Back to Journals » Risk Management and Healthcare Policy » Volume 18
Early Detection of Malignant Cells in Gastric Lavage via Hexokinase 2 and Single-Cell Sequencing for Gastric Cancer Diagnosis
Authors Qian P, Sun J, Zhao Z, Lu P
Received 19 December 2024
Accepted for publication 13 March 2025
Published 25 March 2025 Volume 2025:18 Pages 1011—1021
DOI https://doi.org/10.2147/RMHP.S510123
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kyriakos Souliotis
Peiyu Qian,1,2,* Jie Sun,3,* Zhenya Zhao,4 Peihua Lu5
1Institutes of Biomedical Sciences, Fudan University, Shanghai, 200032, People’s Republic of China; 2Minhang Fudan Medical Education Research Center, Minhang District Central Hospital, Fudan University, Shanghai, 201100, People’s Republic of China; 3Center of Clinical Research, the Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi Medical Center, Nanjing Medical University, Wuxi, 214023, People’s Republic of China; 4International Medical Care Center, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 201620, People’s Republic of China; 5Department of Oncology, the Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi Medical Center, Nanjing Medical University, Wuxi, 214023, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Peihua Lu, Department of Oncology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi Medical Center, Nanjing Medical University, Wuxi, 214023, People’s Republic of China, Tel +8613918164127, Email [email protected] Zhenya Zhao, International medical care Center, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 201620, People’s Republic of China, Tel +8615221091968, Email [email protected]
Objective: Gastric cancer represents a significant global health challenge due to its high prevalence and mortality rates, largely attributed to the limitations of current screening methods, such as endoscopy, which impede early diagnosis. This study presents an innovative method for early detection by identifying exfoliated tumor cells in gastric lavage, aiming to overcome challenges related to patient compliance and the variability in endoscopist expertise.
Methods: Hexokinase 2 (HK2), a metabolic marker, was utilized to identify exfoliated tumor cells with heightened glycolytic activity in gastric lavage fluid. The malignancy of these HK2-positive, high-glycolytic tumor cells was further validated using single-cell sequencing (SCS), specifically through genome-wide copy number variation analysis.
Results: A total of 60 individuals were assessed, including 10 patients with gastric cancer (9 at stage IA and 1 at stage IIA), 26 patients with precancerous lesions, 15 patients with benign gastric conditions, and 9 healthy controls. The HK2 assay demonstrated an 80% diagnostic sensitivity for stages IA and IIA of gastric cancer and a 96% diagnostic specificity in distinguishing benign conditions from healthy controls. Importantly, the assay exhibited 57% sensitivity for cases of severe dysplasia, underscoring its potential for early gastric cancer detection and preventive diagnostics.
Conclusion: The study highlights the feasibility of a novel gastric lavage-based HK2 assay, complemented by SCS for malignancy confirmation, as a highly accurate method for the early detection of gastric cancer. This approach offers a promising alternative to traditional gastroscopy, particularly for early-stage disease, potentially enhancing detection rates and improving patient outcomes.
Keywords: copy number variation, early diagnosis, gastric cancer, single cell sequencing, Warburg effect
Introduction
Gastric cancer ranks as the fifth most prevalent cancer globally and is the fourth leading cause of cancer-related mortality.1 Early-stage gastric cancer often presents without distinct clinical symptoms, leading to a significant number of cases being diagnosed at advanced stages, where the 5-year survival rate is < 30%.2,3 In contrast, patients with gastric cancer diagnosed at an early, resectable stage have a significantly improved 5-year survival rate of over 90%.4,5 Currently, endoscopy followed by histologic biopsy remains the gold standard for diagnosing gastric cancer.6,7 However, its diagnostic accuracy is influenced by the expertise of the endoscopists, with early-stage lesions often exhibiting only subtle mucosal changes or obscured by scabs, leading to missed diagnoses of gastric cancer and precancerous lesions in 20–40% of cases.8,9 Furthermore, the discomfort associated with gastroscopy limits the acceptance of endoscopy as a screening tool.10 Conventional serum biomarkers, such as carcinoembryonic antigen (CEA), carbohydrate antigen 19–9 (CA 19–9), and carbohydrate antigen 72–4 (CA 72–4), exhibit limited diagnostic value for early-stage gastric cancer due to their low sensitivity and specificity.11 Thus, there is a critical need for developing more sensitive, accurate, and non-invasive diagnostic methods that can assist endoscopists in the early detection of gastric cancer, offering significant clinical value.
In recent decades, advancements in liquid biopsy research have rapidly progressed, enabling the development of less invasive and more efficient diagnostic tools.12 However, reliably identifying malignant cells in body fluids remains a significant challenge. The presence of free cancer cells in the peritoneal cavity can signal or contribute to peritoneal metastasis, a poor prognostic indicator in advanced gastric cancer.13,14 Despite this, peritoneal lavage cytology offers limited diagnostic sensitivity, ranging from only 20–30%, primarily due to the scarcity of free tumor cells in peritoneal lavage samples from patients who are at an early-stage.15 Although immunohistochemistry and Polymerase Chain Reaction (PCR) techniques have enhanced diagnostic sensitivity, cytology alone remains suboptimal for accurate diagnosis.16,17
Compared to other solid tumors, circulating tumor cells (CTCs) are rarely found in the peripheral blood of patients with gastric cancer, presenting a challenge for early detection.18 Cancer cells, due to weakened cell-to-cell adhesion, are prone to detachment from primary lesions and are exfoliated into the stomach lumen, while normal cells typically remain intact.19,20 This characteristic facilitates the potential detection of exfoliated tumor cells in gastric lavage fluid, which can be obtained by rinsing the stomach with saline during routine endoscopy. Consequently, the detection of exfoliated tumor cells in gastric lavage fluid is emerging as a promising, non-invasive diagnostic method for the early detection of gastric cancer.21,22
A defining characteristic of cancer cells is their metabolic shift from oxidative phosphorylation to aerobic glycolysis, known as the “Warburg effect”, which supports rapid proliferation and survival. This metabolic reprogramming has been applied in cancer diagnostics, notably through positron emission tomography (PET).23 Hexokinase (HK), the first enzyme in glycolysis, initiates glucose phosphorylation via ATP, producing glucose-6-phosphate. Previous studies have identified hexokinase 2 (HK2), a hexokinase isoform, as a novel biomarker for detecting rare malignant cells characterized by heightened glycolytic activity in peripheral blood, urine, and malignant pleural effusion.24–26 Similarly, increased glycolytic activity and HK2 expression have been found in gastric cancer.27–29
Additionally, genome-wide copy number variations (CNVs) are frequently recognized as a hallmark of cellular malignancy, as they are rarely found in normal tissue. In 1992, Correa proposed a human model of gastric carcinogenesis, which is widely accepted to be a multi-step process: this includes chronic atrophic gastritis, intestinal metaplasia, intraepithelial neoplasia, and the progression to gastric cancer.30
In the 1960s, Japanese researchers first identified precancerous gastric lesions by morphology, describing these lesions as “atypia”.31 Subsequently, in 1975, western researchers introduced the term “dysplasia” to specifically classify these precancerous lesions.32 A 15-year follow-up study of patients with esophageal carcinoma further demonstrated an association between early CNVs and patient prognosis.33
Based on this premise, we hypothesize that the HK2 assay may serve as a diagnostic tool for gastric cancer by identifying rare, exfoliated tumor cells in gastric lavage from patients with early-stage gastric cancer (stages IA and IIA) and those with precancerous lesions. Initially, the HK2 assay demonstrated high accuracy in detecting high-glycolysis tumor cells (hgTCs) within gastric lavage samples. Subsequently, single-cell sequencing (SCS) was used to verify selected putative tumor cells through CNV profile analysis.
Materials and Methods
Patient Selection and Sample Collection
This study was conducted at the Affiliated Wuxi People’s Hospital of Nanjing Medical University between April 2021 and February 2022, in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Institutional Ethics Review Committee (No.KS202087), and written informed consent was secured from all participants.
A total of 60 patients undergoing diagnostic endoscopy were prospectively enrolled. Patients were categorized based on subsequent histopathological results into four groups: early-stage gastric cancer (n = 10), precancerous lesions (n = 26), benign gastric disorders (n = 15), and healthy individuals (n = 9).
Inclusion Criteria: Patients undergoing diagnostic endoscopy for suspected gastric pathology; Age between 18–80 years; No prior history of chemotherapy, radiotherapy, or gastric surgery; Exclusion Criteria: Patients with advanced gastric cancer beyond stage IIA; Presence of other malignancies; Severe systemic diseases or coagulopathy contraindicating endoscopic procedures; Patients who declined to provide informed consent.
To minimize contamination from tumor cells, gastric lavage was performed prior to submucosal resection in all cases. This ensured that exfoliated cells collected in the lavage fluid originated from the stomach lumen rather than surgical manipulation.
Gastric lavage was performed before pathology results were available, as part of the routine diagnostic workflow. A sterile saline solution (50 mL) was introduced into the stomach via the working channel of the endoscope, followed by gentle aspiration to collect lavage fluid. The samples were transported on ice and processed within 12 hours. Histopathological classification was conducted independently by two pathologists following the 8th edition of the International TNM Staging System.
Fabrication and Surface Modification of Polydimethylsiloxane (PDMS) Chips
Microwell chips were fabricated in PDMS using standard microfabrication soft lithography techniques. A silicon wafer was patterned with the photoresist SU-8 2050 to create a mold for replication. The PDMS prepolymer was prepared by mixing the components in a 10:1 ratio and then cast onto the lithographically patterned mold. After curing at 80°C for 2 hours, the PDMS component was separated from the mold. To modify the hydrophobic surface of the PDMS chip and enhance cell adhesion within the microwells, the chip was treated with oxygen plasma in a plasma cleaner for 2 minutes.
Gastric Lavage Sample Processing and HK2-Based Detection of Tumor Cells
Gastric lavage samples were initially filtered through a 10 μm cell strainer followed centrifugation at 400 g for 10 minutes. The supernatant was discarded, and cell pellets were resuspended in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA). To remove red and white blood cells, the cell suspension was filtered through a 5 μm cell strainer. Following recovery in PBS with 0.1% BSA, approximately 105 cells were added to prepared microwell chips for cell fixation and sedimentation. Cells on the slides were permeabilized using 0.5% Triton X-100 for 15 minutes and blocked with 3% BSA and 10% normal goat serum for 1 hour. Cells were incubated overnight at 4 °C with anti-HK2 antibody (1:100 dilution) and anti-pan-CK antibody (1:100 dilution). After washing with PBS, the cells were treated with fluorescein-conjugated secondary antibodies (1:400 dilution) for 1 hour and stained with DAPI for 5 minutes. Imaging of all cells was performed using the ImageXpress Micro XLS Widefield High Content Screening system (Molecular Devices) capturing both bright field and fluorescent channels to identify HK2+/CK+/DAPI+ cells, which were classified as putative high glycolytic tumor cells (hgTCs).
Single-Cell Sequencing of Genome-Wide Copy Number Alterations
Targeted single cells were retrieved using a motorized micromanipulator (XenoWorks) for subsequent single-cell sequencing. A Sutter P-2000 needle-drawing instrument was used to fabricate a 1 mm capillary glass tube, which was then drawn into a glass needle with an approximate diameter of 25 μm. Using image analysis software to identify the locations of the cells, the XenoWorks hydraulic system and robotic arm extracted the targeted positive cells from specific micropores on the chip. These cells were transferred into low-adsorption PCR tubes pre-loaded with 5 μL of cell lysate. Whole genome amplification (WGA) of the isolated single cells was conducted following a previously established protocol,20 using the MALBAC Single Cell WGA Kit (Yikon Genomics).
Statistical Analysis
Data analysis was conducted using GraphPad Prism 8. Comparisons between experimental groups were conducted using a two-tailed unpaired t-test, while Fisher’s exact test was used to analyze clinicopathological characteristics. P < 0.05 was considered statistically significant.
Results
Strategies for HK2 Assay and Single-Cell Sequencing Based on Gastric Lavage
To assess the feasibility and diagnostic accuracy of a gastric lavage-based HK2 assay for gastric cancer, a total of 60 participants were recruited and classified into four cohorts based on conventional histopathological results: early-stage cancer, precancerous lesions, benign diseases, and healthy controls. The clinicopathological characteristics of patients with early gastric cancer and precancerous lesions are detailed in Supplementary Table 1. The workflow for the gastric lavage-based HK2 assay, along with SCS for validation, is depicted in Figure 1.
During endoscopy, a sterile saline solution was introduced into the stomach of the patient through a specialized tube with assistance from nursing staff. Following the rinsing of the gastric cavity, the diluted lavage fluid, containing cells shed from the stomach and potentially from the esophagus, was collected in a liquid tank connected to a collection tube (Figure 1A). Cells from the lavage solution were then processed through cleaning and negative selection before being positioned onto a microchip for immunofluorescence staining.
The microwell chips, fabricated from PDMS, contained approximately 110,000 addressable wells, each 30 μm in diameter and depth, designed for holding cells. These microwells minimize cell loss during immunofluorescence staining and provide precise locations to facilitate the extraction of suspected tumor cells for subsequent genome amplification and sequencing. The identification of putative hgTCs in the gastric lavage samples was achieved using on-slide fluorescence multi-immunostaining for HK2, pan-CK (to exclude leukocytes), and DAPI (for nuclear staining). A test result was considered positive for HK2 when the number of hgTCs (defined as HK2+/CK+/DAPI+ cells) was greater than 1. To confirm malignancy, each putative hgTC was individually retrieved and subjected to low-depth whole-genome sequencing to characterize genome-wide CNV at the single-cell level (Figure 1B).
HK2 Protein Expression and Genomic Copy Number Variations in Gastric Cancer
Initially, the expression levels of HK2 in tumor tissues were compared to those in normal tissues using data from the TCGA database. The results revealed a significant upregulation of HK2 in gastric cancer tissues compared to normal tissues, indicating that HK2 may serve as a malignancy-associated marker in gastric cancer (Figure 2A). Subsequently, the HK2 levels in gastric cancer cell lines were compared to those in human leukocytes (WBC) using the Human Protein Atlas database. The findings demonstrated that the average HK2 levels in gastric cancer cell lines were significantly higher than those observed in WBCs (Figure 2B).
Furthermore, HK2 levels were directly measured in gastric cancer cell lines (HGC27 and AGS) and leukocytes from a healthy donor through immunofluorescent staining. The results confirmed elevated HK2 levels in the gastric cancer cell lines (Figure 2C), consistent with earlier reports. Leukocytes, commonly present in gastric lavage, were effectively distinguished from malignant gastric cancer cells by HK2 staining (Figure 2D).
Somatic CNVs are prevalent in tumors, and recurrent CNV patterns across multiple cells being characteristic of malignancy. In this study, CNV patterns in gastric cancer were analyzed using TCGA data. The mapping revealed that large genomic segments exhibited copy number gains or losses across all four stages of gastric cancer. Notably, CNV patterns were similar across stages T2, T3, and T4, indicating that genomic instability arises early in the development of gastric tumors (Figure 2E).
Diagnostic Accuracy of the Gastric Lavage-Based HK2 Assay for Gastric Cancer
An initial measurement was conducted on 9 gastric lavage samples from 9 healthy donors using the cell-based HK2 test to establish baseline HK2-positive counts. No HK2-positive counts were detected in these samples (Figure 3A and Table 1). This data provided a baseline reference for further comparisons. Subsequently, the cell-based HK2 assay was applied to samples from the benign group, with only 1 of 15 benign samples revealing HK2-positive counts, indicating HK2 as a highly specific marker for distinguishing between malignant and non-malignant gastric samples (Figure 3A and Table 1).
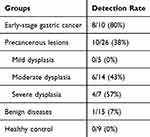 |
Table 1 Detection Rates of HK2-Positive Cells in Gastric Lavage Samples Across Study Groups |
Following this, 26 patients with precancerous lesions and 10 patients with early-stage gastric cancer were included in the study. Detection rates of HK2-positive cells via the gastric lavage-based HK2 assay were 0%, 43%, and 57% for patients in the mild, moderate, and severe dysplasia groups, respectively, among different precancerous lesions (Figure 3A and Supplementary Table 1). The detection rate of HK2-positive cells in early-stage gastric cancer cases was notably high at 80% (Figure 3A and B).
Additionally, HK2-positive malignant cells were statistically larger than leukocytes observed in the gastric lavage samples (Figure 3C). Further analysis of the correlation between HK2 assay results and clinicopathological characteristics in patients with early-stage gastric cancer and precancerous lesions revealed no significant correlation with sex, age, location, or tumor/lesion size (Figure 3D and Supplementary Table 1).
Validation of Malignancy in HK2-Derived hgTCs via Single-Cell Sequencing
Tumor cell genomes are highly unstable and prone to rearrangement. The rearranged genome often exceeds 1 kb in size, with submicroscopic structural changes revealing amplifications or deletions. Previous studies have demonstrated that CNV profiles can be used effectively to identify cancer cells. Gastric precancerous lesions are widely recognized as a risk factor for the development of intestinal-type gastric adenocarcinomas within multistep gastric carcinogenesis models. In this study, single-cell sequencing confirmed the presence of epithelial-derived hgTCs detected by the HK2 assay in gastric lavage samples from patients with moderate to severe dysplasia (Supplementary Table 1).
In one patient with moderate dysplasia (Patient 25), at least 13 HK2+/CK+/CD45−/DAPI+ cells, identified as hgTCs, were found in the gastric lavage sample, with additional CD45 staining used to exclude leukocytes (Figure 3E and Supplementary Figure 1). To confirm the malignancy of hgTCs, six cells were randomly selected for SCS based on a laboratory-established route for CNV analysis. Four out of six hgTCs displayed CNV patterns with gains and losses consistent with those of the primary tissue, providing strong evidence of the lesion origin (Figure 3F).
Additionally, amplification of chromosome 8 in the CNV profile, commonly observed in gastric cancer, further indicated the malignant nature of these cells (Figure 3F). These findings indicate that the HK2 assay, combined with SCS validation, can detect malignant cells in gastric lavage samples with high specificity and accuracy for gastric cancer, even at the precancerous stage.
Discussion
This study demonstrated the potential of a gastric lavage-based Hexokinase 2 (HK2) assay, complemented by single-cell sequencing (SCS), for the early detection of gastric cancer. The results revealed that the HK2 assay achieved an 80% diagnostic sensitivity for early-stage gastric cancer and a 96% specificity in distinguishing benign from malignant cases. Additionally, it identified 57% of cases with severe dysplasia, highlighting its potential in preventive diagnostics.
The findings align with previous studies investigating liquid biopsy approaches for gastric cancer detection. Prior research has explored the role of exfoliated tumor cells in gastric lavage fluid, emphasizing their diagnostic utility due to the detachment of malignant cells from the mucosal layer into the stomach lumen.34 Studies on circulating tumor cells (CTCs) have similarly reported their potential as non-invasive biomarkers for early cancer detection, though their presence in peripheral blood is less frequent for gastric cancer compared to other malignancies.18 Furthermore, gastric juice DNA methylation analyses have demonstrated promising results in differentiating malignant from non-malignant conditions.35 Our study builds on these findings by employing a metabolic biomarker, HK2, to enhance sensitivity and specificity in detecting early gastric malignancies.
Several studies have explored that alcohol dehydrogenase (ADH) activity, particularly class IV ADH, is significantly higher in gastric cancer tissues compared to healthy mucosa, while aldehyde dehydrogenase (ALDH) activity is lower, leading to increased acetaldehyde accumulation.36–38 This metabolic imbalance may contribute to carcinogenesis and suggests that ethanol metabolism plays a role in gastric cancer progression. Increased ADH IV activity has also been observed in other cancers, where it contributes to carcinogenic acetaldehyde production. Additionally, studies analyzing DNA methylation in gastric washings and exosomal DNA in gastric juice have identified epigenetic modifications linked to tumor progression. Our findings reinforce the connection between metabolic and genetic alterations in gastric cancer and support their potential as diagnostic markers. The high detection rate (80%) of early-stage gastric cancer through metabolic analysis of gastric lavage samples further highlights the relevance of non-invasive liquid biopsy techniques in cancer screening.
The HK2-based assay also offers an improvement over conventional peritoneal lavage cytology, which exhibits limited sensitivity (20–30%) in detecting free tumor cells in early-stage gastric cancer patients.15 While endoscopic biopsy remains the gold standard for gastric cancer diagnosis, it is invasive and can lead to a significant number of missed detections, particularly for early-stage lesions that exhibit subtle mucosal changes.9 By contrast, our proposed method offers a non-invasive alternative that can be performed alongside routine endoscopic procedures or independently using a newly developed gastric lavage collection device, improving accessibility and patient compliance.
A key advantage of the HK2 assay is its ability to identify highly metabolically active tumor cells, as HK2 is a known marker of the Warburg effect in cancer metabolism (Supplementary Figure 2).23 This is further supported by our SCS validation, which confirmed the malignant nature of HK2-positive cells through copy number variation (CNV) profiling. Chromosome 8 amplification, commonly associated with gastric cancer, was identified in exfoliated cells, providing additional evidence of their neoplastic origin (Supplementary Figure 1).
This study has some limitations. The sample size is relatively small, and larger multicenter studies are required to validate these findings. Additionally, while SCS provides definitive genomic confirmation of malignancy, it remains a complex and resource-intensive method. Future work should explore the development of a streamlined molecular diagnostic workflow that integrates metabolic profiling with simplified genomic assays.
Conclusion
This study established a novel test to detect exfoliated tumor cells in gastric lavage samples, aimed at diagnosing early-stage gastric cancer and precancerous lesions. Clinical studies, supported by single-cell sequencing, demonstrated that assays targeting elevated malignant cell metabolism can effectively screen for rare exfoliated tumor cells in gastric lavage, achieving high sensitivity and specificity for early gastric cancer and precancerous conditions. This minimally invasive approach holds promise as a pre-screening tool, potentially enhancing early detection when used before routine endoscopic examination and biopsy.
Abbreviations
HK2, Hexokinase 2; hgTCs, high glycolytic tumor cells; SCS, single-cell sequencing; CNV, copy number variation; CEA, carcinoembryonic antigen; CA 19-9, carbohydrate antigen 19-9; CT, computed tomography; CA 72-4, carbohydrate antigen 72-4; PCR Polymerase Chain Reaction; CTCs, circulating tumor cells; PET, positron emission tomography; WGA, whole genome amplification; PBS, Phosphate Buffered Saline; BSA, bovine serum albumin; CK, Cytokeratin; DAPI, 4’,6-diamidino-2-phenylindole; WBC, White blood cell.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). The study was approved by Ethics Committee of the Affiliated Wuxi People’s Hospital of Nanjing Medical University (No.KS202087). Written informed consent was obtained from all participants.
Acknowledgments
We acknowledge the support of the Department of Gastroenterology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Jiangsu Province, China.
This paper has been uploaded to Research Square as a preprint: https://www.researchsquare.com/article/rs-3494487/v1.
Funding
Health Commission of Minhang District, Shanghai, China (2021MHZ080).
Disclosure
The authors declare that they have no competing interests.
References
1. Bray F, Laversanne M, Sung H. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
2. Katai H, Ishikawa T, Akazawa K, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer. 2018;21(1):144–154. doi:10.1007/s10120-017-0716-7
3. Shen L, Shan Y-S, Hu H-M, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14(12):e535–e547. doi:10.1016/S1470-2045(13)70436-4
4. Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic resection compared with gastrectomy to treat early gastric cancer: a systematic review and meta-analysis. PLoS One. 2015;10(12):e0144774. doi:10.1371/journal.pone.0144774
5. Bang CS. Long-term outcomes of endoscopic submucosal dissection of undifferentiated-type early gastric cancer. Clin Endosc. 2021;54(2):143–144. doi:10.5946/ce.2021.044
6. Hong Y, Li X, Liu Z, et al. Predicting tumor invasion depth in gastric cancer: developing and validating multivariate models incorporating preoperative IVIM-DWI parameters and MRI morphological characteristics. Eur J Med Res. 2024;29(1):431. doi:10.1186/s40001-024-02017-w
7. S MH, Cnuhgi C. Cnuhgi C.Endoscopic prediction of depth of tumor invasion in early gastric cancer in the upper third of the stomach. J Clin Oncol. 2023;41(4_suppl):406. doi:10.1200/JCO.2023.41.4_suppl.406
8. Hosokawa O, Hattori M, Douden K, Hayashi H, Ohta K, Kaizaki Y. Difference in accuracy between gastroscopy and colonoscopy for detection of cancer. Hepatogastroenterology. 2007;54(74):442–444.
9. Ren W, Yu J, Zhang ZM, Song YK, Li YH, Wang L. Missed diagnosis of early gastric cancer or high-grade intraepithelial neoplasia. World J Gastroenterol. 2013;19(13):2092–2096. doi:10.3748/wjg.v19.i13.2092
10. Yao K, Uedo N, Muto M, et al. Development of an E-learning system for the endoscopic diagnosis of early gastric cancer: an international multicenter randomized controlled trial. EBioMedicine. 2016;9:140–147. doi:10.1016/j.ebiom.2016.05.016
11. Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the task force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17(1):26–33. doi:10.1007/s10120-013-0259-5
12. Ho HY, Chung KK, Kan CM, Wong SC. Liquid biopsy in the clinical management of cancers. Int J mol Sci. 2024;25(16):8594. doi:10.3390/ijms25168594
13. Chong X, Li Y, Lu J, Feng X, Li Y, Zhang X. Tracking circulating PD-L1-positive cells to monitor the outcome of patients with gastric cancer receiving anti-HER2 plus anti-PD-1 therapy. Hum Cell. 2024;37(1):258–270. doi:10.1007/s13577-023-00990-8
14. Li W, Zhang X, Yang Y, et al. Circulating tumor cells are a good predictor of tumor recurrence in clinical patients with gastric cancer. Sci Rep. 2024;14(1):12758. doi:10.1038/s41598-024-63305-3
15. Cheng R, Peng Y, Sun X, Zhang S, Li P. Circulating tumor cells as diagnostic markers of early gastric cancer and gastric precancerous lesions. Oncology. 2023;101(8):512–519. doi:10.1159/000531323.
16. Shen K, Chen B, Gao W. Integrated single-cell RNA sequencing analysis reveals a mesenchymal stem cell-associated signature for estimating prognosis and drug sensitivity in gastric cancer. J Cancer Res Clin Oncol. 2023;149(13):11829–11847. doi:10.1007/s00432-023-05058-6
17. Hasbahceci M, Akcakaya A, Guler B, Kunduz E, Malya FU, Muslumanoglu M. Use of peritoneal washing cytology for the detection of free peritoneal cancer cells before and after surgical treatment of gastric adenocarcinoma. J Cancer Res Ther. 2018;14(6):1225–1229. doi:10.4103/0973-1482.184518
18. Uenosono Y, Arigami T, Kozono T, et al. Clinical significance of circulating tumor cells in peripheral blood from patients with gastric cancer. Cancer. 2013;119(22):3984–3991. doi:10.1002/cncr.28309
19. Goto O, Shimoda M, Sasaki M, et al. Potential for peritoneal cancer cell seeding in endoscopic full-thickness resection for early gastric cancer. Gastrointest Endosc. 2018;87(2):450–456. doi:10.1016/j.gie.2017.08.036
20. Watanabe Y, Kim HS, Castoro RJ, et al. Sensitive and specific detection of early gastric cancer with DNA methylation analysis of gastric washes. Gastroenterology. 2009;136(7):2149–2158. doi:10.1053/j.gastro.2009.02.085
21. Abe H, Rokutan H, Totoki Y, et al. Lost expression of AT-rich interaction domain 1A in the gastric mucosa-A constituent of field cancerization in the stomach. Pathol Int. 2023;73(6):234–245. doi:10.1111/pin.13320
22. Ji X, Liu W, Wu X, et al. Gastric juice piR-015551: a promising prognostic biomarker for gastric cancer. J Clin Oncol. 2024;42(16_suppl):e16074. doi:10.1200/JCO.2024.42.16_suppl.e16074
23. Gallamini A, Zwarthoed C, Borra A. Positron emission tomography (PET) in oncology. Cancers. 2014;6(4):1821–1889. doi:10.3390/cancers6041821
24. Lei Y, He L, Li Y, Hou J, Zhang H, Li G. PDLIM1 interacts with HK2 to promote gastric cancer progression through enhancing the Warburg effect via Wnt/β-catenin signaling. Cell Tissue Res. 2024;395(1):105–116. doi:10.1007/s00441-023-03840-z
25. Shima T, Taniguchi K, Inomata Y, Arima J, Lee SW. Glycolysis in gastrointestinal stromal tumor: a brief overview. Neoplasia. 2024;55:101022. doi:10.1016/j.neo.2024.101022
26. Yu Y, Jiang Y, Glandorff C, Sun M. Exploring the mystery of tumor metabolism: Warburg effect and mitochondrial metabolism fighting side by side. Cell Signal. 2024;120:111239. doi:10.1016/j.cellsig.2024.111239
27. Ohki A, Abe N, Yoshimoto E, et al. Gastric washing by distilled water can reduce free gastric cancer cells exfoliated into the stomach lumen. Gastric Cancer. 2018;21(6):998–1003. doi:10.1007/s10120-018-0824-z
28. Li R, Mei S, Ding Q, Wang Q, Yu L, Zi F. A pan-cancer analysis of the role of hexokinase II (HK2) in human tumors. Sci Rep. 2022;12(1):18807. doi:10.1038/s41598-022-23598-8
29. Wu J, Hu L, Wu F, Zou L, He T. Poor prognosis of hexokinase 2 overexpression in solid tumors of digestive system: a meta-analysis. Oncotarget. 2017;8(19):32332–32344. doi:10.18632/oncotarget.15974
30. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--first American cancer society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52(24):6735–6740.
31. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–112. doi:10.1007/s10120-011-0041-5.
32. Schlemper RJ, Hirata I, Dixon MF. The macroscopic classification of early neoplasia of the digestive tract. Endoscopy. 2002;34(2):163–168. doi:10.1055/s-2002-19855
33. Killcoyne S, Gregson E, Wedge DC, et al. Genomic copy number predicts esophageal cancer years before transformation. Nat Med. 2020;26(11):1726–1732. doi:10.1038/s41591-020-1033-y
34. Virgilio E, Giarnieri E, Montagnini M, et al. Analyzing gastric lavage of gastric cancer patients: a prospective observational study on cytopathology and determination of intragastric CEA, CA 19.9, CA 72.4, and CA 50. Acta Cytol. 2016;60(2):161–166. doi:10.1159/000445765
35. Yamamoto H, Watanabe Y, Oikawa R, et al. BARHL2 methylation using gastric wash DNA or gastric juice exosomal DNA is a useful marker for early detection of gastric cancer in an H. pylori-independent manner. Clin Transl Gastroenterol. 2016;7(7):e184. doi:10.1038/ctg.2016.40
36. Jelski W, Chrostek L, Szmitkowski M. The activity of class I, III, and IV of alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in gastric cancer. Dig Dis Sci. 2007;52(2):531–535. doi:10.1007/s10620-006-9454-0
37. Jelski W, Chrostek L, Zalewski B, Szmitkowski M. Alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) activity in the sera of patients with gastric cancer. Dig Dis Sci. 2008;53(8):2101–2105. doi:10.1007/s10620-007-0135-4
38. Jelski W, Orywal K, Laniewska M, Szmitkowski M. The diagnostic value of alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) measurement in the sera of gastric cancer patients. Clin Exp Med. 2010;10(4):215–219. doi:10.1007/s10238-010-0097-2
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




