nat Rev Clin Oncol. 2015;12:732–742. doi:10.1038/nrclinonc.2015.169
7. Li YY, Jones SJ. Drug repositioning for personalized medicine. Genome Med. 2012;4:27. doi:10.1186/gm326
8. Linehan A, O’Reilly M, McDermott R, O’Kane GM. Targeting KRAS mutations in pancreatic cancer: opportunities for future strategies. Front Med. 2024;11:1369136. doi:10.3389/fmed.2024.1369136
9. De Lellis L, Veschi S, Tinari N, et al. Drug repurposing, an attractive strategy in pancreatic cancer treatment: preclinical and clinical updates. Cancers. 2021;13:3946. doi:10.3390/cancers13163946
10. Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18:41–58. doi:10.1038/nrd.2018.168
11. Karres D, Lesa G, Ligas F, et al. Can a multistakeholder prioritization structure support regulatory decision making? A review of pediatric oncology strategy forums reflecting on challenges and opportunities of this concept. Clin Pharmacol Ther. 2020;108:553–556. doi:10.1002/cpt.1939
12. Croft NM, de Ridder L, Griffiths AM, et al. Paediatric inflammatory bowel disease: a multi-stakeholder perspective to improve development of drugs for children and adolescents. J Crohns Colitis. 2023;17:249–258. doi:10.1093/ecco-jcc/jjac135
13. Rebelo R, Polónia B, Santos LL, Vasconcelos MH, Xavier CPR. Drug repurposing opportunities in pancreatic ductal adenocarcinoma. Pharmaceuticals. 2021;14:280. doi:10.3390/ph14030280
14. Cros J, Raffenne J, Couvelard A, Poté N. Tumor heterogeneity in pancreatic adenocarcinoma. Pathobiology. 2018;85:64–71. doi:10.1159/000477773
15. Evan T, Wang VM-Y, Behrens A. The roles of intratumour heterogeneity in the biology and treatment of pancreatic ductal adenocarcinoma. Oncogene. 2022;41:4686–4695. doi:10.1038/s41388-022-02448-x
16. Gutiérrez ML, Muñoz-Bellvís L, Orfao A. Genomic heterogeneity of pancreatic ductal adenocarcinoma and its clinical impact. Cancers. 2021;13:4451. doi:10.3390/cancers13174451
17. Martens S, Lefesvre P, Nicolle R, et al. Different shades of pancreatic ductal adenocarcinoma, different paths towards precision therapeutic applications. Ann Oncol. 2019;30:1428–1436. doi:10.1093/annonc/mdz181
18. Michiels E, Madhloum H, Van Lint S, et al. High‐resolution and quantitative spatial analysis reveal intra‐ductal phenotypic and functional diversification in pancreatic cancer. J Pathol. 2024;262:76–89. doi:10.1002/path.6212
19. Yu Y, Yang G, Huang H, et al. Preclinical models of pancreatic ductal adenocarcinoma: challenges and opportunities in the era of precision medicine. J Exp Clin Cancer Res. 2021;40:8. doi:10.1186/s13046-020-01787-5
20. Mulder J, van Rossum T, Mariz S, et al. Orphan medicinal products for the treatment of pancreatic cancer: lessons learned from two decades of orphan designation. Front Oncol. 2021:11. doi:10.3389/fonc.2021.809035.
21. Weth FR, Hoggarth GB, Weth AF, et al. Unlocking hidden potential: advancements, approaches, and obstacles in repurposing drugs for cancer therapy. Br J Cancer. 2023;130(5):703–715. doi:10.1038/s41416-023-02502-9
22. Fierro AG, Pérez R, Chávez-Blanco A, Dominguez-Gomez GDuenas-Gonzalez A, Duenas-Gonzalez A. Does therapeutic repurposing in cancer meet the expectations of having drugs at a lower price? Clin Drug Investig. 2023;43(4):227–239. doi:10.1007/s40261-023-01251-0
23. Carioli G, Malvezzi M, Bertuccio P, et al. European cancer mortality predictions for the year 2021 with focus on pancreatic and female lung cancer. Ann Oncol. 2021;32:478–487. doi:10.1016/j.annonc.2021.01.006
24. Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated projection of US cancer incidence and death to 2040. JAMA Network Open. 2021;4:e214708. doi:10.1001/jamanetworkopen.2021.4708
25. Schultz É, Mignot L, Ward JK, Boaventura Bomfim D, Chabannon C, Mancini J. Public perceptions of the association between drug effectiveness and drug novelty in France during the COVID-19 pandemic. Therapies. 2022;77:693–701. doi:10.1016/j.therap.2022.05.001
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.