Back to Journals » International Journal of Nanomedicine » Volume 20
Eco-Friendly Synthesized Carbon Dots from Chinese Herbal Medicine: A Review
Authors Zhao Y, Li Y, Li D, Yuan H, Shen C
Received 13 October 2024
Accepted for publication 8 February 2025
Published 12 March 2025 Volume 2025:20 Pages 3045—3065
DOI https://doi.org/10.2147/IJN.S497892
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. RDK Misra
Yusheng Zhao, Yucong Li, Dawei Li, Huageng Yuan, Chuanan Shen
Senior Department of Burns and Plastic Surgery, the Fourth Medical Center of Chinese PLA General Hospital, Beijing, 100048, People’s Republic of China
Correspondence: Chuanan Shen, Email [email protected]
Abstract: Chinese herbal medicines and their extracts will produce nano-components of charcoal drugs after high-temperature carbonization, and the process is similar to that of carbon dots (CDs). Chinese herbal medicine-derived CDs (CHM-CDs) are a new carbon-based nanomaterial with a particle size of less than 10 nm discovered in charcoal drugs in recent years. CHM-CDs possess a range of beneficial traits, such as minimal toxicity, strong water solubility, superior biocompatibility, and remarkable photoluminescence capabilities. Additionally, they exhibit multifaceted pharmacological activity in the absence of drug loading. Over the past half-decade, numerous publications have presented evidence suggesting that CHM-CDs exhibit a wide array of pharmacological effects. These primarily encompass hemostatic capabilities, neuroprotection, anti-infective, antitumor, immunomodulatory effects and hypoglycemic activity. Notably, they have been associated with circulatory system, digestive system, nervous system, immune system, endocrine system, urinary system and skeletal system. This article systematically reviews the modern pharmacological effects and potential mechanisms of CHM-CDs, offering insights into current challenges and proposing directions for future advancements. As such, it serves as a vital reference for the clinical application of CHM-CDs.
Keywords: Chinese herbal medicines, carbon dots, pharmacological effects, mechanisms
Introduction
Originally discovered by Scrivens in 2004, carbon dots (CDs) are zero-dimensional nanomaterials featuring a carbon framework and a diameter of under 10 nanometers.1 As an innovative form of carbon-based nanomaterials, CDs are distinguished by their notable features, including strong photoluminescence,2 excellent water solubility,3 good biocompatibility,4 tunable chemical properties,5 and photochemical stability.6 They have sparked significant interest across multiple sectors, including bioimaging,7 biosensing,8 biological monitoring,9 drug delivery10 and cancer therapy.11 With the continuous development of nanomedicine, CDs are expected to become an important tool in future medical diagnosis and treatment.12 Therefore, in-depth research into the biomedical properties of CDs and further improvements in their preparation and functionalization strategies will provide more innovative solutions for the practical applications of nanomedicine.
In recent years, researchers have concentrated on refining the techniques for creating these materials and broadening their range of uses, placing greater emphasis on the investigation of synthetic raw materials derived from both chemical and natural substances. At present, chemically synthesized CDs have the advantages of fast preparation speed and high yield, their potential toxicity and complex synthesis process remain the main limiting factors in their biomedical applications. In contrast, biomass-based CDs show greater potential due to their low toxicity and high biocompatibility.13 Furthermore, by precisely controlling the synthesis conditions, the physicochemical properties of the CDs can be adjusted to meet specific application requirements. Researchers are turning their attention to green precursors with specific therapeutic properties after considering the need for low toxicity and clinical safety application.14 Compared with chemically derived CDs, Chinese herbal medicine-derived CDs (CHM-CDs) have the advantages of abundant raw material sources, simple preparation methods, good biocompatibility, good water solubility, low toxicity, and low cost, and are an ideal CDs precursor material selection.15,16 More notably, Chinese herbal medicine (CHM) is rich in a variety of active ingredients that make them a direct route to heteroatoms.
As illustrated in Figure 1, currently, hydrothermal method and pyrolysis method with the advantage of one-bond synthesis are the main means of preparing CHM-CDs, which further broadens the application range of CHM-CDs. Different process parameters change the chemical bond splitting mode of various chemical components in the original CHM, which in turn leads to changes in the particle size, crystal structure and biological activity of the formed CHM-CDs.17 CHM-CDs eco-friendly synthesized from different precursors and under different conditions exhibit varying characteristics, primarily in terms of size, charge, and chemical groups.18 Due to their high surface area and sp2 kernel characteristics, CHM-CDs can be combined with various hydrophobic molecules through π-π* accumulation or electrostatic interaction, which further increases water solubility.19,20 Therefore, the poorly soluble CHM is carbonized to extract its derived CHM-CDs, which can improve the physical properties such as poor water solubility while retaining the original biological activity or reducing toxic side effects, and improve the bioavailability of CHM.
 |
Figure 1 The flowchart for the synthesis process of CHM-CDs. |
In this review, we systematically focused on discussing the modern pharmacological activities and mechanisms of CHM-CDs. Due to the different content of active ingredients in Chinese herbal medicines, the CHM-CDs derived from them also exhibits a variety of biological activities. The pharmacological activities and potential mechanisms of CHM-CDs were discussed in terms of its effects on circulatory system, digestive system, nervous system, immune system, endocrine system, urinary system and skeletal system. Finally, we discuss the prospects and challenges of CHM-CDs.
Precursor Sources of CHM-CDs
The precursor of CHM-CDs is derived from charcoal Chinese herbal medicine (C-CHM), a product of pyrolysis of natural green CHM. C-CHM is a distinctive form of CHM exhibiting broad pharmacological effects, formulated through a similar carbonization process applied to different base herbal medicine. It has a long history of wide clinical application. Its applications span a wide array of common disorders in internal, external, gynecological, and pediatric medicine, including but not limited to hemorrhage, trauma, carbuncles, stroke, rheumatic disorders, measles, eczema, menstrual disorders in women, cough in children, convulsions, and food stagnation. Its unequivocal efficacy has been validated through generations of clinical practice and is continually substantiated by contemporary pharmacological research.21,22
Records of the Medicinal Use of C-CHM
The earliest known utilization of C-CHM is recorded in the Fifty-Two Diseases Prescription, a seminal work from the Qin and Han dynasties. As the most ancient extant medical prescription book, it carries a historical legacy surpassing two thousand years. The Fifty-Two Diseases Prescription recorded 31 kinds of charcoal medicine, including human hair, Fuligo Plantae, Velvet Antler, and Achyranthis Bidentatae Radix, which were mainly used to treat more than 10 kinds of diseases such as wounds, abscesses, epilepsy, and urinary retention. The preparation technology of C-CHM began to be used in the Han Dynasty, the record of “stir-fried charcoal retained nature” in Synopsis of the Golden Chamber is considered to be the first quality standard for the preparation of C-CHM. During the Song and Yuan Dynasty, Miraculous book of Ten Recipes recorded the famous hemostatic formula called Ten Ashes Formula for the treatment of hemoptysis in consumptive disease. The theory of stir-fried carbon for hemostasis was preliminarily established and continues to be extensively employed in contemporary clinical practice. The Ming and Qing dynasties offer a more comprehensive documentation of C-CHM, during which the practical application and theory of stir-fried carbon for hemostasis were further refined. As of 2024, the Pharmacopoeia of the People’s Republic of China has included 27 variations of C-CHM. In conclusion, the application of C-CHM represents a significant innovation, with its precise therapeutic effects contributing to its lasting relevancy. Nonetheless, due to the current deficiency in understanding the efficacious substances and mechanisms underlying charcoal medicines, their utilization remains somewhat constrained.
Breakthrough in CHM-CDs
Over the years, researchers have tried to elucidate the mechanism of enhancement or change of medicinal properties after high-temperature carbonization of C-CHM from the perspective of small molecule compounds, which is roughly divided into the following aspects: (1) the changes of calcium and magnesium ions play a hemostatic effect;23 (2) the change of the content of small molecules such as tannin and flavonoids is closely related to the change of their biological activity;24 (3) generation of activated carbon.25 These viewpoints are only applicable to the inference that a small number of CHM produce medicinal effects after carbonization, and there is still a lack of unified and recognized basic research standards for effective materials. Therefore, many researchers speculate that new substances may be formed during the process of stir-frying charcoal, and the emergence of these substances provides a potential breakthrough for understanding their “stir-fried charcoal retained nature” characteristics. High-temperature carbonization is the core step in forming the active ingredients of charcoal medicine, which was detailed in the ancient text Lei Gong’s Treatise on the Preparation of Medicines. It describes the process of placing traditional Chinese medicinal materials in a sealed container and processing them under high-temperature, oxygen-deficient conditions, with very strict control over temperature and time, similar to the modern technique for preparing CDs. From the perspective of preparation craftsmanship, linking the potential new substances that may appear during the preparation of charcoal medicine with the emerging nanomaterial CDs offers an innovative viewpoint and scientific research method for exploring the key material basis behind the “stir-fried charcoal retained nature” effect of traditional Chinese medicine. The integration of advanced technologies such as nanomedicine with the natural products of traditional medicine offers a unique opportunity for the complementarity between traditional and modern medical practices.26,27 The discovery of CHM-CDs is an innovative breakthrough in the material basic research of C-CHM, which is no longer limited to the thinking constraints of the original small molecule compounds as pharmacodynamic active ingredients, and solves the scientific problems of the basis of C-CHM from the perspective of nanomaterials.
The clinical application of C-CHM has always been a focus of attention in the field of traditional Chinese medicine, and carbonization is a key process for improving its pharmaceutical properties. The evolution of this preparation method has transitioned from traditional methods to more scientifically validated methods, combining modern technology with ancient wisdom. According to the existing research, the green synthetic CHM-CDs shows a wide range of pharmacological activities, with good water solubility, simple synthesis method, non-toxicity, environmentally friendly and low cost. At present, international cooperation in the field of CHM-CDs is still in its infancy. Professor Zhao Yan’s team at Beijing University of Chinese Medicine, where I worked during my doctoral studies, started and continued to study the biological activity of CHM-CDs, and is also the author with the most paper output and citation times.18 The discovery of CHM-CDs represents an innovative breakthrough in the study of the material basis of C-CHM, breaking free from the restrictive thought that only small molecule compounds are the active components of pharmacological effects, and providing a new perspective to answer the scientific puzzle of the material basis of C-CHM. The discovery and development of CHM-CDs have opened up new avenues for clinical treatment and provided a promising direction for the application of these ancient therapies in contemporary medicine.
Pharmacological Effect of CHM-CDs
Previous studies28,29 have demonstrated that CHM-CDs serve as the fundamental material basis for the C-CHM activity. Different types of CHM-CDs exhibit variations in their physicochemical properties and biological activities, and show distinct therapeutic effects on specific diseases. As shown in Table 1 and Figure 2, current research indicates that CHM-CDs hold significant potential in the treatment of diseases in the circulatory, digestive, nervous, immune, endocrine, urinary and skeletal systems.30,31 This section aims to explore the pharmacological characteristics and mechanisms of action of these CHM-CDs in order to optimize their clinical application and promote their translational use.
 |
Table 1 Pharmacological Effects of Carbon Dots Derived from Chinese Herbal Medicine |
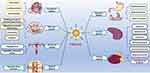 |
Figure 2 Schematic of the main pharmacological effects for CHM-CDs. |
Impact on the Circulatory System
Hemostasis Effect
C-CHM, as a special processed Chinese medicine product under high temperature conditions, was first used in ancient times to the treatment of hemoptysis. Currently, many scholars have confirmed that CHM-CDs were an effective material basis for hemostatic effect in charcoal drugs, and their potential mechanisms have been preliminarily explored.32,33 Coagulation and anticoagulation system, fibrinolysis and anti-fibrinolysis system have always been regarded as a contradictory and mutually restrictive dynamic equilibrium system in blood.34 Once the above balance is broken, the body will have bleeding or thrombotic diseases. Among them, the process of hemostasis involves promoting vasoconstriction, activating the coagulation system, inhibiting the activity of the fibrinolytic system, and promoting platelet (PLT) aggregation.35 Due to the participation of various coagulation factors in the coagulation process, there are three pathways to activate a series of coagulation factors in the stage before the formation of thrombin, namely the intrinsic coagulation pathway, the extrinsic coagulation pathway and the common coagulation pathway.36 Activated partial thromboplastin time (APTT) and prothrombin time (PT) are associated with intrinsic and extrinsic coagulation pathways, respectively, while the common coagulation pathway and the activity of promoting the conversion of FIB to fibrin in plasma are related with thrombin time (TT) and fibrinogen (FIB).
CDs derived from Pollen Typhae,37 Junci Medulla,38 Schizonepetae Herba,39 Schizonepetae Spica,40 Cirsium Setosum,41 Cirsii Japonici,42 Selaginella Tamariscina,43 Descurainiae Semen,44 Phellodendri Cortex,45 Scutellariae Radix,46 Lotus Leaf,47 Dryopteridis Crassirhizomatis Rhizoma48 and egg yolk oil49 significantly shorten the bleeding time of traumatic hemorrhagic animal models and have a significant hemostatic effect. In addition, the hemostatic effect of Junci Medulla Carbonisata - based CDs on snake venom-induced hemorrhage model in mice is also outstanding. There is a common trend for the reported mechanisms by which CHM-CDs exert hemostatic effects, namely, activation of the fibrinogen system and promotion of platelet aggregation to exert hemostatic effects. However, the overall effects are not completely consistent. Among them, the CDs derived from Junci Medulla,38 Schizonepetae Herba,39 Cirsium Setosum41 and Selaginella Tamariscina43 can reduce the PT value, indicating that they can stimulate the extrinsic coagulation pathway to exert a hemostatic effect. The CDs in Pollen Typhae,37 Scutellariae Radix46 and egg yolk oil49 can reduce the APTT value, indicating that they can stimulate the endogenous coagulation pathway to achieve hemostasis. More interestingly, the CDs derived from Schizonepetae Spica,40 Cirsii Japonici,42 Phellodendri Cortex,45 and Dryopteridis Crassirhizomatis Rhizoma48 had no significant effect on APTT and TT values, which may be related to the properties of CDs prepared from different sources, such as surface charge, type of group.
Cardioprotective Effect
Acute myocardial infarction is a common cardiovascular disease, mainly caused by the rapid occlusion of the coronary arteries leading to insufficient oxygen supply.50 Dong et al51 reported that CDs derived from Curcumae Radix can effectively alleviate symptoms of isoproterenol-induced myocardial infarction in rats, reducing the elevation of the ST segment on electrocardiograms and the area of myocardial infarction. Additionally, CRC-CDs can increase the ejection fraction and shorten the fractional shortening of the heart. Their mechanism of action may be related to enhancing the antioxidant capacity of cardiac tissue and reducing apoptosis of cardiomyocytes.
Anti-Frostbite Effect
Frostbite caused by cold environments can cause varying degrees of damage to cells and tissues, but there is a lack of corresponding interventions.52 Kong et al53 reported that AAFC-CDs can significantly improve stiffness in frostbitten mice. In multiple experiments simulating the refreezing and rewarming cycle of ice baths, AAFC-CDs effectively reduced tissue damage and ear frostbite caused by cold, and showed the ability to improve the body’s tolerance to frostbite and alleviated the stiffness caused by frostbite. Further research found that AAFC-CDs may reduce the concentration of inflammatory factors and provide energy to the body under frostbite conditions by reducing the increase in blood sugar caused by frostbite.
Impact on the Digestive System
Hepatoprotective Effect
The liver is a key hub of many physiological processes, and plays many important roles in metabolism, detoxification, and hematopoietic production.54 Liver injury is defined as acute liver dysfunction caused by viral infection, hepatotoxicity, toxic substances, or hepatic ischemia-reperfusion.55 Alcoholic liver injury is mainly the result of oxidative stress, inflammatory mediators and nutritional imbalance directly or indirectly induced by ethanol and its derivatives metabolism.56 Zhao et al57 reported that CDs derived from Vaccariae Semen alleviated the abnormal contents of ALT, AST, TBA and ALP in alcohol-induced liver injury mice. Carbon tetrachloride (CCl4) can induce lipid peroxidation by activating liver microparticle somatic pigment P450 to produce free radicals and covalently bind to macromolecules in liver cells.58 It has been reported that CDs derived from Paeoniae Radix Alba,59 Vaccaria Semen60 and Curcumae Radix61 improved the abnormal condition of ALT, AST, TBA and TBIL in CCl4-induced liver fibrosis mice, and the mechanism of its anti-liver fibrosis may be related to reducing inflammation and regulating TGF-β/Smad signaling pathway. Moreover, Junci Medulla-based CDs (JMC-CDs)38 can inhibit the increase of ALT, AST, ALP and TBIL induced by snake venom in the process of liver injury induced by snake venom. Platycodon Grandiflorum-based CDs62 and Salvia Miltiorrhiza-based CDs63 can suppress the abnormal levels of ALT, AST, TBA, and TBIL in the serum of mice with hyperbilirubinemia, effectively alleviating liver damage caused by hyperbilirubinemia. These results lay the foundation for the development of CHM-CDs as a new type of hepatoprotective drug and also provide experimental evidence for the clinical nanomedicine treatment of liver injury diseases.
Gastroprotective Effect
The pathogenesis of gastric ulcer is mainly the imbalance between the defense mechanism of gastric mucosa and external invasive factors, including gastric acid, pepsin, infection, genetics, constitution, environment, diet, lifestyle, and neuropsychiatric factors.64 It has been reported65–67 that CDs derived from Radix Sophorae Flavescentis, Nelumbinis Rhizomatis Nodus and Glycyrrhizae Radix et Rhizoma (GRR-CDs) had a certain protective effect on the acute gastric ulcer model of alcohol-induced rats, which can inhibit the inflammatory response of gastric tissue by reducing the level of NF-κB and the concentration of tumor necrosis factor (TNF-α) and interleukin (IL)-6, while the aforementioned CHM-CDs increased the activity levels of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), GSH and reduce the contents of malondialdehyde (MDA), nitric oxide (NO) and inducible nitric oxide synthase (iNOS) to relieve symptoms of alcohol-induced oxidative stress levels. In addition, Lu et al68–70 reported that CDs derived from Atractylodes Macrocephala and Fuligo Plantae have anti-stress and anti-alcoholic gastric ulcers effects, with an inhibition rate ranging from 60% to 90%. Therefore, the aforementioned CHM-CDs not only alleviated abnormal levels of inflammatory factors and oxidative stress, but also reduced excessive neuroendocrine responses caused by stress and alcohol, regulated energy metabolism and intestinal flora structure, which improved the damage of gastric ulcers to the body. In summary, the intrinsic mechanism of CHM-CDs in the treatment of gastric ulcer disease is mainly related to the regulation of NF-κB signaling pathway, the improvement of the anti-inflammatory and antioxidant effects of the ROS system, and the regulation of the structure of intestinal flora, which provides an experimental basis for the clinical application of CHM-CDs in the treatment of gastric ulcer.
Colon Protective Effect
Ulcerative colitis (UC) is a chronic and nonspecific intestinal inflammation involving the colon, rectum, and submucosa, with clinical manifestations such as tenesmus, mucopurulent bloody stools, abdominal pain, and diarrhea.71 In recent years, relevant studies have indicated that CDs derived from Coptidis Rhizoma72 and Rhei Radix Rhizoma73 have a significant alleviating effect on dextran sodium sulphate-induced ulcerative colitis in mice. The mechanism of action may be related to enhancing the intestinal mucosal barrier function, improving inflammatory levels, and regulating the composition of the gut microbiota. Bai and Chen et al74,75 reported that CDs derived from Platycladi Cacumen and Rehmanniae Radix effectively relieved the symptoms of UC in rats caused by 2,4,6-trinitrobenzenesulfonic acid, and improve the degree of blood in the stool and diarrhea. Moreover, the aforementioned CHM-CDs decreased the levels of TNF-α, IL-6 and MPO, and increased the contents of IL-10, which indicated that CHM-CDs can regulate the balance between pro-inflammatory and anti-inflammatory factors to improve symptoms of UC. These results imply that CHM-CDs have the potential to become nanomedicines for the treatment of bowel diseases.
Impact on the Nervous System
Inhibiting Cerebral Ischemia Reperfusion Injury
Ischemia-reperfusion injury includes primary injury caused by ischemia and secondary injury caused by reperfusion.76 Once the brain undergoes ischemia/reperfusion, the tissue cells may undergo calcium overload, oxidative stress, endoplasmic reticulum stress, apoptosis, autophagy, and other reactions, which will affect the normal physiological structure and function of brain tissue.77 The breakdown of the blood-brain barrier (BBB) is a key event in the development of ischemic stroke. The pathology caused by ischemic stroke increases the permeability of the BBB and further leads to brain swelling.78 Zhang et al79 isolated a novel type of CDs derived from Crinis Carbonisatus (CrCi-CDs) and utilized a rat model of middle cerebral artery occlusion (MCAO) to verify the neuroprotective effect of the CrCi-CDs. The results showed that CrCi-CDs could significantly reduce ischemic injury volume and BBB permeability in MCAO rats, improve neurological deficits, reduce the levels of tumor necrosis factor TNF-α and IL-6, and inhibit the excitatory neurotransmitter aspartate and glutamate (Glu), increase serotonin (5-HT) levels, suggesting that the underlying mechanisms may be related to anti-inflammatory effects and inhibition of neuroexcitatory toxicity.
Alleviating Traumatic Brain Injury
Traumatic brain injury is a central nervous system disorder caused by external forces acting on the brain, and its secondary injuries involve a complex cascade of reactions such as damage to the BBB, edema, and inflammation.80 The integrity of the BBB is closely related to tight junction proteins, among which claudins and ZO-1 are considered markers for judging the integrity of the BBB.81 Luo et al82 reported that CDs synthesized hydrothermally from Semen Pruni Persicae and Carthamus Tinctorius L. could improve the recovery of neurological function after traumatic brain injury in mice, reduce the permeability of the blood-brain barrier, brain edema, and neuronal damage, and upregulate the expression levels of claudin-5 and ZO-1 in brain tissue. The aforementioned results indicate that the CDs mentioned have significant therapeutic effects in promoting the repair of the BBB.
Antianxiety Effect
Anxiety disorder is a kind of nervous disease mainly characterized by anxiety. It is manifested by persistent anxiety, tension, panic, and restlessness, accompanied by autonomic nervous disorder, muscle tension and motor restlessness.83 Interestingly, the results of elevated plus maze test and open field test showed that CDs derived from cigarette mainstream smoke had obvious anxiolytic and certain sedative effects on mice.84 The underlying mechanism may be that CHM-CDs decreased the levels of Glu in the brain and promoted the production of norepinephrine (NE), as well as decreased the contents of dopamine (DA) in serum of mice, which provides new avenues for the development of anti-anxiety drugs. In addition, Chen and Cui et al85,86 confirmed that CDs from Os Draconis and Chrysanthemum morifolium Ramat increased the central activity time of the open field test in mice, showed more frequent activity in the light compartment and the open arms in light/dark box test and elevated plus maze test, and shorted the feeding latency of mice in the novelty-suppressed feeding test, which may be associated with a significant increase in serum levels of 5-HT and NE as well as a decrease in concentrations of corticotropin-releasing hormone (CRH), adrenocorticotropin (ATCH) and corticosterone (CORT).
Analgesic Effect
After the body is stimulated by pain, it will release a variety of endogenous opioid peptides, mainly enkephalin and β-endorphin, which combine with the corresponding receptors to produce analgesic effect.87 Pain control involves the interaction between various regulatory systems, including not only endogenous opioid peptides, but also the release of 5-HT, PGE2, bradykinin and other neurotransmitters related to the pain control system.88 It was reported that CDs derived from Zingiberis Rhizome89 (ZR-CDs) and Terra Flava Usta90 could increase the pain tolerance time of mice to thermal stimulation and reduce the number of writhing caused by chemical components such as acetic acid. ZR-CDs can increase the levels of enkephalin and β-endorphin in serum of mice and contribute to the increase of enkephalin level in brain tissue during heat stimulation analgesia, which indicates that the regulation of brain opioid peptide system is involved in ZR-CDs-induced analgesia. In addition, ZR-CDs increased the level of 5-HT in the brain and decreased the level of 5-HT in the serum, suggesting that the dual regulatory effect of 5-HT in the CNS versus the periphery induced by ZR-CDs treatment may be related to the activation of different 5-HT receptor subtypes. The exploration of the analgesic mechanism of CHM-CDs currently only involves research at the level of opioid peptides and neurotransmitters such as 5-HT, and further systematic research at the molecular level is still required.
Impact on the Immune System
Antivirus Effect
Research on highly effective antiviral drugs is crucial to prevent infection transmission and reduce losses. CHM-CDs have the polyvalent property of high surface-to-volume ratio and can allow the attachment of multiple ligands, which makes them well able to interfere with virus attachment and prevent the virus from entering cell.91,92 Tong et al93 reported that CDs derived from glycyrrhizic acid synthesized by hydrothermal method has a large surface area and contact site, which can inhibit the invasion and replication of porcine reproductive and respiratory syndrome virus, stimulate the antiviral innate immune response, and alleviate the accumulation of intracellular reactive oxygen species caused by PRRSV infection. The mechanism may be related to stimulating cells to regulate the expression of host limiting factors directly related to PRRSV proliferation such as DDX53 and NOS3 genes. Lin et al94 reported that CDs derived from curcumin could effectively block enterovirus 71 (EV71) attached to the cell membrane of human rhabdomyosarcoma cells and inhibit the generation of ROS and PGE2 in the RD cells induced by EV71 by scavenging free radicals. The mechanism may be related to inhibiting the translation of EV71 and EV71-induced eukaryotic translation initiation factor 4 gamma (eIF4G) and reducing the expression level of phosphorylated p38 kinase. Meanwhile, in vivo animal experiments further demonstrated that the CHM-CDs can significantly reduce mouse mortality and protect newborn mice from virus-induced hind limb paralysis. Therefore, CHM-CDs showed remarkable antiviral activity and multi-site inhibition mechanism, which provided research direction and scientific basis for alternative treatment of viral infection.
Antibacterial Effect
Wang et al95 reported that CDs derived from Artemisia Argyi (AAFC-CDs) can selectively inactivate gram-negative bacteria and play a certain antibacterial role. AAFC-CDs had a strong inhibitory effect on a variety of gram-negative bacteria and even some drug-resistant gram-negative bacteria, and the anti-gram-negative bacteria efficiency can reach 100% at a concentration of 150 mg/mL, but the killing effect on gram-positive bacteria was poor. In terms of its antibacterial mechanism, AAFC-CDs inhibited the activity of UDP-3-O-(acyl)-N-acetylglucosamine deacylase (LpxC), which was related to cell wall synthesis of Gram-negative bacteria. Moreover, AAFC-CDs also influenced the α-helical structure of LpxC. It was speculated that AAFC-CDs may affect the activity of LpxC by changing the secondary structure of LpxC. The above results make it possible for AAFC-CDs to become a nano antibiotic with specific bactericidal properties, which is of great significance for the development of natural biomass antibacterial nanomaterials and the treatment of gram-negative bacterial infections. Lu et al96 reported that CDs synthesized by hydrothermal method using citric acid and curcumin as raw materials had sterilization rates of 100% and 80% against gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) and gram-positive bacteria (Staphylococcus aureus and Pseudomonas aeruginosa), respectively, and inhibited the biofilm formation of gram-negative bacteria and gram-positive bacteria, which indicated that the CHM-CDs had good broad-spectrum antibacterial activity and antibacterial film activity. Additionally, the CDs extracted from onions97 significantly reduced the drip loss, total volatile basic nitrogen values, and total viable bacterial counts in Atlantic mackerel, extending its shelf life by two days. This indicates the potential of the CHM-CDs as a bacteriostatic agent for aquatic products.
Antitumor Effect
Yao et al98 used ginsenoside Re as raw material to prepare a new kind of photoluminescent Re-CDs by one-step hydrothermal synthesis method. The research results showed that, compared with ginsenoside Re, Re-CDs had stronger inhibitory effect on the proliferation of cancer cells (A375, HepG2 and MCF-7) and lower toxicity to normal cells (293T, L-02, MCF-10A and Human normal skin fibroblasts). Its anticancer activity is mainly related to increasing the level of ROS and inhibiting tumor cell proliferation and induction of apoptosis through caspase-mediated pathways, which has good antitumor activity. The Ginger-based CDs (GI-CDs) synthesized by Li et al99 using ginger as raw material showed anti-tumor effects. The in vitro activity study found that GI-CDs induced intracellular the levels of ROS by up-regulating the expression of p53 gene and produced obvious cytotoxicity to HepG2 cells at high concentrations. Meanwhile, in vivo studies have also found that GI-CDs can be retained at the tumor site through the enhanced permeability and retention effect of solid tumors, which can inhibit tumor growth and exhibit obvious anti-liver cancer activity. According to Yao et al,100 CDs derived from the natural product chlorogenic acid can recruit immune cells to activate a systemic antitumor immune response, significantly inhibiting tumor growth in HepG2 tumor-bearing mice. Additionally, Xu et al101 reported that CDs synthesized using jujube as raw materials could promote the proliferation of red blood cells by modulating hypoxia response and enhancing the phosphorylation level of STAT5, and have no significant impact on the proliferation and metastasis of tumors, indicating the great potential of the CHM-CDs in the treatment of cancer-related anemia. Hence, the satisfactory antitumor activity of CHM-CDs is of great significance for tumor prevention and targeted therapy, and is worthy of further study.
Alleviating Sepsis
Sepsis is a systemic inflammatory response syndrome caused by bacteria and other pathogenic microorganisms invading the body and propagating and releasing toxins in the blood, tissues, and organs, which can lead to acute organ dysfunction in severe cases.102 Zhao et al103,104 reported that CDs from Armeniacae Semen Amarum and Descurainiae Semen were able to alleviate symptoms of lipopolysaccharide (LPS)-induced lung injury, accompanied by a demonstrated reduction of the levels of IL-6, IL-1β, TNF-α, MDA and myeloperoxidase (MPO) and increasing the contents of IL-10, SOD and GSH, suggesting that CHM-CDs may work by reducing inflammation levels and enhancing antioxidant capacity. LPS-induced systemic inflammatory response is usually accompanied by fever or hypothermia.105 Wu et al106 showed that CDs from Lonicerae japonicae Flos can reduce the levels of TNF-α, IL-1β and IL-6 in serum to a certain extent to regulate the abnormal temperature. The mechanism may be to further block the production of prostaglandin E2 (PGE2) to control the increase in body temperature, or to reduce the production of TNF-α as an endogenous cooling factor to alleviate the symptoms of hypothermia, which acts as a bidirectional regulator of LPS-induced hypothermia or febrile symptoms.
Improving Psoriasis
Psoriasis is an autoimmune-mediated chronic skin inflammation characterized by well-circumscribed red plaques and adhering silvery-white scales.107 The histopathologic features of psoriasis are abnormal thickening of the epidermis, downward extension of the reticulum ridge, and accumulation of neutrophils.108 Psoriasis relies on the release of mediators from immune cells such as T cells, macrophages, mast cells and granulocytes to coordinate its pre-cutaneous pathological changes.109 It is reported that PCC-CDs110 can effectively improve the appearance, psoriasis area and severity index scores, and histopathological morphology of dorsal skin tissue and right ear in imiquimod-induced psoriasis model mice, which may be related to inhibition of M1 polarization and relative promotion of M2 polarization in macrophages.
Impact on the Endocrine System
Hypoglycemic Effect
The experimental results of hyperglycemia model111,112 showed that CDs derived from Jiaosanxian and Fructus Crataegi (CFC-CDs) have the effect of regulating blood sugar, which can regulate blood sugar to normal level after 90–120 minutes without causing hypoglycemia. CFC-CDs had a significant inhibitory effect on sucrase and maltase in the small intestine of mice. During the experiment, the median inhibition concentration (IC50) of sucrase was 0.73 mg/mL, and the IC50 of maltase was 0.26 mg/mL. These results indicated that small doses of CFC-CDs can be effective, and the inhibitory effect on maltase was stronger. The inhibitory mode of CFC-CDs against sucrase and maltase may be a partially noncompetitive type. Noncompetitive inhibitors bind to the enzyme/substrate complex and affect the disassembly of the enzyme/substrate to form a product.113 Part of the non-competitive inhibitor is thought to be released from the enzyme during the breakdown of the enzyme/substrate complex into products. This may be related to the intrinsic properties of CDs, which lead to their different interactions with enzymes. These findings suggest that CHM-CDs have potential value as hypoglycemic agents.
Regulating Hormone Levels
Menopausal syndrome refers to a range of physiological and psychological symptoms caused by the gradual decline of ovarian function and the decrease in estrogen levels in women as they enter menopause, such as hot flashes, night sweats, emotional fluctuations, sleep disturbances, vaginal dryness, and loss of sexual desire. Zhang et al114 reported that GRR-CDs can increase the level of estradiol in serum of ovariectomized female mice to a certain extent, reduce the levels of follicle stimulating hormone and luteinizing hormone, suggesting that CHM-CDs can alleviate menopausal syndrome by regulating hormone levels.
Impact on the Urinary System
Nephroprotective Effect
It has been reported115 that snake venom may harm kidney physiology directly through nephrotoxic components or by activating or modulating immune and inflammatory mediators, involving related biomedical indicators including serum creatinine (SCR), blood urea nitrogen (BUN), urine total protein (UTP), and microproteinuria (MALB), IL-1β, IL-10, monocyte chemoattractant protein-1 (MCP-1) and PLT. Zhang et al116 reported that CDs derived from Phellodendri Chinensis Cortex (PCC-CDs) greatly improved the direct cytotoxic response and inflammatory response of mouse kidneys caused by snake venom. From the results, PCC-CDs alleviated the increase of SCR, BUN, UTP and MALB levels caused by snake venom and promoted the recovery of PLT, which can improve the symptoms of urine and serum biochemical indexes and PLT reduction induced by snake venom related to kidney dysfunction to a certain extent. Dong et al117 reported that CDs derived from Astragali Radix have a significant alleviating effect on aristolochic acid-induced acute kidney injury, the mechanism of which may be related to the regulation of Akt/Mdm2/p53 signaling pathway to enhance anti-apoptotic ability. Additionally, Wang et al118 reported that CDs derived from Pollen Typhae demonstrated significant activity in improving the levels of BUN and CRE, urine volume, and renal histopathological morphology in rats with rhabdomyolysis-induced acute kidney injury. The mechanism may be related to the reduction of inflammatory responses and oxidative stress levels. The results suggest that CHM-CDs have potential applications as an adjunct in the treatment of acute kidney injury diseases caused by snake venom.
Treating Gouty Arthritis
Gout is a pathological state of purine nucleotide metabolism characterized by elevated uric acid levels, monosodium urate crystal deposition, and uric acid-induced periarticular inflammatory responses.119 Wang et al120,121 reported that CDs derived from Puerariae Lobatae Radix and Aurantii Fructus Immaturus exhibited good anti-gout effects in a rat model of hyperuricemia and gouty arthritis, accompanied by a reduction in uric acid levels and joint inflammation, and relief of joint swelling. The CHM-CDs mentioned above downregulated the levels of uric acid by inhibiting the activity of xanthine oxidase (XOD), and their ability to inhibit the activity of XOD was further verified by in vitro experiments, which may be related to the effect of nano-components on the catalytic site of the enzyme. In addition, The CHM-CDs also ameliorated inflammatory responses by reducing the levels of IL-1β and TNF-α in rats with monosodium urate crystal-induced inflammation. The existing research is only a preliminary evaluation of the anti-hyperuricemia and anti-inflammatory effects of the aforementioned CHM-CDs, and the further mechanism and biological activity of their pharmacological effects remain to be further explored. The anti-hyperuric acid activity exhibited by the CHM-CDs makes it promising to be an effective drug for clinical prevention and treatment of gouty arthritis.
Impact on the Skeletal System
Radioactive skeletal damage is one of the common side effects of radiotherapy for head and neck malignancies. As a severe complication that may arise following radiotherapy, osteoradionecrosis can lead patients to experience symptoms such as swelling, pain, exposure of the jawbone, and pathological mandibular fractures.122 Guo et al123 reported that CDs derived from Lycium barbarum can effectively alleviate bone damage caused by radiation, mediating the injury and senescence of bone marrow mesenchymal stem cells induced by radiation, and regulating the balance between osteogenesis and adipogenesis. The mechanism may involve the CDs increasing the N6-methyladenosine levels in irradiated bone marrow mesenchymal stem cells by enhancing methyltransferase-like 3, thereby inhibiting senescence and reducing radiation-induced bone damage.
Others
Kong et al reported that CDs derived from Scutellariae Radix Carbonisatac124 reduced c48/80-induced mast cell degranulation in RBL-2H3 cells, and down-regulated cytokine expression, suggesting that this CHM-CDs have significant anti-allergic effects. Zhao et al125 reported that CDs derived from Granati Pericarpium had obvious therapeutic effects on diarrhea index, diarrhea latency and intestinal transit function of diarrhea model mice, which was enough to reduce diarrhea index and diarrhea latency time, and had an antagonistic effect on small intestinal hypermotility caused by senna decoction. However, the current antidiarrheal effect of CHM-CDs only focuses on the effect evaluation, and the in-depth mechanism research still needs to be further explored.
Oxidative stress refers to the imbalance between oxidation and antioxidant systems in the body, which produces a large number of reactive oxygen species (ROS) intermediates.126 ROS plays a vital role in regulating various physiological functions of the biological organism.127 Jia et al128 reported that CDs derived from Black Soya Beans (N-CDs) were responsive to 2.2-diphenyl-1-picrylhydrazyl (DPPH) and superoxide anion radicals, showing certain antioxidant capacity. It was found that the scavenging rate of DPPH and superoxide anion radical upregulated with the increase of the concentration of N-CDs, which was mainly related to the electron transfer between the carboxyl, amino and hydroxyl active groups on the surface of N-CDs and the free radical. Sharma et al129 reported that CDs synthesized from Red Cabbage as a precursor carbon source had clearance rates of 61%, 56% and 91% for DPPH, hydroxyl, and potassium permanganate radicals, respectively, indicating that exhibited good antioxidant activity. Wei et al130 reported that CDs derived from Gynostemma mitigated oxidative damage in zebrafish caused by H2O2. The study found that CHM-CDs reduced the contents of ROS and MDA, and promoted the expression levels of associated antioxidant genes mRNA (glutamate cysteine ligase catalytic subunit, glutathione s-transferase P1, quinone oxidoreductase-1, Cu/Zn-superoxide dismutase), which further reduces oxidative stress levels.
Discussion
In recent years, CDs have become one of the important directions in the field of traditional Chinese medicine research due to their unique optical properties, good biocompatibility, and broad application prospects. CHM-CDs and CHM differ significantly in terms of material form, structure, preparation methods, functions, and application areas. While CHM primarily serves as a therapeutic tool in traditional medicine, CHM-CDs are nano-sized materials extracted from herbs using modern technologies. CHM-CDs possess unique physicochemical properties, which extend their potential applications in fields such as biomedicine, imaging, and drug delivery.13 It is worth noting that the properties of CDs are influenced by the source herb, and thus different CHM-CDs exhibit significant differences in morphology, optical performance, surface functional groups, and chemical composition.131In terms of morphology, the shape and size of CHM-CDs are typically affected by the properties of the raw materials and the conditions of the synthesis process (such as temperature, reaction time, pH, etc)., leading to different structures for different CHM-CDs.16 Regarding optical properties, the fluorescence performance of CDs is generally determined by their molecular structure and the types of surface functional groups. CHM-CDs may exhibit variations in emission wavelength, quantum yield, and fluorescence stability.19 As for surface functional groups, the types and distribution of these groups on CHM-CDs are usually closely related to the components of the herbs. For example, herbs rich in polyphenols (such as Lycium barbarum)123may produce CDs with a high content of hydrophilic groups like hydroxyl and carboxyl groups, making them more stable in water. On the other hand, herbs abundant in amino acids (such as Crinis Carbonisatus)79 may lead to CDs with more amino groups on their surfaces. Furthermore, the natural chemical substances contained in different CHM, such as flavonoids, saponins, polysaccharides, essential oils, and amino acids, are retained during the synthesis process of CDs, which in turn influences their chemical properties, structural characteristics, and functional performance.
As a novel carbon-based nanomaterial, CHM-CDs have been shown to have multifaceted pharmacological activities, including hemostatic capabilities, neuroprotection, anti-infective, antitumor, immunomodulatory effects and hypoglycemic activity (Table 1 and Figure 2). As far as existing research reports are concerned, CHM-CDs derived from the same CHM may have multifaceted pharmacological activities. For instance, JMC-CDs have been found to possess both hemostatic and hepatoprotective effects.38 PCC-CDs not only demonstrated a hemostatic effect and the capacity to mitigate kidney damage induced by snake venom, but also were found to ameliorate imiquimod-mediated psoriasis-like inflammation in murine models by regulating M1/M2 macrophage polarization.45,110,116 AAFC-CDs have exhibited anti-frostbite and selective antibacterial effect.53,95 These findings are derived from the current experimental research, and it can be postulated that that: (1) the confirmed CHM-CDs may have additional pharmacological activities yet to be discovered; (2) Other CHMs that have not yet been studied may also possess unique pharmacological activities, and (3) CHM-CDs derived from the same CHM may harbor additional pharmacological effects. To validate these postulates necessitates further comprehensive investigation by researchers.
In addition, preliminary studies have been made on the mechanism underlying the pharmacological effects of CHM-CDs. The mechanism of hemostatic effect of CHM-CDs may be related to both endogenous and exogenous coagulation pathways. CHM-CDs have been found to regulate signaling pathways and cytokines to offer organ tissue protection. They can also inhibit neuroexcitatory toxicity and increase levels of 5-HT and NE, to thereby playing a neuroprotective and analgesic role. Additionally, CHM-CDs regulate a multi-site inhibition mechanism to generate antiviral and antibacterial effects. They regulate inflammatory cytokine levels to produce anti-inflammatory effects, and exhibit immunomodulatory effects by inhibiting macrophage M1 polarization and relatively promoting M2 polarization. CHM-CDs inhibited the activity of sucrase and maltase to regulate blood sugar levels, indicating that CHM-CDs have the potential to be developed as drugs to treat a wide range of diseases. Nevertheless, in vivo studies on CHM-CDs mostly focus on efficacy results, with in-depth exploration of their pharmacodynamic mechanisms being a focal point of future research. Therefore, researchers still need to continue to conduct in-depth research to verify the specific pathways and targets of CHM-CDs in treating individual diseases, as well as to analyze the correlation between the pharmacological effects of CHM-CDs and the implicated signaling pathways and specific targets.
CHM-CDs, as an emerging therapeutic modality, offer numerous advantages over conventional Western pharmaceuticals, including their natural origin, excellent biocompatibility, multifunctionality, and eco-friendly attributes. Since CHM-CDs are derived from CHM, they typically demonstrate favorable biocompatibility, allowing for safe metabolism and clearance from the body, thereby minimizing the risk of immune responses.29,30 In contrast, certain conventional Western drugs, particularly synthetic chemical agents, may provoke immune reactions or long-term toxicological side effects. Furthermore, the environmentally sustainable synthesis of CHM-CDs does not rely on harmful chemical reagents or heavy metal catalysts, aligning with green chemistry principles. In comparison, the manufacturing processes of some Western pharmaceuticals may involve high energy consumption or the use of hazardous chemicals. Additionally, CHM-CDs are derived from abundant and easily accessible resources, resulting in a relatively cost-efficient production process. In comparison to some high-cost Western drugs, CHM-CDs present a more favorable cost-benefit ratio.
Nevertheless, although CHM-CDs have demonstrated promising efficacy in vitro and in animal models, their clinical effectiveness and long-term safety still require comprehensive validation. Furthermore, while the synthesis of CHM-CDs is relatively straightforward, scaling up production to a high-quality standard remains a significant challenge. The consistency in stability and potency across different batches of CHM-CDs needs to be thoroughly assessed. Additionally, as water-soluble nanomaterials, the stability of CHM-CDs in acidic and enzymatic conditions, along with the potential for further degradation upon interaction with the human body, warrants further investigation. Biocompatibility is another critical concern, as adverse effects and improper accumulation of the drug may disrupt normal physiological processes. Therefore, a more in-depth evaluation of their biocompatibility is essential. At present, the clinical research and application of CHM-CDs are in the nascent stages, with promising pharmacological activity across several domains. Consequently, the development of safe, stable, and efficacious CHM-CDs remains an urgent priority.
The precursors of CHM-CDs are diverse and include a variety of herbs, each with unique properties that can contribute to the characteristics of the resultant CHM-CDs. The selection of appropriate precursors is crucial for the synthesis of CHM-CDs with specific physicochemical properties and bioactivities. The exploration of different precursors facilitates the expansion of the application spectrum of CHM-CDs and the development of new carbon-based nanomaterials with potential applicability across diverse fields, including biomedicine. At present, to improve the preparation efficiency and quality of CHM-CDs, it is imperative to optimize the existing preparation methods, including the improvement of high-temperature carbonization and hydrothermal synthesis, the two main preparation methods for CHM-CDs. Adjusting various process parameters can directly alter the chemical bond cleavage patterns of various chemical components in the original traditional Chinese medicine, thereby affecting the particle size, crystal structure, and bioactivity of CHM-CDs. However, the preparation process of CHM-CDs faces several challenges, mainly including: (1) quality control during the carbonization production process; (2) lack of definitive quality inspection criteria post CHM carbonization; (3) the need for upgrading and optimization existing equipment, which otherwise leads to inconsistent structural and pharmacological performance of CHM-CDs. Therefore, how to determine the optimal processing process, pursue quantitative indicators, and upgrade and optimize equipment remains the focus of subsequent in-depth research. Firstly, future investigations should prioritize refining the synthesis methodologies and exploring more advanced, efficient, and controlled approaches to ensure the stability and reproducibility of CHM-CDs. In addition, establishing strict quality standards and testing systems is essential to regularly verify different batches of CHM-CDs to ensure their safety and effectiveness in clinical applications. Additionally, the development of diverse dosage forms, such as controlled-release formulations, injectable preparations, and transdermal systems, should be pursued to further enhance the clinical translation and application of CHM-CDs.
The safety and side effects of drugs are always key issues in clinical applications, especially in the research of novel materials like CHM-CDs. It is foreseeable that the application of CHM-CDs in biomedicine depends on ensuring their biological safety. In addition to cytotoxicity and hematotoxicity, attention should also be paid to the metabolic process and deposition of CHM-CDs in the body, especially their long-term toxicity. Currently, most research primarily focuses on the impact of CHM-CDs on cell viability, while studies on allergic reactions, cellular oxidative damage, and DNA damage are relatively few. Furthermore, the interaction between CHM-CDs and the immune system, as well as their in vivo toxicity, long-term toxicity, and distribution within the body, still require further investigation. Specifically, whether they can be effectively metabolized and excreted by organs such as the kidneys and liver remain to be explored. Therefore, future research should strengthen systematic evaluations of the cytotoxicity, immune responses, metabolic processes, and long-term toxicity of CHM-CDs, and establish a more comprehensive safety assessment system to provide scientific evidence for clinical applications.
In summary, CHM-CDs, as nanoscale pharmaceutical entities, possess considerable developmental value. Future investigations ought to delve into the optimization of the preparation process, the elucidation of pharmacodynamic mechanisms, the determination of clinical indications, and the exploration of novel dosage forms. Such research efforts will lay the foundation for their potential application in the management of complex pathologies, including but not limited to neoplasms, cerebrovascular accidents, and gout.
Conclusion
Within the domain of carbon nanomaterials, CDs have risen to prominence as a superior option for applications in biomedicine. With a commitment to the principles of green chemistry, CHM-CDs have garnered significant attention. Their synthesis primarily employs methods such as pyrolysis and hydrothermal synthesis, utilizing precursors like C-CHM, extracts, and small molecule compounds derived from CHM. This review article encapsulates the recent findings on the pharmacological activities of CHM-CDs and explores their underlying mechanisms, which encompass hemostatic capabilities, neuroprotection, anti-infective, antitumor, immunomodulatory effects and hypoglycemic activity. Notably, they have been associated with circulatory system, digestive system, nervous system, immune system, endocrine system, urinary system and skeletal system. Nonetheless, forthcoming research still confronts challenges in both the fundamental understanding and practical application of these materials.
Abbreviations
CDs, carbon dots; CHM-CDs, Chinese herbal medicine-derived CDs; C-CHM, charcoal Chinese herbal medicine; platelet, PLT; APTT, activated partial thromboplastin time; PT, prothrombin time; TT, thrombin time; FIB, fibrinogen; CCl4, carbon tetrachloride; JMC-CDs, CDs derived from Junci Medulla; GRR-CDs, CDs derived from Glycyrrhizae Radix et Rhizoma; TNF-α, tumor necrosis factor; IL, interleukin; CAT, catalase; GSH-Px, glutathione peroxidase; NO, nitric oxide; iNOS, inducible nitric oxide synthase; SCR, serum creatinine; BUN, blood urea nitrogen; UTP, urine total protein; MALB, microproteinuria; MCP-1, monocyte chemoattractant protein-1; PCC-CDs, CDs derived from Phellodendri Chinensis Cortex; BBB, blood-brain barrier; CrCi-CDs, CDs derived from Crinis Carbonisatus; Glu, glutamate; 5-HT, serotonin; MCAO, middle cerebral artery occlusion; NE, norepinephrine; DA, dopamine; CRH, corticotropin-releasing hormone; ATCH, adrenocorticotropin; CORT, corticosterone; LPS, lipopolysaccharide; SOD, superoxide dismutase; MDA, malondialdehyde; MPO, myeloperoxidase; PGE2, prostaglandin E2; XOD, xanthine oxidase; UC, Ulcerative colitis; ROS, reactive oxygen species; N-CDs, CDs derived from Black Soya Beans; DPPH, 2.2-diphenyl-1-picrylhydrazyl; ZR-CDs, CDs derived from Zingiberis Rhizome; CFC-CDs, CDs derived from Fructus Crataegi; IC50, the median inhibition concentration; EV71, enterovirus 71; eIF4G, eukaryotic translation initiation factor 4 gamma; AAFC-CDs, CDs derived from Artemisia Argyi; LpxC, UDP-3-O-(acyl)-N-acetylglucosamine deacylase.
Acknowledgments
We greatly appreciate the support of Senior Department of Burns and Plastic Surgery, the Fourth Medical Center of Chinese PLA General Hospital. This study is funded by the National Natural Science Foundation of China (82272279, 82072169).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Szczepankowska J, Khachatryan G, Khachatryan K. et al. Carbon dots-types, obtaining and application in biotechnology and food technology. Int J mol Sci. 2023;24(19):14984. doi:10.3390/ijms241914984
2. Singh P, Bhankar V, Kumar S, et al. Biomass-derived carbon dots as significant biological tools in the medicinal field: a review. Adv Colloid Interface Sci. 2024;328:103182. doi:10.1016/j.cis.2024.103182
3. Zhao J, Yao J, Wang Y, et al. A red fluorescent carbon dots with good water solubility for rapid detection of Al3+++ in actual samples. Luminescence. 2024;39(2):e4666. doi:10.1002/bio.4666
4. Kamble P, Malavekar D, Tiwari AP. Natural biowaste derived fluorescent carbon quantum dots: synthesis, characterization and biocompatibility study. J Fluoresc. 2024;34(1):191–201. doi:10.1007/s10895-023-03244-w
5. Zhou Y, Zhang W, Leblanc RM. Structure-property-activity relationships in carbon dots. J Phys Chem B. 2022;126(51):10777–10796. doi:10.1021/acs.jpcb.2c06856
6. Lee DY, Haider Z, Krishnan SK, et al. Oxygen-enriched carbon quantum dots from coffee waste: extremely active organic photocatalyst for sustainable solar-to-H2O2 conversion. Chemosphere. 2024;361:142330. doi:10.1016/j.chemosphere.2024.142330
7. Deb A, Chowdhury D. Biogenic carbon quantum dots: synthesis and applications. Curr Med Chem. 2024;31(25):3899–3924. doi:10.2174/0929867330666230608105201
8. Magdy G, Elmansi H, Belal F, et al. Doped Carbon Dots as Promising Fluorescent Nanosensors: synthesis, Characterization, and Recent Applications. Curr Pharm Des. 2023;29(6):415–444. doi:10.2174/1381612829666221103124856
9. Bosu S, Rajamohan N, Sagadevan S, et al. Biomass derived green carbon dots for sensing applications of effective detection of metallic contaminants in the environment. Chemosphere. 2023;345:140471. doi:10.1016/j.chemosphere.2023.140471
10. Kaurav H, Verma D, Bansal A, et al. Progress in drug delivery and diagnostic applications of carbon dots: a systematic review. Front Chem. 2023;11:1227843. doi:10.3389/fchem.2023.1227843
11. Xu J, Ning J, Wang Y, et al. Carbon dots as a promising therapeutic approach for combating cancer. Bioorg Med Chem. 2022;72:116987. doi:10.1016/j.bmc.2022.116987
12. Xu J, Huang B, Lai C, et al. Advancements in the synthesis of carbon dots and their application in biomedicine. J Photochem Photobiol B. 2024;255:112920. doi:10.1016/j.jphotobiol.2024.112920
13. Sharma A, Choi HK, Lee HJ. Carbon Dots for the Treatment of Inflammatory Diseases: an Appraisal of In Vitro and In Vivo Studies. Oxid Med Cell Longev. 2023;2023:3076119. doi:10.1155/2023/3076119
14. Radnia F, Mohajeri N, Zarghami N. New insight into the engineering of green carbon dots: possible applications in emerging cancer theranostics. Talanta. 2020;209:120547. doi:10.1016/j.talanta.2019.120547
15. Luo WK, Zhang LL, Yang ZY, et al. Herbal medicine derived carbon dots: synthesis and applications in therapeutics, bioimaging and sensing. J Nanobiotechnology. 2021;19(1):320. doi:10.1186/s12951-021-01072-3
16. Li D, Xu KY, Zhao WP, et al. Chinese medicinal herb-derived carbon dots for common diseases: efficacies and potential mechanisms. Front Pharmacol. 2022;13:815479. doi:10.3389/fphar.2022.815479
17. Zeng M, Wang Y, Liu M, et al. Potential efficacy of herbal medicine-derived carbon dots in the treatment of diseases: from Mechanism to Clinic. Int J Nanomed. 2023;18:6503–6525. doi:10.2147/IJN.S431061
18. Ai S, Li Y, Zheng H, et al. Collision of herbal medicine and nanotechnology: a bibliometric analysis of herbal nanoparticles from 2004 to 2023. J Nanobiotechnology. 2024;22(1):140. doi:10.1186/s12951-024-02426-3
19. Qiang R, Huang H, Chen J, et al. Carbon quantum dots derived from herbal medicine as therapeutic nanoagents for rheumatoid arthritis with ultrahigh lubrication and anti-inflammation. ACS Appl Mater Interfaces. 2023;15(32):38653–38664. doi:10.1021/acsami.3c06188
20. Wu T, Li M, Li T, et al. Natural biomass-derived carbon dots as a potent solubilizer with high biocompatibility and enhanced antioxidant activity. Front Mol Biosci. 2023;10:1284599. doi:10.3389/fmolb.2023.1284599
21. He Y, Fan Q, Shi J, et al. Advances in Clinical Application and Hemostatic Mechanism of Charcoal Drugs of Chinese Materia Medica. Chin J Exp Traditional Med Formulae. 2021;27:201–208.
22. Qiao R, Liu S, Bai Y, et al. Research on change discipline of main composition and chromaticity value and hemostatic effect of Scutellariae Radix in process of frying charcoal. Chin Traditional Herbal Drugs. 2024;55(15):5083–5092.
23. Li S, Jia S, Yang L, et al. The study of changes in microstructure and hemostatic components of Crataegus pinnatifida Bge. Before and After Carbonization. Lishizhen Med Mater Med Res. 2019;30:1352–1354.
24. Zhang Q, Wang Y, Gao D, et al. Comparing coagulation activity of Selaginella tamariscina before and after stir-frying process and determining the possible active constituents based on compositional variation. Pharm Biol. 2018;56(1):67–75. doi:10.1080/13880209.2017.1421673
25. Wu L, Tan L, Gong F, et al. Promoting effect of the Maillard reaction products produced during the stir-frying process of Hordei Fructus Germinatus on the intestinal absorption of active ingredients in Hordei Fructus Germinatus. Food Sci Biotechnol. 2021;30(5):631–642. doi:10.1007/s10068-021-00911-1
26. Zhang J, Hu K, Di L, et al. Traditional herbal medicine and nanomedicine: converging disciplines to improve therapeutic efficacy and human health. Adv Drug Deliv Rev. 2021;178:113964. doi:10.1016/j.addr.2021.113964
27. Zeng M, Guo D, Fernández-Varo G, et al. The integration of nanomedicine with traditional Chinese medicine: drug delivery of natural products and other opportunities. Mol Pharm. 2023;20(2):886–904. doi:10.1021/acs.molpharmaceut.2c00882
28. Liu Y, Zhang L, Cai H, et al. Biomass-derived carbon dots with pharmacological activity for biomedicine: recent advances and future perspectives. Sci Bull. 2024;69(19):3127–3149. doi:10.1016/j.scib.2024.08.011
29. Zhang J, Zou L, Li Q, et al. Carbon dots derived from traditional Chinese medicines with bioactivities: a rising star in Clinical Treatment. ACS Appl Bio Mater. 2023;6(10):3984–4001. doi:10.1021/acsabm.3c00462
30. Zhang YL, Wang YL, Yan K, et al. Nanostructures in Chinese herbal medicines (CHMs) for potential therapy. Nanoscale Horiz. 2023;8(8):976–990. doi:10.1039/D3NH00120B
31. Wu D, Wang S, Yu G, Chen X. Cell Death Mediated by the Pyroptosis Pathway with the Aid of Nanotechnology: prospects for Cancer Therapy. Angew Chem Int Ed Engl. 2021;60(15):8018–8034. doi:10.1002/anie.202010281
32. Chen Z, Ye SY, Yang Y, et al. A review on charred traditional Chinese herbs: carbonization to yield a haemostatic effect. Pharm Biol. 2019;57(1):498–506. doi:10.1080/13880209.2019.1645700
33. Humaera NA, Fahri AN, Armynah B, et al. Natural source of carbon dots from part of a plant and its applications: a review. Luminescence. 2021;36(6):1354–1364. doi:10.1002/bio.4084
34. Tripodi A. Thrombin generation: a global coagulation procedure to investigate hypo- and hyper-coagulability. Haematologica. 2020;105(9):2196–2199. doi:10.3324/haematol.2020.253047
35. Budkowska M, Lebiecka A, Marcinowska Z, et al. The circadian rhythm of selected parameters of the hemostasis system in healthy people. Thromb Res. 2019;182:79–88. doi:10.1016/j.thromres.2019.08.015
36. Xue Y, Li S, Liu W, et al. The mechanisms of sulfated polysaccharide drug of propylene glycol alginate sodium sulfate (PSS) on bleeding side effect. Carbohydr Polym. 2018;194:365–374. doi:10.1016/j.carbpol.2018.04.048
37. Yan X, Zhao Y, Luo J, et al. Hemostatic bioactivity of novel Pollen Typhae Carbonisata-derived carbon quantum dots. J Nanobiotechnology. 2017;15(1):60. doi:10.1186/s12951-017-0296-z
38. Cheng J, Zhang M, Sun Z, et al. Hemostatic and hepatoprotective bioactivity of Junci Medulla Carbonisata-derived carbon dots. Nanomedicine. 2019;14(4):431–446. doi:10.2217/nnm-2018-0285
39. Zhang M, Zhao Y, Cheng J, et al. Novel carbon dots derived from Schizonepetae Herba Carbonisata and investigation of their haemostatic efficacy. Artif Cells Nanomed Biotechnol. 2018;46(8):1562–1571. doi:10.1080/21691401.2017.1379015
40. Sun Z, Lu F, Cheng J, et al. Haemostatic bioactivity of novel Schizonepetae Spica Carbonisata-derived carbon dots via platelet counts elevation. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S308–S317. doi:10.1080/21691401.2018.1492419
41. Luo J, Zhang M, Cheng J, et al. Hemostatic effect of novel carbon dots derived from Cirsium setosum Carbonisata. RSC Adv. 2018;8(66):37707–37714. doi:10.1039/C8RA06340K
42. Wang Y, Kong H, Liu X, et al. Novel carbon dots derived from Cirsii Japonici Herba Carbonisata and their haemostatic effect. J Biomed Nanotechnol. 2018;14(9):1635–1644. doi:10.1166/jbn.2018.2613
43. Zhao Y, Zhang Y, Kong H, et al. Haemostatic nanoparticles-derived bioactivity of from Selaginella Tamariscina Carbonisata. Molecules. 2020;25(3):446. doi:10.3390/molecules25030446
44. Zhao Y, Tian Y, Li W, et al. Nano-components derived from Descurainiae Semen Carbonisatum and its hemostatic mechanism. Acta Pharma Sin. 2022;57(02):492–499.
45. Liu X, Wang Y, Yan X, et al. Novel Phellodendri Cortex (Huang Bo)-derived carbon dots and their hemostatic effect. Nanomedicine. 2018;13(4):391–405. doi:10.2217/nnm-2017-0297
46. Zhang M, Cheng J, Luo J, et al. Protective effects of Scutellariae Radix Carbonisata-derived carbon dots on blood-heat and hemorrhage rats. Front Pharmacol. 2023;14:1118550. doi:10.3389/fphar.2023.1118550
47. Luo J, Zhao Y, Cheng J, et al. Nanoparticles derived from Lotus Leaf Carbonisatus and their hemostatic effect. Northwest Pharma J. 2019;34:499–504.
48. Xiong W, Zhao Y, Cheng J, et al. Novel carbon dots derived from Dryopteridis Crassirhizomatis Rhizoma Carbonisatum and their hemostatic effect. Chin Traditional Herbal Drugs. 2019;50(6):1388–1394.
49. Zhao Y, Zhang Y, Liu X, et al. Novel carbon quantum dots from egg yolk oil and their haemostatic effects. Sci Rep. 2017;7(1):4452. doi:10.1038/s41598-017-04073-1
50. Fan J, Ren M, Chen W, et al. Celastrol relieves myocardial infarction-induced cardiac fibrosis by inhibiting NLRP3 inflammasomes in rats. Int Immunopharmacol. 2023;121:110511. doi:10.1016/j.intimp.2023.110511
51. Dong L, Zhao Y, Luo J, et al. Carbon dots derived from Curcumae Radix and their heartprotective effect. Int J Nanomed. 2024;19:3315–3332. doi:10.2147/IJN.S444125
52. Lorentzen AK, Davis C, Penninga L. Interventions for frostbite injuries. Cochrane Database Syst Rev. 2020;12(12):CD012980.
53. Kong H, Zhao Y, Zhu Y, et al. Carbon dots from Artemisiae Argyi Folium Carbonisata: strengthening the anti-frostbite ability. Artif Cells Nanomed Biotechnol. 2021;49(1):11–19. doi:10.1080/21691401.2020.1862134
54. Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017;27(21):R1147–R1151. doi:10.1016/j.cub.2017.09.019
55. Aramjoo H, Mohammadparast-Tabas P, Farkhondeh T, et al. Protective effect of Sophora pachycarpa seed extract on carbon tetrachloride-induced toxicity in rats. BMC Complement Med Ther. 2022;22(1):76. doi:10.1186/s12906-022-03554-9
56. Singal AK, Bataller R, Ahn J, et al. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol. 2018;113(2):175–194. doi:10.1038/ajg.2017.469
57. Zhao Y, Li Y, Chen Y, et al. Discovery of Vaccaria Segetalis Carbonisatum nano-components and their hepatoprotective effect. Chin Traditional Herbal Drugs. 2021;52(22):6825–6833.
58. Guo Y, Liang X, Meng M, et al. Hepatoprotective effects of Yulangsan flavone against carbon tetrachloride (CCl4)-induced hepatic fibrosis in rats. Phytomedicine. 2017;33:28–35. doi:10.1016/j.phymed.2017.07.005
59. Zhao Y, Zhang Y, Kong H, et al. Carbon dots from Paeoniae Radix Alba Carbonisata: hepatoprotective effect. Int J Nanomed. 2020;15:9049–9059. doi:10.2147/IJN.S281976
60. Zhao Y, Dai E, Dong L, et al. Available and novel plant-based carbon dots derived from Vaccaria Semen carbonisata alleviates liver fibrosis. Front Mol Biosci. 2023;10:1282929. doi:10.3389/fmolb.2023.1282929
61. Zhao Y, Kong H, Li Y, et al. Inhibitory effects of Curcumae Radix carbonisata-based carbon dots against liver fibrosis induced by carbon tetrachloride in mice. Artif Cells Nanomed Biotechnol. 2024;52(1):23–34. doi:10.1080/21691401.2023.2239522
62. Chen R, Ma H, Li X, et al. A novel drug with potential to treat hyperbilirubinemia and prevent liver damage induced by hyperbilirubinemia: carbon dots derived from Platycodon Grandiflorum. Molecules. 2023;28(6):2720. doi:10.3390/molecules28062720
63. Guo Y, Chen R, Cao T, et al. Discovery of nano-components of Salvia Miltiorrhiza Carbonisatum and their protective effect on liver injury induced by hyperbilirubinemia in mice. Guiding Journal of Traditional Chinese Medicine and Pharmacy. 2023;29:6–11.
64. Chiu PW. Endoscopic Management of Peptic Ulcer Bleeding: recent Advances. Clin Endosc. 2019;52(5):416–418. doi:10.5946/ce.2018.182
65. Hu J, Luo J, Zhang M, et al. Protective effects of Radix Sophorae Flavescentis Carbonisata-based carbon dots against ethanol-induced acute gastric ulcer in rats: anti-inflammatory and antioxidant activities. Int J Nanomed. 2021;16:2461–2475. doi:10.2147/IJN.S289515
66. Luo J, Hu J, Zhang M, et al. Gastroprotective effects of Nelumbinis Rhizomatis Nodus-derived carbon dots on ethanol-induced gastric ulcers in rats. Nanomedicine. 2021;16(19):1657–1671. doi:10.2217/nnm-2020-0472
67. Liu Y, Zhang M, Cheng J, et al. Novel carbon dots derived from Glycyrrhizae Radix et Rhizoma and their anti-gastric ulcer effect. Molecules. 2021;26(6):1512. doi:10.3390/molecules26061512
68. Lu F, Ma Y, Huang H, et al. Edible and highly biocompatible nanodots from natural plants for the treatment of stress gastric ulcers. Nanoscale. 2021;13(14):6809–6818. doi:10.1039/D1NR01099A
69. Zhai C, Lu F, Du X, et al. Green carbon dots derived from Atractylodes macrocephala: a potential nanodrug for treating alcoholic gastric ulcer. Colloids Surf B Biointerfaces. 2023;230:113492. doi:10.1016/j.colsurfb.2023.113492
70. Zhao Y, Cheng G, Gao Y, et al. Green synthetic natural carbon dots derived from Fuligo Plantae with inhibitory effect against alcoholic gastric ulcer. Front Mol Biosci. 2023;10:1223621. doi:10.3389/fmolb.2023.1223621
71. Pavan E, Damazo AS, Arunachalam K, et al. Copaifera malmei Harms leaves infusion attenuates TNBS-ulcerative colitis through modulation of cytokines, oxidative stress and mucus in experimental rats. J Ethnopharmacol. 2021;267:113499. doi:10.1016/j.jep.2020.113499
72. Mou Y, Bai X, Ma H, et al. Protective effect of carbon dots derived from scrambled Coptidis Rhizoma against ulcerative colitis in mice. Front Mol Biosci. 2023;10:1253195. doi:10.3389/fmolb.2023.1253195
73. Zhang Y, Zhao J, Zhao Y, et al. The Rhei radix rhizoma-based carbon dots ameliorates dextran sodium sulphate-induced ulcerative colitis in mice. Artif Cells Nanomed Biotechnol. 2023;51(1):180–191. doi:10.1080/21691401.2023.2197947
74. Zhang M, Liu Y, Qu H. Protective Effect of Nanoparticles from Platycladi Cacumen Carbonisata on 2,4,6-Trinitrobenzene Sulfonic Acid (TNBS)-Induced Colitis in Rats. J Biomed Nanotechnol. 2022;18(2):422–434. doi:10.1166/jbn.2022.3248
75. Chen R, Zhao J, Kong R, et al. Discovery of dried Rehmanniae Radix Carbonisata nano-components and their therapeutic effect on ulcerative colitis. Chin Traditional Herbal Drugs. 2023;54:16):5172–5181.
76. Enzmann G, Kargaran S, Engelhardt B. Ischemia-reperfusion injury in stroke: impact of the brain barriers and brain immune privilege on neutrophil function. Ther Adv Neurol Disord. 2018;11:1756286418794184. doi:10.1177/1756286418794184
77. Halladin NL. Oxidative and inflammatory biomarkers of ischemia and reperfusion injuries. Dan Med J. 2015;62(4):B5054.
78. Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14(3):133–150. doi:10.1038/nrneurol.2017.188
79. Zhang Y, Wang S, Lu F, et al. The neuroprotective effect of pretreatment with carbon dots from Crinis Carbonisatus (carbonized human hair) against cerebral ischemia reperfusion injury. J Nanobiotechnology. 2021;19(1):257. doi:10.1186/s12951-021-00908-2
80. Lu Q, Xiong J, Yuan Y, et al. Minocycline improves the functional recovery after traumatic brain injury via inhibition of aquaporin-4. Int J Biol Sci. 2022;18(1):441–458. doi:10.7150/ijbs.64187
81. Cash A, Theus MH. Mechanisms of blood-brain barrier dysfunction in traumatic brain injury. Int J mol Sci. 2020;21(9):3344. doi:10.3390/ijms21093344
82. Luo W, Zhang L, Li X, et al. Green functional carbon dots derived from herbal medicine ameliorate blood-brain barrier permeability following traumatic brain injury. Nano Res. 2022;15(10):9274–9285. doi:10.1007/s12274-022-4616-8
83. Chen H, Gu L, Yang Y, et al. GABA and 5-HT systems are involved in the anxiolytic effect of Gan-Mai-Da-Zao decoction. Front Neurosci. 2019;12:1043. doi:10.3389/fnins.2018.01043
84. Zhao Y, Lu F, Zhang Y, et al. Water-soluble carbon dots in cigarette mainstream smoke: their properties and the behavioural, neuroendocrinological, and neurotransmitter changes they induce in mice. Int J Nanomed. 2021;16:2203–2217. doi:10.2147/IJN.S291670
85. Chen Y, Xiong W, Zhang Y, et al. Carbon dots derived from Os Draconis and their anxiolytic effect. Int J Nanomed. 2022;17:4975–4988. doi:10.2147/IJN.S382112
86. Cui L, Zhang Q, Zhang Y, et al. Anxiolytic effects of Chrysanthemum morifolium Ramat Carbonisata-based carbon dots in mCPP-induced anxiety-like behavior in mice: a nature-inspired approach. Front Mol Biosci. 2023;10:1222415. doi:10.3389/fmolb.2023.1222415
87. Misra U, Kalita J, Tripathi G, et al. Role of β endorphin in pain relief following high rate repetitive transcranial magnetic stimulation in migraine. Brain Stimul. 2017;10(3):618–623. doi:10.1016/j.brs.2017.02.006
88. Haroutiunian S, Kagan L, Yifrach-Damari I, et al. Enhanced antinociceptive efficacy of epidural compared with i.v. methadone in a rat model of thermal nociception. Br J Anaesth. 2014;112(1):150–158. doi:10.1093/bja/aet234
89. Zhang M, Cheng J, Zhang Y, et al. Green synthesis of Zingiberis Rhizoma-based carbon dots attenuates chemical and thermal stimulus pain in mice. Nanomedicine. 2020;15(9):851–869. doi:10.2217/nnm-2019-0369
90. Zhao Y, Luo J, Xing H, et al. The discovery of new carbon point in Terra Flava Usta and its analgesic effect. J Chinese Med Mater. 2019;42(12):2882–2886.
91. Innocenzi P, Stagi L. Carbon-based antiviral nanomaterials: graphene, C-dots, and fullerenes. A perspective. Chem Sci. 2020;11(26):6606–6622. doi:10.1039/D0SC02658A
92. Chen L, Liang J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater Sci Eng C Mater Biol Appl. 2020;112:110924. doi:10.1016/j.msec.2020.110924
93. Tong T, Hu H, Zhou J, et al. Glycyrrhizic-Acid-Based Carbon Dots with High Antiviral Activity by Multisite Inhibition Mechanisms. Small. 2020;16(13):e1906206. doi:10.1002/smll.201906206
94. Lin C, Chang L, Chu H, et al. High amplification of the antiviral activity of Curcumin through transformation into carbon quantum dots. Small. 2019;15(41):e1902641. doi:10.1002/smll.201902641
95. Wang H, Zhang M, Ma Y, et al. Selective inactivation of Gram-negative bacteria by carbon dots derived from natural biomass: Artemisia argyi leaves. J Mater Chem B. 2020;8(13):2666–2672. doi:10.1039/C9TB02735A
96. Lu F, Ma Y, Wang H, et al. Water-solvable carbon dots derived from curcumin and citric acid with enhanced broad-spectrum antibacterial and antibiofilm activity. Mater Today Commun. 2021;26:102000. doi:10.1016/j.mtcomm.2020.102000
97. Lin R, Cheng S, Tan M. Green synthesis of fluorescent carbon dots with antibacterial activity and their application in Atlantic mackerel (Scomber scombrus) storage. Food Funct. 2022;13(4):2098–2108. doi:10.1039/D1FO03426J
98. Yao H, Li J, Song Y, et al. Synthesis of ginsenoside Re-based carbon dots applied for bioimaging and effective inhibition of cancer cells. Int J Nanomed. 2018;13:6249–6264. doi:10.2147/IJN.S176176
99. Li C, Ou C, Huang C, et al. Carbon dots prepared from ginger exhibiting efficient inhibition of human hepatocellular carcinoma cells. J Mater Chem B. 2014;2(28):4564–4571. doi:10.1039/c4tb00216d
100. Yao L, Zhao M, Luo Q, et al. Carbon quantum dots-based nanozyme from coffee induces cancer cell ferroptosis to activate antitumor immunity. ACS Nano. 2022;16(6):9228–9239. doi:10.1021/acsnano.2c01619
101. Xu Y, Wang B, Zhang M, et al. Carbon dots as a potential therapeutic agent for the treatment of cancer-related anemia. Adv Mater. 2022;34(19):e2200905. doi:10.1002/adma.202200905
102. Ye R, Liu Z. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp Mol Pathol. 2020;113:104350. doi:10.1016/j.yexmp.2019.104350
103. Zhao Y, Zhang Y, Kong H, et al. Protective effects of carbon dots derived from Armeniacae Semen Amarum Carbonisata against acute lung injury induced by lipopolysaccharides in rats. Int J Nanomed. 2022;17:1–14. doi:10.2147/IJN.S338886
104. Zhao Y, Li W, Cao T, et al. Research on the material basis and mechanism of Descurainiae Semen Carbonisatum in the treatment of acute lung injury based on nanotechnology. Chin Traditional Herbal Drugs. 2021;52(20):6188–6196.
105. Garami A, Steiner AA, Romanovsky AA. Fever and hypothermia in systemic inflammation. Handb Clin Neurol. 2018;157:565–597. doi:10.1016/B978-0-444-64074-1.00034-3
106. Wu J, Zhang M, Cheng J, et al. Effect of Lonicerae japonicae Flos Carbonisata-derived carbon dots on rat models of fever and hypothermia induced by lipopolysaccharide. Int J Nanomed. 2020;15:4139–4149. doi:10.2147/IJN.S248467
107. González C, Franco M, Londoño A, et al. Breaking paradigms in the treatment of psoriasis: use of botulinum toxin for the treatment of plaque psoriasis. Dermatol Ther. 2020;33(6):e14319. doi:10.1111/dth.14319
108. Lai C, Su Y, Lin K, et al. Natural Modulators of Endosomal Toll-Like Receptor-Mediated Psoriatic Skin Inflammation. J Immunol Res. 2017;2017:7807313. doi:10.1155/2017/7807313
109. Sato Y, Ogawa E, Okuyama R. Role of innate immune cells in psoriasis. Int J mol Sci. 2020;21(18):6604. doi:10.3390/ijms21186604
110. Zhang M, Cheng J, Hu J, et al. Green Phellodendri Chinensis Cortex-based carbon dots for ameliorating imiquimod-induced psoriasis-like inflammation in mice. J Nanobiotechnology. 2021;19(1):105. doi:10.1186/s12951-021-00847-y
111. Sun Z, Lu F, Cheng J, et al. Hypoglycemic bioactivity of novel eco-friendly carbon dots derived from traditional Chinese medicine. J Biomed Nanotechnol. 2018;14(12):2146–2155. doi:10.1166/jbn.2018.2653
112. Lu F, Zhang Y, Cheng J, et al. Maltase and sucrase inhibitory activities and hypoglycemic effects of carbon dots derived from charred Fructus crataegi. Mater Res Express. 2019;6(12):125005. doi:10.1088/2053-1591/ab4fd8
113. Blat Y. Non-competitive inhibition by active site binders. Chem Biol Drug Des. 2010;75(6):535–540. doi:10.1111/j.1747-0285.2010.00972.x
114. Zhang Y, Chen Y, Bai X, et al. Glycyrrhizae radix et Rhizoma-derived carbon dots and their effect on menopause syndrome in ovariectomized mice. Molecules. 2023;28(4):1830. doi:10.3390/molecules28041830
115. Yoshida EH, Dini MMJ, Campanholi J, et al. Acute kidney injury caused by the intraperitoneal injection of Bothrops jararaca venom in rats. Nat Prod Res. 2020;34(17):2533–2538. doi:10.1080/14786419.2018.1543675
116. Zhang M, Cheng J, Sun Z, et al. Protective effects of carbon dots derived from Phellodendri Chinensis Cortex Carbonisata against deinagkistrodon acutus venom-induced acute kidney injury. Nanoscale Res Lett. 2019;14(1):377. doi:10.1186/s11671-019-3198-1
117. Dong L, Cao T, Guo Y, et al. Aristolochic Acid Nephropathy: a novel suppression strategy of carbon dots derived from Astragali Radix Carbonisata. J Biomed Nanotechnol. 2022;18(8):1963–1974. doi:10.1166/jbn.2022.3403
118. Wang X, Wu T, Yang Y, et al. Ultrasmall and highly biocompatible carbon dots derived from natural plant with amelioration against acute kidney injury. J Nanobiotechnology. 2023;21(1):63. doi:10.1186/s12951-023-01795-5
119. Roberts RL, Wallace MC, Phipps-Green AJ, et al. ABCG2 loss-of-function polymorphism predicts poor response to allopurinol in patients with gout. Pharmacogenomics J. 2017;17(2):201–203. doi:10.1038/tpj.2015.101
120. Wang X, Zhang Y, Zhang M, et al. Novel carbon dots derived from Puerariae lobatae Radix and their anti-gout effects. Molecules. 2019;24(22):4152. doi:10.3390/molecules24224152
121. Wang S, Zhang Y, Kong H, et al. Antihyperuricemic and anti-gouty arthritis activities of Aurantii Fructus Immaturus Carbonisata-derived carbon dots. Nanomedicine. 2019;14(22):2925–2939. doi:10.2217/nnm-2019-0255
122. Wang Z, Xu J, Wan J, et al. Vascular analysis of soft tissues around the bone lesion in osteoradionecrosis, medication-related osteonecrosis, and infectious osteomyelitis of the jaw. J Craniofac Surg. 2022;33(7):e750–e754. doi:10.1097/SCS.0000000000008697
123. Guo Z, Wang Z, Liu Y, et al. Carbon dots from Lycium Barbarum attenuate radiation-induced bone injury by inhibiting senescence via METTL3/Clip3 in an m6A-dependent manner. ACS Appl Mater Interfaces. 2023;15(17):20726–20741. doi:10.1021/acsami.3c01322
124. Kong H, Zhao Y, Cao P, et al. The bioactivity of Scutellariae Radix Carbonisata-derived carbon dots: antiallergic effect. J Biomed Nanotechnol. 2021;17(12):2485–2494. doi:10.1166/jbn.2021.3200
125. Zhao Y, Li L, Li W, et al. Material basis of Granati Pericarpium Carbonisatum for antidiarrheal effect from perspective of nanomaterials. Chin Traditional Herbal Drugs. 2021;52(05):1335–1342.
126. Liu J, Ma L, Zhang G, et al. Recent progress of surface modified nanomaterials for scavenging reactive oxygen species in organism. Bioconjug Chem. 2021;32(11):2269–2289. doi:10.1021/acs.bioconjchem.1c00402
127. Yang B, Chen Y, Shi J. Reactive oxygen species (ROS)-based nanomedicine. Chem Rev. 2019;119(8):4881–4985. doi:10.1021/acs.chemrev.8b00626
128. Jia J, Lin B, Gao Y, et al. Highly luminescent N-doped carbon dots from black soya beans for free radical scavenging, Fe3+ sensing and cellular imaging. Spectrochim Acta A Mol Biomol Spectrosc. 2019;211:363–372. doi:10.1016/j.saa.2018.12.034
129. Sharma N, Das GS, Yun K. Green synthesis of multipurpose carbon quantum dots from red cabbage and estimation of their antioxidant potential and bio-labeling activity. Appl Microbiol Biotechnol. 2020;104(16):7187–7200. doi:10.1007/s00253-020-10726-5
130. Wei X, Li L, Liu J, et al. Green synthesis of fluorescent carbon dots from Gynostemma for bioimaging and antioxidant in zebrafish. ACS Appl Mater Interfaces. 2019;11(10):9832–9840. doi:10.1021/acsami.9b00074
131. Sun L, Zhang R, Zhang T, et al. Synthesis, applications and biosafety evaluation of carbon dots derived from herbal medicine. Biomed Mater. 2023;18(4):8. doi:10.1088/1748-605X/acdeb8
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

