Back to Journals » International Journal of Nanomedicine » Volume 19
Effect of C60 Fullerene on Muscle Injury-Induced Rhabdomyolysis and Associated Acute Renal Failure
Authors Prylutskyy Y, Nozdrenko D, Omelchuk O, Prylutska S, Motuziuk O, Soroсa V, Vareniuk I, Stetska V , Bogutska K , Ritter U, Piosik J
Received 17 May 2024
Accepted for publication 19 July 2024
Published 6 August 2024 Volume 2024:19 Pages 8043—8058
DOI https://doi.org/10.2147/IJN.S468013
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sachin Mali
Yuriy Prylutskyy,1 Dmytro Nozdrenko,1 Olexandr Omelchuk,2 Svitlana Prylutska,3 Olexandr Motuziuk,1,2 Vasil Soroсa,1 Igor Vareniuk,1 Viktoria Stetska,1 Kateryna Bogutska,1 Uwe Ritter,4 Jacek Piosik5
1ESC ”institute of Biology and Medicine”, Taras Shevchenko National University of Kyiv, Kyiv, Ukraine; 2Faculty of Biology and Forestry, Lesya Ukrainka Volyn National University, Lutsk, Ukraine; 3Faculty of Plant Protection, Biotechnology and Ecology, National University of Life and Environmental Science of Ukraine, Kyiv, Ukraine; 4Institute of Chemistry and Biotechnology, Technical University of Ilmenau, Ilmenau, Germany; 5Intercollegiate Faculty of Biotechnology, University of Gdansk, Gdańsk, Poland
Correspondence: Jacek Piosik, Email [email protected]
Introduction: Rhabdomyolysis, as an acute stage of myopathy, causes kidney damage. It is known that this pathology is caused by the accumulation of muscle breakdown products and is associated with oxidative stress. Therefore, the present study evaluated the effect of intraperitoneal administration (dose 1 mg/kg) of water-soluble C60 fullerenes, as powerful antioxidants, on the development of rat kidney damage due to rhabdomyolysis caused by mechanical trauma of the muscle soleus of different severity (crush syndrome lasting 1 min under a pressure of 2.5, 3.5, and 4.5 kg/cm2, respectively).
Methods: Using tensometry, biochemical and histopathological analyses, the biomechanical parameters of muscle soleus contraction (contraction force and integrated muscle power), biochemical indicators of rat blood (concentrations of creatinine, creatine phosphokinase, urea and hydrogen peroxide, catalase and superoxide dismutase activity), glomerular filtration rate and fractional sodium excretion value, as well as pathohistological and morphometric features of muscle and kidney damages in rats on days 1, 3, 6 and 9 after the initiation of the injury were studied.
Results: Positive changes in biomechanical and biochemical parameters were found during the experiment by about 27– 30 ± 2%, as well as a decrease in pathohistological and morphometric features of muscle and kidney damages in rats treated with water-soluble C60 fullerenes.
Conclusion: These findings indicate the potential application of water-soluble C60 fullerenes in the treatment of pathological conditions of the muscular system caused by rhabdomyolysis and the associated oxidative stress.
Keywords: muscle soleus, С60 fullerene, rhabdomyolysis, biomechanical parameters of muscle contraction, blood biochemical indicators, histopathological analysis
Introduction
Acute renal failure (ARF) is a condition in which there is a rapid decline in kidney function, in particular, there is a decrease or even complete absence of urine production, which leads to the accumulation of nitrogen metabolism products in the blood. Recently, a significant number of ARF cases have been associated with rhabdomyolysis of skeletal muscle. Rhabdomyolysis is a clinical syndrome of muscle disorder caused by the destruction of skeletal muscle fibers, large artery occlusion, epileptic status, infections, etc. It is characterized by the release of skeletal muscle cell contents into the bloodstream, including myoglobin, labile iron ions, and various toxic metabolites,1 followed by a potentially lethal accumulation of toxins, including potassium, lactic acid, and myoglobin, leading to multiple organ failure, primarily acute kidney injury.2 This pathology affects up to 46% of hospitalized patients and 80% of patients requiring intensive care for rhabdomyolysis.3 Even with effective medical care, the mortality rate exceeds 25%.4 Over the past decade, the incidence of rhabdomyolysis has increased 10-fold,5 particularly for wounded military personnel due to ballistic trauma to skeletal muscle,6,7 who are 5 times more likely to develop rhabdomyolysis than civilians.8 Global mortality from rhabdomyolysis is estimated at 10% to 46% depending on the severity, cause, early treatment, and the presence of comorbidities and complications.9
Currently, there is no specific treatment for rhabdomyolysis. Dialysis remains the main treatment for patients with ARF caused by rhabdomyolysis. Dialysis is believed to remove myoglobin released from damaged muscles and metabolic toxins from the bloodstream. Standard medical interventions are usually massive hydration, urine alkalization, and forced diuresis. It is reported that 28% to 65% of patients who develop rhabdomyolysis require mandatory dialysis therapy.10
Note that traumas of various natures can cause acute kidney injury (AKI). It is the early diagnosis of AKI, in particular, thanks to the use of nanoparticles11 and recently discovered biomarkers,12 ensures its effective therapy in the future. The burst of reactive oxygen species (ROS) plays a key role in the pathological progression of AKI. Therefore, mitochondrial-targeted antioxidant therapy is very promising because mitochondria are the main source of ROS in AKI. It was shown that antioxidant nanodrugs, in particular ultra-small Tungsten-based nanodots, with actively targeted mitochondria have achieved encouraging success in many oxidative stress-induced diseases.13 Many new antioxidant nanodrugs with intrinsic kidney targeting through the control of size, shape, and surface properties have opened exciting prospects for the treatment of AKI.14,15
ARF causes a general or regional decrease in renal blood flow. Subsequent renal hypoxia and ischemia contribute to the formation of ROS, which damage biomolecules and membranes, including renal tubular cells. It has been shown that various exogenous antioxidants have a significant effect on the course of ARF induced by rhabdomyolysis. For example, ascorbate (vitamin C) reduces rhabdomyolysis kidney damage in humans.16 It has been shown that the increase in the level of lipid peroxidation products (LPO) and the activity of antioxidant enzymes (superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase) was reversed by the administration of D-panthenol.17 Its use improved renal morphology and reduced ARF markers through antioxidant effects and normalization of mitochondrial metabolism. It was found that allopurinol administration attenuated renal dysfunction in the ARF model associated with rhabdomyolysis by reducing oxidative stress (systemic, renal and muscle), apoptosis, and inflammation. Allopurinol also reduced oxidative stress in injured muscles, attenuated muscle damage/inflammation, and accelerated muscle recovery.18 Acetaminophen has been shown to reduce kidney damage and improve kidney function by counteracting ROS and free radical formation in a rat model of rhabdomyolysis.19 Study20 found that N-acetylcysteine improves renal function. It has been shown that biocompatible and bioavailable carbon nanoparticles C60 fullerenes with actively targeted mitochondria21 are powerful scavengers of ROS induced by muscle damage: their application in vivo resulted in significant positive therapeutic effects after the initiation of ischemic injury,22 fatigue,23 atrophy,24 and skeletal muscle injury,25 as well as pesticide poisoning.26 Finally, in our previous work,27 we demonstrated a protective effect of water-soluble C60 fullerene on the development of ARF in a rat model of glycerol-induced rhabdomyolysis.
Based on the above-mentioned data, the aim of this work was to study the effect of water-soluble C60 fullerenes on the development of rhabdomyolysis caused by muscle soleus mechanical trauma of different degrees of severity and associated ARF by evaluating biomechanical parameters of muscle contraction (contraction force and integrated muscle power) and biochemical parameters of laboratory animals blood (concentrations of creatinine, creatine phosphokinase (CPK), urea and hydrogen peroxide, CAT and SOD activity), as well as glomerular filtration rate (GFR) and fractional excretion of sodium (FENa). Additionally, the pathohistological and morphometric features of muscle and kidney damage in rats after the initiation of the injury were evaluated.
Materials and Methods
Material Preparation
An aqueous solution of pristine C60 fullerene with a purity of more than 99.95% has been prepared according the method.28 Briefly, it is based on the transfer of C60 molecules from toluene to water followed by sonication. Microscopic and spectroscopic data indicate that an aqueous solution of C60 fullerene at a maximum concentration of 0.15 mg/mL is a typical colloid containing both single C60 molecules (~0.7 nm in diameter) as well as their nanoaggregates (up to ~100 nm)29 and remains highly stable (zeta potential value is −25.3 mV30) for 18 months at a temperature +4 °C.
It is important to note that due to its nanosized almost spherical shape, high chemical stability, and unique physical properties, C60 fullerene is of the greatest interest for biomedical research.31 In particular, C60 fullerene exhibits a strong reducing ability, readily attaching up to six electrons simultaneously. Due to this, it acts in in vito and in vivo systems as a powerful scavenger of free radicals,32,33 the overproduction of which leads to many pathologies.
Animal Model
All experiments were performed on rats in accordance with the international principles of the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (Strasbourg, 1986), Article 26 of the Law of Ukraine “On the Protection of Animals from Cruelty” (No. 3447-IV, February 21, 2006) as well as the European Union Directive (2010/63/EU) “On the protection of animals used for scientific purposes”. The research protocols were approved by the Bioethics Committee of the ESC ‘Institute of Biology and Medicine’ of the Taras Shevchenko National University of Kyiv (Protocol No. 2 of September 2, 2022).
Experiments were performed on male Wistar rats weighing 150–180 g, randomly divided into the following groups:
- control group (n = 7);
- model pathology groups of 1 (n=7), 2 (n=7), and 3 (n=7) severity degrees;
- model pathology groups of 1 (n=7), 2 (n=7), and 3 (n=7) severity degrees after administration of water-soluble C60 fullerenes.
Muscle trauma was induced by compressing the muscle for 1 min with a clamp under pressure of 2.5 (1st degree of severity), 3.5 (2nd degree of severity) and 4.5 (3rd degree of severity) kg/cm2.34 The applied crush syndrome led to a systemic manifestation of pathological changes due to the destruction of muscle cells, in particular, the release of muscle cell components (creatine kinase, myoglobin) into the extracellular environment, which served as a marker of a muscle injury severity.
It should be noted that the applied dose of water-soluble C60 fullerenes of 1 mg/kg was chosen as the most effective when studying their antioxidant effect in other models of muscle pathologies.22–26 In addition, it is significantly lower than the LD50 value, which was 600 mg/kg in the case of oral administration to rats32 and 721 mg/kg in the case of intraperitoneal administration to mice.30 An aqueous solution of C60 fullerene was administered intraperitoneally daily throughout the experiment, starting on the first day immediately after the initiation of muscle injury.
Animals were anesthetized by intraperitoneal injection of nembutal (40 mg/kg). The rat soleus muscle was freed from the surrounding tissues. Its tendon part was cut across the distal part of the muscle, which was connected to the force sensors. Efferents were stimulated with electrical pulses of 2 ms duration, generated by an ADC (analog-to-digital converter) pulse generator through platinum electrodes.22–26
Biomechanical and Biochemical Analysis
In the process of analyzing the biomechanical results, the following parameters were used: minimum and maximum muscle contraction forces when performing 6 s of stimulation pool and integrated muscle power (calculated area under the force curve), which is an indicator of its overall performance.22–26
The content of creatinine, CPK, urea, and hydrogen peroxide, CAT, and SOD activity in the blood of experimental animals as muscle and kidney dysfunction markers were determined using clinical diagnostic equipment - biochemical analyzers RNL-200 and JN-1101-TR2 (Netherlands).22–26
GFR was determined by the clearance of endogenous creatinine:35 GFR (μL/min)=(V·CrU)/CrB, where V is urine volume (μL/min; minute diuresis), CrU (μmole/l) and CrB (μmole/l) are the concentrations of creatinine in urine and blood, respectively.
The FENa value (the ratio between glomerular filtration of sodium and its reabsorption) was used to assess sodium transport in the kidneys:36 FENa(%)=100%×(CrB×NaU)/(NaB×CrU), where NaU and NaB are the concentrations of sodium in urine and blood, respectively.
Histopathological Analysis
The samples of a kidney and a muscle were separated and fixed in 10% formalin, embedded in paraffin, cut into 5 µm sections, and stained with hematoxylin and eosin (H&E) for general histopathological analyses.37 Sections of the muscle were additionally stained with hematoxylin and picrofuchsin by van Gieson for detecting connective tissue and muscle fibers. Digital microphotographs of stained sections were taken at ×100 and ×400 magnification using a computer-assisted image analyzing system (consisting of Olympus BX41 microscope and Olympus C-5050 Zoom digital camera). The histopathological profiles of the muscles and kidneys were determined by light microscopy observation. Then, the muscle fiber diameters and the area occupied by connective tissue in the muscle bundles were measured using ImageJ software. In addition, the cross-sectional area of renal glomeruli and diameter of renal tubes were measured in the kidneys using ImageJ software.
Statistical Analysis
Statistical evaluation of the results was performed using the procedure of analysis of variances (ANOVA) with mixed design. Two between-group factors were supposed: 1) injury (three levels – 1st, 2nd, and 3rd degree of severity); 2) C60 fullerene treatment (two levels – no and use of C60). The factor of time was supposed a within-group with four levels (1, 3, 6, and 9 days after muscle injury initiation). The Shapiro–Wilk W-test was used to test for normality. Levene’s test was used to assess the equality of variances across groups. Multiple pairwise comparisons between different groups and conditions were performed by the Bonferroni post-hoc test. The differences between the groups were considered significant at P < 0.05. The statistical evaluation was performed by the software package Statistica 8.0 (Dell, USA).
Results and Discussion
Biomechanics of Muscle Contraction
Figure 1 shows the generation curves of muscle soleus contraction force on days 1, 3, 6, and 9 after muscle injury initiation of 1, 2, and 3 degrees of severity. Thus, on the first day after the injury, integrated muscle power decreased to 69 ± 3%, 43 ± 2%, and 24 ± 1% of control values at 1, 2, and 3 degrees of severity, respectively (Figure 1A and C). On the third day, these indices amounted to 29 ± 1%, 17 ± 1%, and 11 ± 1%, respectively, in comparison with the control (Figure 1A and C), which confirms the data on the maximum dysfunction of the injured muscle on the third day after the injury.38,39 On the sixth day, a significant increase in the integrated power of the muscle was recorded up to 62 ± 2%, 55 ± 3%, and 41 ± 3% at 1, 2, and 3 degrees of severity, respectively (Figure 1A and C). Finally, on the ninth day, the value of this parameter was 83 ± 5%, 79 ± 3%, and 75 ± 3%, respectively, relative to the control (Figure 1A and C), which is consistent with the data on the recovery of muscle function on the ninth day after injury. It should be emphasized that during a long period (2–3 months) the performance of the injured muscle does not fully recover:40,41 in our case, its level on the ninth day after injury initiation was 75–80 ± 3%% of the control and remained so during 1 month.
The injections of C60 fullerene aqueous solution increased integrated muscle power by 18 ± 1%, 13 ±1%, and 14 ± 2% at severity grades 1, 2, and 3, respectively, on the first day after injury and by 23 ± 2%, 18 ± 2% and 11 ± 1%, respectively, on the third day (Figure 1B and C). On the sixth and ninth day, these parameters did not change significantly (Figure 1B and C) relative to the injury group. It should be noted that there was a slight difference in the positive effect of C60 fullerene between the different severity levels of muscle injury on both the first and ninth days of the experiment. Thus, C60 fullerene aqueous solution injections increased the level of integrated muscle power by 15–25 ± 2% relative to the injury group on the ninth day of the experiment.
When performing single-joint movements, such biomechanical indices as the generation levels of maximum and minimum force of muscle contraction are the main markers of muscle dysfunction in the development of pathological processes.22–26 The positive effect of C60 fullerene injections on the level of minimal force (the most sensitive marker of muscle dysfunction) did not exceed 10 ± 2% relative to the injury group on the first day of the experiment (Figure 2A). On the third day (the day of most maximal dysfunction of the injured muscle), this index increased significantly and was 29 ± 2%, 73 ± 3%, and 75 ± 4% at severity grades 1, 2, and 3, respectively (Figure 2B). On the sixth and ninth day, it amounted to 45–50 ± 2% relative to the injury group (Figure 2C and D). The change in the level of maximal force of muscle contraction at injections of C60 fullerene amounted to 12–16 ± 1% relative to the injury group at all time periods of the experiment (Figure 2). The obtained data testify to the suppressive effect of the aqueous solution of C60 fullerene on free-radical processes in the injured muscle, thus promoting myocytes to generate the minimum force at a higher level. The insignificant positive effect of C60 fullerene on the level of maximal force generation in the injured muscle may be related to the free-radical processes that occur inside the injured myocytes and prevent actomyosin interactions.
Thus, biomechanical studies show that C60 fullerene aqueous solution injections significantly reduce the level of development of pathologic processes in injured muscle, which, in turn, may influence the level of ARF-initiated rhabdomyolysis.
Biochemical Analysis
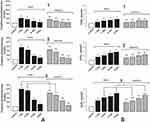
Blood creatinine level is an important marker of muscle injury as well as ARF.42 In rhabdomyolysis’ ARF, it can increase 2 (with 1 degree of injury severity), 3 (with 2 degree of injury severity), 4 times or higher (with 3 degree of injury severity) from baseline.2 The data obtained (Figure 3A) confirm the significant increase in creatinine level with increasing severity of muscle injury. However, on the third day of the experiment, the creatinine level decreased compared to the first day after injury initiation by 11 ± 1%, 14 ± 1%, and 23 ± 1% at 1, 2, and 3 degrees of severity, respectively, relative to the control. In our opinion, this is due to the cessation of the release of intracellular myocyte contents into the circulating blood. On the sixth and ninth day after the injury, creatinine levels increased dramatically by 150–170 ± 5% and 300–450 ± 5% at 1 and 3 injury severity levels, respectively. Such an increase indicates the development of ARF caused by rhabdomyolysis.
C60 fullerene aqueous solution injections lead to a 14–22 ± 1%, 21–24 ± 2%, and 30–40 ± 2% decrease in the level of creatinine at the 1st, 2nd, and 3rd degree of injury severity, respectively, relative to the injury group during the experiment (Figure 3A).
GFR is an important indicator that characterizes the ability of the kidneys to perform their main function - to clear plasma and produce urine.43 During the development of rhabdomyolysis’ ARF outside the proximal convoluted tubule (in the thick ascending part of the loop of Henle), urinary myoglobin combines with Tamm-Horsfall protein to form a precipitate. pH-dependent precipitate forms tubular casts that obstruct the distal tubules.44 Obstruction is thought to increase intra-canalicular pressure above interstitial pressure, reducing vascular inflow and perfusion, promoting inflammation, and directly reducing GFR by altering Starling forces, and the effect of antioxidants on this process may reduce ARF development.43,44
Decrease in GFR value was recorded starting from the third day after injury initiation (Figure 3B). On the ninth day, the difference from control values was 21 ± 1%, 43 ± 4%, and 67 ± 4% at 1, 2, and 3 degrees of injury severity, respectively. When applying C60 fullerene injections, this index increased approximately by 17 ± 1%, 21 ± 1%, and 27 ± 1% at 1, 2, and 3 degrees of injury severity, respectively, relative to the injury group. At the same time, it should be noted a slight difference in the GFR values (within the error) on the sixth and ninth day of the experiment (Figure 3B).
When ARF develops, the concentration in the blood of nitrogenous slag, which can only be excreted by the body with urine, increases significantly, which is a sign of deteriorating kidney function. Urea is one of the end products of protein metabolism that contains nitrogen. The result of a blood urea test is one of the main indicators of the quality of glomerular filtration in ARF development.
An increase in urea levels was observed starting from the third day of the experiment and amounted to 83–156 ± 5%, 89–211 ± 5%, and 203–340 ± 7% of the control at 1, 2, and 3 muscle injury severity degrees, respectively, on days 3–9 of the experiment (Figure 4A). C60 fullerene injections decreased urea concentration by 17–18 ± 1%, 20–22 ± 1%, and 24–27 ± 2% at 1, 2, and 3 degrees of injury severity, respectively, during the experiment (Figure 4A).
Also, one of the important quantitative indicators of the degree of ARF development is sodium transport assessment, since the mechanism of free-radical inhibition of reabsorption in rhabdomyolysis is associated with the inhibition of sodium-potassium ATPase transport system, and the action of antioxidants can reduce the development of cascade pathological reactions.45 On the third day of the experiment, the FENa value increased by 31 ± 1%, 98 ± 3%, and 141 ± 4% relative to the control at 1, 2, and 3 injury severity, respectively (Figure 4B). On the sixth and ninth day, this index increased by 45–271 ± 5% and 128–387 ± 7% relative to the control at 2 and 3 injury severity, respectively. Injections of C60 fullerene aqueous solution reduced FENa value by 13–15 ± 1%, 20–23 ± 1%, and 25–32 ± 2% at 1, 2, and 3 muscle injury severity, respectively, relative to the injury group during the experiment (Figure 4B).
Thus, the application of C60 fullerene demonstrated significant (15–35 ± 2%) positive effects on the main markers of ARF induced by rhabdomyolysis. Note that their most significant reduction is observed in severe forms of rhabdomyolysis on the ninth day after muscle injury initiation.
Recently, CPK concentration measurement in blood has become a clinical test to determine the level of rhabdomyolysis development due to its rapidity and cheapness.5 The relationship between CPK level increase and ARF development in patients with muscle injuries has been established.46 CPK is an enzyme from the energy supply system of skeletal muscle cells, which catalyzes the transfer of phosphate group from ATP to creatinine molecule to form the high-energy compound creatinine phosphate. In case of mechanical muscle damage, there is a release of this enzyme from the cells and, accordingly, an increase in its concentration in the blood.
CPK levels on the first day after injury rose by 112 ± 3%, 234 ± 7%, and 414 ± 7% compared with control values at 1, 2, and 3 degrees of muscle injury severity, respectively (Figure 5A). CPK levels decreased on the following days of the experiment and on the ninth day were 13–25 ± 1% of control values, despite an increase in ARF development on the sixth and ninth days.
C60 fullerene injections significantly reduced CPK levels, which were 24 ± 1%, 31 ± 2% and 34 ± 2% and 17 ± 1%, 36 ± 2% and 42 ± 2% at 1, 2, and 3 degrees of muscle injury severity, respectively, on the first and third day of the experiment relative to the injury group. On the ninth day, the positive effect was insignificant due to the small level of CPK increase relative to the control during this period (Figure 5A).
Pathological inflammatory processes occurring immediately after injury are a source of ROS and contribute to the intensification of LPO processes.38,47 During the physiological recovery of muscle tissue, oxygen entering the tissues initiates xanthine and hypoxanthine oxidation by xanthine oxidase, which leads to the formation of a large amount of superoxide anion radical and hydrogen peroxide. In addition, inflammatory processes increase the expression of adhesive molecules on the endothelium. Activated neutrophils attracted to the focus of damage also release hydrogen peroxide and provoke pathological free-radical processes.
Throughout the experiment, an increase in the level of H2O2 was recorded, which is associated with the muscle fiber destruction and the development of inflammatory processes. The increase in H2O2 concentration on the first and third day was 24–28 ± 1%, 42–79 ± 2%, and 98–124 ± 3% at 1, 2, and 3 injury severity levels, respectively, relative to the control (Figure 5B). Further increases in H2O2 concentration of 10 ± 1%, 14 ± 1% and 19 ± 1% and 15 ± 1%, 26 ± 2% and 33 ± 2% on the sixth and ninth day of the experiment, respectively, indicated the development of rhabdomyolysis’ ARF.
At C60 fullerene aqueous solution injections there is a decrease in H2O2 concentration by 11–12 ± 1%, 15–18 ± 1%, and 22–29 ± 1% at 1, 2, and 3 degrees of muscle injury severity, respectively, relative to the injury group during the experiment (Figure 5B).
The increase in H2O2 level during muscle fiber injury leads to an increase in CAT activity, which performs a protective antioxidant function by catalyzing the decomposition of hydrogen peroxide into water, oxygen and SOD. The most powerful natural antioxidant and enzyme of the first link of antioxidant defense is SOD, which performs the dismutation reaction of superoxide anion radicals and transforms them into less reactive hydrogen peroxide molecules.
SOD activity on the first day of the experiment increased by 25 ± 1%, 39 ± 4%, and 45 ± 2% at the 1st, 2nd, and 3rd degree of muscle injury severity, respectively, relative to the control (Figure 6A). On the third day, this index decreased by 11–14 ± 1%, which seems to be associated with a decrease in inflammatory processes in the muscle tissue. However, on the sixth and ninth day, SOD activity increased dramatically by 23–29 ± 1%, 39–41 ± 2%, and 56–59 ± 2% at injury severity grades 1, 2, and 3, respectively, compared to the third day of the experiment, which is most likely due to the development of ARF caused by rhabdomyolysis.
C60 fullerene injections decreased SOD activity by 12 ± 1% and 15 ± 1% relative to the injury group on the first and third day of the experiment. On the sixth and ninth day, the positive effects of C60 fullerene injections were 10 ± 1%, 15 ± 1%, and 29 ± 2% at 1, 2, and 3 degrees of muscle injury severity, respectively, relative to the injury group (Figure 6A).
CAT activity on the first day of the experiment increased by 111 ± 6%, 123 ± 6%, and 215 ± 8% at 1, 2, and 3 degrees of muscle injury severity, respectively, relative to the control (Figure 6B). On the third day, this index, as well as SOD activity, decreased by 15 ± 1%, 27 ± 2%, and 34 ± 6%, which seems to be associated with a decrease in inflammatory processes in muscle tissue. On the sixth and ninth day, CAT activity increased sharply by 27–31 ± 2%, 65–79 ± 3%, and 81–94 ± 3% at 1, 2, and 3 injury severity levels, respectively, compared to the third day of the experiment.
The positive therapeutic effect of C60 fullerene aqueous solution injections on CAT activity was within the changes of SOD activity in all investigated time ranges and degrees of muscle injury (Figure 6A and B). So, C60 fullerene injections decreased CAT activity by 11 ± 1%, 24 ± 1%, and 33 ± 2% relative to the injury group on day one and by 10 ± 1%, 11 ± 1%, and 14 ± 1% on day three of the experiment. On the sixth and ninth day, the positive effects of C60 fullerene injections were 10 ± 1%, 12 ± 2% and 16 ± 2% and 10 ± 1%, 11 ± 1% and 14 ± 1% at 1, 2, and 3 degrees of muscle injury severity, respectively, relative to the injury group (Figure 6B).
Histopathological Analysis
Muscles
No deviations from the normal histological structure of the muscle were observed in the control group. Groups of muscle fibers are visible, separated by thin layers of connective tissue. Inside each muscle fiber, myofibrils are clearly visible (Figure 7A).
In rats with muscle injury, muscle fibers are destroyed and connective tissue is heavily proliferated at the site of injury (Figure 7B, D and F). In other parts of the muscle, the normal histological structure of the muscle fibers is disturbed. Some of the fibers are hypochromatic, with the signs of edema. Some fibers are hyperchromatic, with the signs of destruction. The structure of myofibrils inside the muscle fibers is disturbed. There is a disturbance in the transverse striation of the fiber. Fibers may decrease in diameter. Connective tissue also grows: the area occupied by connective tissue increases in all study groups (Table 1). In cases of the 1st and the 2nd degrees of muscle injury severity (Figure 7B and D), the increase in connective tissue volume is mainly due to the matrix ground substance of connective tissue, and in the case of the 3rd degree (Figure 7F) – mainly due to the growth of collagen fibers. The severity of the above pathological and morphometric changes varies in different experimental groups (Table 1).
 |
Table 1 Morphometrical Features of Muscle and Kidney in Tested Rats |
The area occupied by connective tissue in rats with the 1st degree of muscle injury severity is 80–110 ± 7% higher than in the control group. The diameter of muscle fibers is 16 ± 2% smaller compared to the control group in rats that did not receive water-soluble C60 fullerenes but does not differ from the control values in rats that received them (Table 1).
In rats with the 2nd degree of muscle injury severity, which received water-soluble C60 fullerenes, the area occupied by connective tissue is 20 ± 2% smaller and the diameter of muscle fibers is 30 ± 2% larger (and does not differ from the control) compared to rats that did not receive them (Table 1).
In rats with the 3rd degree of muscle injury severity, which received water-soluble C60 fullerenes, the area occupied by connective tissue is 31 ± 2% smaller and the diameter of muscle fibers is 12 ± 1% larger compared to rats that did not receive them (Table 1).
Thus, in rats treated with water-soluble C60 fullerenes, connective tissue does not grow so much, and the diameter of muscle fibers does not decrease (in cases of the 1st and 2nd severity of muscle injury), or decreases to a lesser extent (in case of the 3rd severity of muscle injury). The degree of destructive pathological changes in muscle tissue is also less (Figure 7C, E and G).
Kidney
In control rats, the kidneys have a normal histological structure (Figure 8A). In rats with muscle injury (Figure 8B, D and F), retraction of glomerular tuft is observed in part of the renal glomeruli. As a result, the cross-sectional area of the renal glomeruli decreases. In addition, in cases of 3rd degree of muscle injury severity (Figure 8F), a slight hemorrhage is observed in some renal glomeruli. The diameter of the renal tubes may decrease (Table 1).
Thus, noticeable histopathological changes in the kidneys are not observed after muscle injury. However, morphometric parameters may decrease (this is evidence of a decrease in the functional activity of the kidney). The severity of these changes varies in the different experimental groups. For example, the diameter of renal tubules in rats with 3rd degree of muscle injury severity, which received water-soluble C60 fullerenes, is bigger by 10 ± 1% compared to rats with injury (Table 1; Figure 8G), which did not receive them. In rats with 1st and 2nd degrees of muscle injury severity, which received water-soluble C60 fullerenes, the diameter of renal tubules did not differ from the control values (Table 1; Figure 8C and E).
Summarizing, the above-described positive effects of exposure to C60 fullerenes on the ARF caused by rhabdomyolysis are related precisely to their powerful antioxidant properties.25,26 C60 molecules effectively inactivate the ROS, protecting nephron membranes from oxidation, and, thus, reduce the severity of ARF.
Conclusion
Thus, during the experiment, there is a positive change of the studied biomechanical and biochemical parameters by about 27–30 ± 2% with C60 fullerene aqueous solution administration at a low therapeutic dosage. Besides, they were confirmed by the data of histopathological analysis. This indicates that there is compensatory activation by C60 fullerenes of the endogenous antioxidant system of the organism in the process of posttraumatic changes of the muscle soleus caused by mechanical damage of different degrees of severity. Thus, C60 fullerenes, as powerful antioxidants, reduce the development of both rhabdomyolysis and ARF induced by it. We can also assume the therapeutic effect of C60 fullerenes on the acceleration of the process of repair of muscle structures damaged by rhabdomyolysis, which is confirmed by the previously obtained data on their efficient action on the functions of the antioxidant system of the organism at various pathological processes.22,24,25
Based on the obtained results, it can be suggested that the use of the aqueous solution of C60 fullerene as a therapeutic agent is able to correct pathological conditions of the muscular and excretory system arising from skeletal muscle injuries.
Acknowledgments
We acknowledge support for the publication costs by the Open Access Publication Fund of the University of Gdansk, Poland. This research was supported by the National Research Foundation of Ukraine (2022.01/0004).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Hudkova О, Krysiuk І, Drobot L, Latyshko Н. Rhabdomyolysis attenuates activity of semicarbazide sensitive amine oxidase as the marker of nephropathy in diabetic rats. Ukr Biochem J. 2022;94(1):23–32. doi:10.15407/ubj94.01.023
2. Candela N, Silva S, Georges B, et al. Short- and long-term renal outcomes following severe rhabdomyolysis: a French multicenter retrospective study of 387 patients. Ann Intensive Care. 2020;10(1):27. doi:10.1186/s13613-020-0645-1
3. Melli G, Chaudhry V, Cornblath DR. Rhabdomyolysis: an evaluation of 475 hospitalized patients. Medicine. 2005;84(6):377–385. doi:10.1097/01.md.0000188565.48918.41
4. Nielsen FE, Cordtz JJ, Rasmussen TB, Christiansen CF. The Association Between Rhabdomyolysis, Acute Kidney Injury, Renal Replacement Therapy, and Mortality. Clin Epidemiol. 2020;12:989–995. doi:10.2147/CLEP.S254516
5. Boden BP, Isaacs DJ, Ahmed AE, Anderson SA. Epidemiology of Exertional Rhabdomyolysis in the United States: analysis of NEISS Database 2000 to 2019. Phys Sportsmed. 2022;50(6):486–493. doi:10.1080/00913847.2021.1956288
6. Shin EH, Sabino JM, Nanos GP, Valerio IL. Ballistic trauma: lessons learned from Iraq and Afghanistan. Semin Plast Surg. 2015;29(01):10–19. doi:10.1055/s-0035-1544173
7. Division AF. Exertional rhabdomyolysis among active component members of the U.S. Armed Forces, 2019–2023. MSMR. 2024;31:9–14.
8. Hill OT, Wahi MM, Carter R, et al. Rhabdomyolysis in the US Active Duty Army, 2004–2006. Med Sci Sports Exerc. 2012;44(3):442–449. doi:10.1249/MSS.0b013e3182312745
9. Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013;144(3):1058–1065. doi:10.1378/chest.12-2016
10. de Meijer AR, Fikkers BG, de Keijzer MH, et al. Serum creatine kinase as predictor of clinical course in rhabdomyolysis: a 5-year intensive care survey. Intensive Care Med. 2003;29(7):1121–1125. doi:10.1007/s00134-003-1800-5
11. Ma Y, Cai F, Li Y, et al. A review of the application of nanoparticles in the diagnosis and treatment of chronic kidney disease. Bioact Mater. 2020;5(3):732–743. doi:10.1016/j.bioactmat.2020.05.002
12. Xiao Z, Huang Q, Yang Y, et al. Emerging early diagnostic methods for acute kidney injury. Theranostics. 2022;12(6):2963–2986. doi:10.7150/thno.71064
13. Huang Q, Yang Y, Zhao T, et al. Passively-targeted mitochondrial tungsten-based nanodots for efficient acute kidney injury treatment. Bioact Mater. 2022;21:381–393. doi:10.1016/j.bioactmat.2022.08.022
14. Wang L, Zhang Y, Li Y, et al. Recent advances in engineered nanomaterials for acute kidney injury theranostics. Nano Res. 2021;14(4):920–933. doi:10.1007/s12274-020-3067-3
15. Chen Q, Nan Y, Yang Y, et al. Nanodrugs alleviate acute kidney injury: manipulate RONS at kidney. Review Bioact Mater. 2022;22:141–167. doi:10.1016/j.bioactmat.2022.09.021
16. Dennis JM, Witting PK. Protective role for antioxidants in acute kidney disease. Nutrients. 2017;9(7):718. doi:10.3390/nu9070718
17. Semenovich DS, Plotnikov EY, Lukiyenko EP, et al. Protective effect of D-panthenol in rhabdomyolysis-induced acute kidney injury. Int J Mol Sci. 2022;23(20):12273. doi:10.3390/ijms232012273
18. Gois PHF, Canale D, Volpini RA, et al. Allopurinol attenuates rhabdomyolysis-associated acute kidney injury: renal and muscular protection. Free Radic Biol Med. 2016;101:176–189. doi:10.1016/j.freeradbiomed.2016.10.012
19. Heard KJ. Acetylcysteine for Acetaminophen poisoning. N Engl J Med. 2008;359(3):285–292. doi:10.1056/NEJMct0708278
20. Shimizu MHM, Coimbra TM, de Araujo M, et al. N-acetylcysteine attenuates the progression of chronic renal failure. Kidney Int. 2005;68(5):2208–2217. doi:10.1111/j.1523-1755.2005.00677.x
21. Foley S, Crowley C, Smaihi M, et al. Cellular localisation of a water-soluble fullerene derivative. Biochem Biophys Res Commun. 2002;294(1):116–119. doi:10.1016/S0006-291X(02)00445-X
22. Nozdrenko D, Matvienko T, Vygovska O, et al. Protective effect of water-soluble C60 fullerene nanoparticles on the ischemia-reperfusion injury of the muscle soleus in rats. Int J Mol Sci. 2021;22(13):6812. doi:10.3390/ijms22136812
23. Prylutskyy Y, Nozdrenko D, Gonchar O, et al. C60 fullerene attenuates muscle force reduction in a rat during fatigue development. Heliyon. 2022;8(12):e12449. doi:10.1016/j.heliyon.2022.e12449
24. Nozdrenko D, Prylutska S, Bogutska K, et al. Effect of C60 fullerene on recovery of muscle soleus in rats after atrophy induced by achillotenotomy. Life. 2022;12(3):332. doi:10.3390/life12030332
25. Nozdrenko D, Matvienko T, Vygovska O, et al. Post-traumatic recovery of muscle soleus in rats is improved via synergistic effect of C60 fullerene and TRPM8 agonist menthol. Appl Nanosci. 2021;12(3):467–478. doi:10.1007/s13204-021-01703-z
26. Nozdrenko D, Abramchuk O, Prylutska S, et al. Analysis of biomechanical parameters of muscle soleus contraction and blood biochemical parameters in rat with chronic glyphosate intoxication and therapeutic use of C60 fullerene. Int J Mol Sci. 2021;22(9):4977. doi:10.3390/ijms22094977
27. Omelchuk O, Prylutska S, Nozdrenko D, et al. C(60) fullerene attenuates the signs of acute renal failure in rats under rhabdomyolysis due to inhibition of oxidative stress. Ukr Biochem J. 2023;95(5):61–75. doi:10.15407/ubj95.05.061
28. Prylutska SV, Matyshevska OP, Grynyuk II, et al. Biological effects of C 60 fullerenes in vitro and in a model system. Mol Cryst Liq Cryst. 2007;468(1):265–274. doi:10.1080/15421400701230105
29. Prilutski Y, Durov SS, Yashchuk VN, et al. Theoretical predictions and experimental studies of self-organized C60 nanoparticles in water solution and on the support. Europ Phys J D. 1999;9(1):341–343. doi:10.1007/s100530050452
30. Prylutska SV, Grebinyk AG, Lynchak OV, et al. In vitro and in vivo toxicity of pristine C 60 fullerene aqueous colloid solution. Fulleren Nanotub Carbon Nanostruct. 2019;27(9):715. doi:10.1080/1536383X.2019.1634055
31. Moussa F. [60]Fullerene and derivatives for biomedical applications. Nanobiomaterials. 2018:113–136.
32. Gharbi N, Pressac M, Hadchouel M, et al. [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005;5(12):2578–2585. doi:10.1021/nl051866b
33. Eswaran SV. Water Soluble Nanocarbon Materials: a Panacea for All? Current Sci. 2018;114(09):1846–1850. doi:10.18520/cs/v114/i09/1846-1850
34. de Souza J, Gottfried C. Muscle injury: review of experimental models. J Electromyogr Kinesiol. 2013;23(6):1253–1260. doi:10.1016/j.jelekin.2013.07.009
35. Kampmann JP, Hansen JM. Glomerular filtration rate and creatinine clearance. Br J Clin Pharmacol. 1981;12(1):7–14. doi:10.1111/j.1365-2125.1981.tb01848.x
36. Kumar D, Bagarhatta R. Fractional excretion of sodium and its association with prognosis of decompensated heart failure patients. J Clin Diagn Res. 2015;9(4):OC01–OC03. doi:10.7860/JCDR/2015/11532.5736
37. Suvarna KS, Layton C, Bancroft JD. Bancroft’s Theory and Practice of Histological Techniques.
38. Stewart IJ, Faulk TI, Sosnov JA, et al. Rhabdomyolysis among critically ill combat casualties associations with acute kidney injury and mortality. J Trauma Acute Care Surg. 2016;80(3):492–498. doi:10.1097/TA.0000000000000933
39. Harrois A, Libert N, Duranteau J. Acute kidney injury in trauma patients. Curr Opin Crit Care. 2017;23(6):447–456. doi:10.1097/MCC.0000000000000463
40. Stewart IJ, Sosnov JA, Howard JT, Chung KK. Acute kidney injury in critically injured combat veterans: a retrospective cohort study. Am J Kidney Dis. 2016;68(4):564–570. doi:10.1053/j.ajkd.2016.03.419
41. Eriksson M, Brattström O, Mårtensson J, et al. Acute kidney injury following severe trauma: risk factors and long-term outcome. J Trauma Acute Care Surg. 2015;79(3):407–412. doi:10.1097/TA.0000000000000727
42. Tarazona V, Figueiredo S, Hamada S, et al. Admission serum myoglobin and the development of acute kidney injury after major trauma. Ann Intensive Care. 2021;11(1):140. doi:10.1186/s13613-021-00924-3
43. Sheerin NS, Sacks SH. Leaked protein and interstitial damage in the kidney: is complement the missing link? Clin Exp Immunol. 2002;130(1):1–3. doi:10.1046/j.1365-2249.2002.01979.x
44. Zager A. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996;49(2):314–326. doi:10.1038/ki.1996.48
45. Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol. 2006;290(2):F262–F272. doi:10.1152/ajprenal.00099.2005
46. Talving P, Karamanos E, Skiada D, et al. Relationship of creatine kinase elevation and acute kidney injury in pediatric trauma patients. J Trauma Acute Care Surg. 2013;74(3):912–916. doi:10.1097/TA.0b013e318278954e
47. Perkins ZB, Haines RW, Prowle JR. Trauma-associated acute kidney injury. Curr Opin Crit Care. 2019;25(6):565–572. doi:10.1097/MCC.0000000000000655
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.