Back to Journals » Journal of Pain Research » Volume 18
Durability of Supplemental Nucleus Pulposus Allograft in Patients with Lumbar Discogenic Pain
Authors Costandi S, Beall DP , Davis TT, Amirdelfan K, Naidu RK , DePalma MJ, Yoon ES, Fleming JW, Block JE , Mekhail N
Received 18 January 2025
Accepted for publication 1 April 2025
Published 9 April 2025 Volume 2025:18 Pages 1901—1908
DOI https://doi.org/10.2147/JPR.S516571
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Dawood Sayed
Shrif Costandi,1 Douglas P Beall,2 Timothy T Davis,3 Kasra Amirdelfan,4 Ramana K Naidu,5 Michael J DePalma,6 Edward S Yoon,7 Jacob W Fleming,8 Jon E Block,9 Nagy Mekhail1
1Pain Management, Cleveland Clinic, Cleveland, OH, USA; 2Comprehensive Specialty Care, Edmond, OK, USA; 3Source Healthcare, Santa Monica, CA, USA; 4Boomerang Healthcare, Inc., Walnut Creek, CA, USA; 5MarinHealth Spine Institute, Larkspur, CA, USA; 6Virginia iSpine Physicians, Richmond, VA, USA; 7Interventional Radiology, Hospital for Special Surgery, New York, NY, USA; 8Vascular and Interventional Specialists, Dallas, TX, USA; 9Private Practice, San Francisco, CA, USA
Correspondence: Jon E Block, Private Practice, 2210 Jackson Street, Ste. 401, San Francisco, CA, 94115, USA, Tel +1 415-775-7947, Email [email protected]
Background: The objective of this study was to determine the degree of improvement in lumbar discogenic pain severity and associated back impairment in patients with chronic axial low back pain treated with intradiscally delivered allogeneic nucleus pulposus (NP) at up to two vertebral levels (L1-S1).
Methods: Prospective, single-arm clinical study conducted at 6 sites in the US involving 28 participants with discogenic pain (mean age: 44 ± 13 yrs) and modified Pfirrmann grade 3– 7 on magnetic resonance imaging. This report includes the final participant follow up at 24 months post procedure. Back pain severity was evaluated using an 11-point numeric rating scale (NRS) and back function using the Oswestry Disability Index (ODI). Minimal clinically important difference (MCID) and substantial clinical benefit (SCB) were set at ≥ 30% and ≥ 50% over baseline, respectively. The patient acceptable symptom state (PASS) threshold for pain severity was ≤ 3.
Results: The average improvement in back pain severity from 7.1 ± 1.6 at baseline to 3.6 ± 2.9 at 24 months was 43% (p< 0.001). Approximately 64% (14 of 22) of participants achieved both the MCID and SCB in back pain at 24 months, while nearly 55% (12 of 22) reported a 24-month back pain severity score of ≤ 3. The corresponding average decrease in ODI values was 53% (p< 0.001) with 73% (16 of 22) of participants achieving the MCID. At baseline approximately 82% (23 of 28) of participants reported severe or crippled back impairment compared to 18% (4 of 22) at 24 months (p< 0.001). There was no association between modified Pfirrmann grade, number of levels treated or Modic changes and any outcome (range: p=0.12 to 0.43).
Conclusion: This study provides evidence of clinically significant pain relief and functional improvement through 24 months of follow up after a single allogeneic NP supplementation procedure in patients with lumbar discogenic pain.
Keywords: nucleus pulposus, allograft, discogenic, back pain, intradiscal, degenerative disc disease
Introduction
There is a substantial research and development effort underway to evaluate minimally invasive intradiscal therapies to treat lumbar discogenic pain associated with intervertebral disc degeneration,1 and several products are currently being evaluated in pivotal clinical trials as a basis for regulatory approval.2 Much of the impetus for this effort stems from our understanding that degeneration of the lumbar spine begins within the intervertebral disc,3 which eventually precipitates further degenerative changes in the posterior spinal elements such as the facet joints and neural foramina.4 Therefore, it remains imperative to restore the natural structure of the disc as a means of preserving a healthy and functional spine for as long as possible to avert recurrent symptoms and the possibility of spinal surgery.
One promising approach is the direct supplementation of degenerated nucleus pulposus (NP) tissue with allogeneic NP. Based on the ability of the proteoglycans within the NP tissue to bind water, the strategy is to enhance the mechanical cushioning properties of the disc.5,6 Processed with minimal manipulation as an allograft, there is no better source of replacement proteoglycans than disc material itself.
The clinical effectiveness of intradiscal delivery of allogeneic NP is gauged not only by the degree of pain amelioration and functional improvement but also by the durability of the treatment effect. Therapeutic durability provides a key buffer that has the potential to retard further degenerative changes across the vertebral motion segment. This could have an important downstream advantage of delaying the need for more invasive interventions such as disc arthroplasty, interbody fusion with instrumentation or posterior column interventions to alleviate chronic symptoms.
Herein, we provide the 24-month patient reported outcomes following a single intradiscal procedure using a commercially available allogeneic NP supplement. All patients suffered chronic symptoms of lumbar discogenic pain refractory to conservative care prior to intradiscal treatment.
Methods
We conducted a prospective, single arm, multicenter clinical study at 6 sites in the US. The primary objective of this study was to determine the degree of improvement in lumbar discogenic pain severity and associated back disability in patients with chronic axial low back pain treated with intradiscally delivered allogeneic NP at up to two vertebral levels (L1-S1). This report includes the final participant follow up at 24 months post procedure. This trial was prospectively registered at ClinicalTrials.gov on December 30, 2021 (NCT05201287). The number of enrolled participants was in accordance with sample size requirements for feasibility studies.7
Study inclusion criteria were: age ≥ 18 years; body mass index (BMI) of < 35 kg/m2; and chronic lumbar discogenic pain of ≥ 6 months duration unresponsive to conservative management. Discogenic pain was defined using established signs and symptoms at physical exam.8 Specifically, all patients demonstrated axial midline low back pain in the absence of lower extremity motor/sensory/reflex changes with or without non-radicular/non-sciatic referred leg pain. Additional inclusion criteria included sitting intolerance, pain with forward flexion, and positive pain provocation using the sustained hip flexion maneuver.9 Study eligibility required a baseline back pain severity score of ≥6 on an 11-point (0 to 10) numeric rating scale (NRS) and a back function score of ≥40 to points on the Oswestry Disability Index (ODI). Moderate-to-severe degeneration of up to two intervertebral discs from L1 to S1 was confirmed by magnetic resonance imaging (MRI) based on a modified Pfirrmann grade 3–7 degree of disc degeneration, with or without Modic changes (grades 1 or 2).10 Discography was not required to confirm eligibility. Patients with other types/sources of low back pain such as facetogenic, vertebrogenic, neurocompressive, sacroiliac or radicular pain were excluded. All patients provided informed consent. The study was reviewed and approved by an independent institutional review board (IRB), Sterling IRB (Atlanta, GA, USA).
The target intervertebral disc(s) was treated with a single dose of VIA Disc NP (VIVEX Biologics, Inc., Miami, FL USA). This commercially available product consists of human allogeneic NP disc tissue that is lyophilized, and cryomilled to particles ≤106 µm in size. The micronized allograft tissue is then aliquoted into a volume size of 100 mg (± 10%), aseptically sealed, and terminally sterilized via electron-beam irradiation. The product is reconstituted at the time of the procedure with 2 mL of sterile saline for delivery into the target intervertebral disc(s) through a 20G cannula.
The procedure is undertaken with the patient under moderate conscious sedation using a local anesthetic at the cannula entry site. Fluoroscopic guidance is used to ensure correct placement of the delivery cannula. Briefly, a small gauge delivery cannula is advanced through Kambin’s triangle into the center of the intervertebral disc. A single intradiscal dose is administered to the affected disc(s) according to the product Instructions for Use (IFU).
Post-procedure clinical follow up was undertaken at 1, 3, 6, 12 and 24 months to evaluate patient reported outcomes and the occurrence of adverse events.
Patient reported outcomes are presented as means (95% CI) at baseline and at each follow-up interval. The overall improvement in clinical outcomes over baseline was assessed using repeated measures analysis of variance (ANOVA). The difference between baseline values and the 24-month endpoint was confirmed using the paired t-test, 2-tailed. NRS and ODI 24-month responder rates were calculated based on a minimal clinically important difference (MCID) of ≥30%. Substantial clinical benefit (SCB) of ≥50% improvement over baseline was computed for NRS11–13 Additionally, baseline and 24-month ODI values were categorized by functional impairment severity as minimal (0–20), moderate (21–40), severe (41–60), and crippled (61–80) and compared using the Wilcoxon signed rank test. The 24-month responder rate for NRS patient acceptable symptom state (PASS) score was also computed with a success threshold set at ≤3.14 Cross-tabulations were used to explore the association between all patient reported outcomes and baseline Pfirrmann grade (3–7), numbers of levels treated (1 vs 2) and presence/absence of Modic changes using Fisher’s exact test, 2-tailed. Adverse events were captured at each post-procedure follow up interval.
Results
Fifty-four patients were prescreened for potential study eligibility based on case history and 28 patients met all inclusion and exclusion criteria and were enrolled as study participants. Twenty-two participants provided complete 24-month patient reported outcomes. Table 1 provides background characteristics for all patients. Table 2 provides mean values for back pain and back function at each follow up interval.
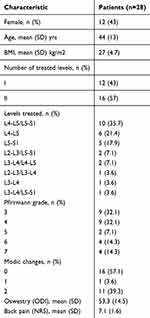 |
Table 1 Background Characteristics |
 |
Table 2 Mean (±SD) Back Pain and Back Function Values by Follow up Interval |
Study participants experienced an average overall improvement in back pain severity of 43% across all post-procedure follow up intervals (p<0.001). Figure 1 provides the mean (95% CI) NRS values at each interval reflecting a statistically significant decrease from baseline (7.1, 95% CI [6.5, 7.7]) to 24 months (3.6, 95% CI [2.3, 4.9]) (p<0.001). Approximately 64% (14 of 22) of participants achieved or exceeded the MCID in back pain severity at 24 months, with all of these participants (14 of 22) also realizing SCB reflecting a ≥ 50% improvement over pre-procedure pain levels. Almost 55% (12 of 22) of participants reported a 24-month back pain severity score of ≤3.
There was corresponding clinical improvement in back function scores across all post-procedure follow up intervals with an average decrease in ODI values of 53% (p<0.001) (Figure 2). Mean ODI values improved from 53 (95% CI [48, 59]) at baseline to 21 (95% CI [13, 29]) at 24 months (p<0.001). At the 24-month follow up visit, approximately 73% (16 of 22) of study participants reported a MCID in back function, reflecting an improvement of at least 30% compared to pre-procedural levels. Figure 3 illustrates a comparison of the baseline and 24-month distributions in ODI functional impairment severity scores. At baseline approximately 82% (23 of 28) of participants reported that their back impairment was severe or crippled. By 24 months, the percentage of patients reporting severe/crippled impairment was reduced to 18% (4 of 22), and the difference in the distributions was statistically significant (p<0.001).
There was no association between modified Pfirrmann grade, number of levels treated or presence/absence of Modic changes and any of the pain or functional outcomes (range: p=0.12 to 0.43).
There were 4 adverse events categorized as possibly related to the NP product and 4 categorized as possibly related to the procedure. All of these events were considered mild or moderate in severity and were resolved by 24 months postprocedure. There were 2 serious adverse events, which were categorized as definitely related to the procedure, and required additional medical care to manage. There were no secondary surgical interventions.
Discussion
The findings of this study confirm the previous 6-month follow up results in the same group of patients with maintenance of treatment effect extended to two years.15 At this final follow up interval, almost two-thirds of the participants reported a substantial clinical benefit (≥50%) in pain relief with more than one half exhibiting minimal residual low back pain with a score ≤3.16 These encouraging results were achieved after a single minimally invasive allogeneic NP supplementation procedure and bodes well for this approach to preserving the natural function of the intervertebral disc.
The recent issuance of specific International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnostic codes for lumbosacral discogenic pain associated with degenerative disc disease by the National Center for Health Statistics (NCHS) underscores the importance of developing and evaluating intradiscal therapies that effectively treat patients suffering from discogenic low back pain.17 Direct supplementation of tissue lost to degenerative disc disease with allogeneic NP represents a straightforward homologous replacement approach to retard or prevent further degeneration of the anterior column of the spine. Minimally invasive intradiscal NP treatment represents an enormous opportunity to improve spine care by delaying or avoiding surgical intervention and enhancing quality of life in patients with discogenic back pain.
The limitations of this study include a small sample size, lack of a concurrent active or placebo control group and absence of follow up imaging evidence of potential disc structural changes. These issues limit the generalizability of the encouraging clinical findings but provide ample support for subsequent investigations that address these shortcomings. We do speculate, however, that the two-year duration of treatment effect observed in the current study mitigates the possibility of a marked placebo effect as the primary driver of efficacy.
The NP allograft used in this study is a minimally manipulated, off-the-shelf product that provides a nonsurgical option that can be delivered through a cannula under fluoroscopic guidance without altering the normal anatomy of the spine. The two-year findings of this study suggest that clinical adoption of this procedure may help to bridge the current treatment gap for patients experiencing chronic moderate-to-severe lumbar discogenic pain and delay the need for more invasive surgical interventions.
Conclusion
Pathologic degeneration of the intervertebral disc can result in chronic lumbar discogenic pain. Direct supplementation of degenerated nucleus pulposus (NP) tissue with allogeneic NP results in sustained symptomatic improvement over a two-year duration of clinical followup. This represents an opportunity to bridge the treatment gap between failed conservative care and spine surgery for patients with lumbar discogenic pain.
Data Sharing Statement
Requests for data sharing can be made by contacting the corresponding author. Individual participant data that underlie the results reported in this article will be made available (after deidentification) from 9 to 36 months after article publication. Data sharing will be limited to investigators whose proposed use of the data has been approved by an independent review committee identified for this purpose.
Institutional Review Board Statement
All patients provided informed consent. The study was reviewed and approved by an independent institutional review board (IRB), Sterling IRB (Atlanta, GA, USA). The trial was conducted in accordance with the Declaration of Helsinki and prospectively registered at ClinicalTrials.gov on December 30, 2021 (NCT05201287). Permission was granted from the 6 clinical sites for participation/access to data during the course of the study.
Acknowledgments
Financial support for this work was provided by Vivex Biologics (Miami, FL, USA). Statistical analyses were conducted by Liwei Chen (Catalyst Clinical Research, Wilmington, NC USA).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by VIVEX Biologics, Inc., Miami, FL USA.
Disclosure
DPB is a scientific advisor to Vivex Biologics; received grants or contracts from Medtronic, Medical Metrics, Avanos, Relievant, Boston Scientific, Stryker, Sollis Pharmaceuticals, Simplify Medical, Lenoss Medical, Spine BioPharma, Eliem Therapeutics, Smart Soft, Tissue Tech, Vivex, Stratus Medical, Restorative Therapies, Kolon, TissueGene, Companion Spine, DiscGenics; royalties from VIVEX and IZI; consulting fees from Medtronic, Spineology, Merit Medical, Johnson & Johnson, IZI, Techlamed, Peterson Enterprises, Medical Metrics, Avanos, Boston Scientific, Sollis Pharmaceuticals, Simplify Medical, Stryker, Lenoss Medical, Spine BioPharma, Piramal, ReGelTec, Nanofuse, Spinal Simplicity, Pain Theory, Spark Biomedical, Micron Medical Corp, Bronx Medical, Smart Soft, Tissue Tech, RayShield, Stayble, Thermaquil, Vivex, Stratus Medical, Genesys, Abbott, Eliquence, SetBone Medical, Amber Implants, Cerapedics, Neurovasis, Varian Medical Systems, Companion Spine, DiscGenics, Discure, SpinaFX, PainTEQ; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Artio, Sophiris, Eleven Biotherapeutics, Flow Forward, Lenoss Medical, ReGelTech, Spark Biomedical; and support for attending meetings and/or travel from Medtronic, ReGelTec, Nanofuse, Talosix, Spinal Simplicity, Pain Theory, Spark Biomedical, Smart Soft, Tissue Tech, Bronx Medical, Thermaquil, Vivex, Genesys, SetBone Medical, Amber Implants, Cerapedics, SpinaFX. TTD received consulting fees from Abbott, Boston Scientific, Biotronik; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbott, Boston Scientific, Biotronik. KA reports minor options from Vivex, outside the submitted work. RKN received consulting fees from Vivex, Boston Scientific, Ferring Pharmaceuticals and Abbott. MJD is a scientific advisor to Vivex Biologics; received grants or contracts from Spine BioPharma, Restorative, Novartis, SPR, Saol, Paradigm; royalties from Springer; patents from iSpine Ingenuity. SC received grants or contracts from the Cleveland Clinic. ESY received consulting fees from Neurovasis. JWF received support for attending meetings and/or travel from Medtronic, Stryker, Nevro, Seattle Science Foundation, HMP Global, American Society of Neuroradiology, American Society of Spine Radiology; received stock or stock options from BackTable LLC. JEB received support for medical writing from Vivex Biologics; consulting fees from Vivex. NM received consulting fees from Vivex Biologics. The authors report no other conflicts of interest in this work.
References
1. Lorio MP, Tate JL, Myers TJ, Block JE, Beall D. Perspective on intradiscal therapies for lumbar discogenic pain: state of the science, knowledge gaps, and imperatives for clinical adoption. J Pain Res. 2024;17:1171–1182. doi:10.2147/JPR.S441180
2. Schneider BJ, Hunt C, Conger A, et al. The effectiveness of intradiscal biologic treatments for discogenic low back pain: a systematic review. Spine J. 2022;22(2):226–237. doi:10.1016/j.spinee.2021.07.015
3. Kushchayev SV, Glushko T, Jarraya M, et al. ABCs of the degenerative spine. Insights Imaging. 2018;9(2):253–274. doi:10.1007/s13244-017-0584-z
4. Fine N, Lively S, Seguin CA, Perruccio AV, Kapoor M, Rampersaud R. Intervertebral disc degeneration and osteoarthritis: a common molecular disease spectrum. Nat Rev Rheumatol. 2023;19(3):136–152. doi:10.1038/s41584-022-00888-z
5. Humzah MD, Soames RW. Human intervertebral disc: structure and function. Anat Rec. 1988;220(4):337–356. doi:10.1002/ar.1092200402
6. Iatridis JC, MacLean JJ, O’Brien M, Stokes IAF. Measurements of proteoglycan and water content distribution in human lumbar intervertebral discs. Spine. 2007;32(14):1493–1497. doi:10.1097/BRS.0b013e318067dd3f
7. Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10(2):307–312. doi:10.1111/j.2002.384.doc.x
8. Lorio MP, Beall DP, Calodney AK, Lewandrowski KU, Block JE, Mekhail N. Defining the patient with lumbar discogenic pain: real-world implications for diagnosis and effective clinical management. J Pers Med. 2023;13(5):821. doi:10.3390/jpm13050821
9. DePalma MJ, Ketchum J, Queler E, et al. Does sustained hip flexion, pelvic rock, or location of low back pain predict the etiology of low back pain? An interim analysis of 170 consecutive low back pain cases. Pain Med. 2009;10:947–955.
10. Abel F, Altorfer FCS, Rohatgi V, Gibbs W, Chazen JL. Imaging of discogenic and vertebrogenic pain. Radiol Clin North Am. 2024;62(2):217–228. doi:10.1016/j.rcl.2023.10.003
11. Glassman SD, Copay AG, Berven SH, Polly DW, Subach BR, Carreon LY. Defining substantial clinical benefit following lumbar spine arthrodesis. J Bone Joint Surg Am. 2008;90(9):1839–1847. doi:10.2106/JBJS.G.01095
12. Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain. Towards international consensus regarding minimal important change. Spine. 2008;33(1):90–94. doi:10.1097/BRS.0b013e31815e3a10
13. Asher AM, Oleisky ER, Pennings JS, et al. Measuring clinically relevant improvement after lumbar spine surgery: is it time for something new? Spine J. 2020;20(6):847–856. doi:10.1016/j.spinee.2020.01.010
14. Pham T, Tubach F. Patient acceptable symptomatic state (PASS). Joint Bone Spine. 2009;76(4):321–323. doi:10.1016/j.jbspin.2009.03.008
15. Beall DP, Davis TT, Amirdelfan K, et al. Nucleus pulposus allograft supplementation in patients with lumbar discogenic pain: initial 6-month outcomes from a prospective clinical pilot study. Pain Physician. 2024;27:E865–E871.
16. Fekete TF, Haschtmann D, Kleinstuck FS, Porchet F, Jeszenszky D, Mannion AF. What level of pain are patients happy to live with after surgery for lumbar degenerative disorders? Spine J. 2016;16(4 Suppl):S12–18. doi:10.1016/j.spinee.2016.01.180
17. Lorio MP, Yuan HA, Beall DP, Block JE, Andersson GBJ. The role of isass in evolving the spine code landscape: lumbar discogenic pain receives specific ICD-10-CM code. Int J Spine Surg. 2024;18(4):353–354. doi:10.14444/8622
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Perspective on Intradiscal Therapies for Lumbar Discogenic Pain: State of the Science, Knowledge Gaps, and Imperatives for Clinical Adoption
Lorio MP, Tate JL, Myers TJ, Block JE, Beall DP
Journal of Pain Research 2024, 17:1171-1182
Published Date: 18 March 2024




