Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Effect of Common Cold on Serum Clozapine Concentrations in Hospitalized Patients with Schizophrenia
Authors Cao Y , Xia Q, Liang J, Wang J, Shan F, Dai B
Received 19 April 2024
Accepted for publication 6 August 2024
Published 13 August 2024 Volume 2024:20 Pages 1563—1570
DOI https://doi.org/10.2147/NDT.S473973
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Taro Kishi
Yin Cao,1– 4 Qingrong Xia,1– 4 Jun Liang,1– 4 Jiequan Wang,1– 4 Feng Shan,1– 4 Biao Dai1– 4
1Department of Pharmacy, Affiliated Psychological Hospital of Anhui Medical University, Hefei, People’s Republic of China; 2Department of Pharmacy, Hefei Fourth People’s Hospital, Hefei, People’s Republic of China; 3Psychopharmacology Research Laboratory, Anhui Mental Health Center, Hefei, People’s Republic of China; 4Department of Clinical Pharmacy, Anhui Clinical Research Center for Mental Disorders, Hefei, People’s Republic of China
Correspondence: Jun Liang, Department of Pharmacy, Hefei Fourth People’s Hospital, 316 Huangshan Road, Hefei, 230000, People’s Republic of China, Email [email protected]
Objective: The present study aims to investigate the effect of common cold on the serum clozapine concentrations in hospitalized patients with schizophrenia.
Methods: A total of 65 schizophrenic patients with common cold receiving clozapine treatment were retrospectively enrolled. The demographic data, medication situation, clozapine concentration, and parameters of routine haematological and biochemical laboratory tests were obtained from the medical record system. The serum clozapine concentration and clozapine concentration/dose (C/D) ratios between the baseline period and cold period were compared by paired-sample t tests. Association between the changes in serum concentration and C/D ratios of clozapine and changes in white blood cell (WBC) and neutrophil (NE) counts was evaluated using Pearson correlation analysis.
Results: The serum clozapine concentration (t = − 9.856, P < 0.001) and clozapine C/D ratios (t = − 10.071, P < 0.001) were found to be significantly elevated in the cold period compared to the baseline period. Moreover, the changes in the serum clozapine concentration were found to be significantly elevated in female patients compared to male patients (t = − 2.483, P = 0.017). Furthermore, changes in the serum clozapine concentration were positively correlated to the changes in WBC (r = 0.303, P = 0.014) and NE (r = 0.315, P = 0.011) counts. Similarly, changes in clozapine C/D ratios were positively correlated to the changes in WBC (r = 0.275, P = 0.027) and NE (r = 0.328, P = 0.008) counts.
Conclusion: The serum clozapine concentrations in patients with schizophrenia during the common cold period were increased, which might by related to the elevated WBC and NE counts.
Keywords: clozapine, blood drug concentration, schizophrenia, white blood cell count, neutrophil count, common cold
Introduction
Schizophrenia is a chronic and severe psychiatric disorder that significantly impairs social and occupational functioning, thereby profoundly affecting patients’ quality of life.1,2 Compared to the general population, individuals with schizophrenia have a reduced life expectancy by 15–20 years and exhibit a 2 to 3-fold increased prevalence of metabolic syndrome,3,4 as well as a 1.5-fold higher risk for coronary heart disease and cerebrovascular disease.5,6 Clozapine, a second-generation atypical antipsychotic, has demonstrated efficacy in the treatment of refractory schizophrenia, acute psychotic disorder, and drug-induced psychosis.7–9 Research indicates a significant correlation between the serum concentration of clozapine and its therapeutic efficacy.10 The Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology strongly endorse the use of therapeutic drug monitoring (TDM) for clozapine, a recommendation substantiated by numerous reports and controlled clinical trials.11 In clinical practice, low concentrations of clozapine exhibit limited efficacy, whereas high concentrations substantially elevate the risk of adverse reactions.12 The incidence of these adverse reactions has consequently restricted the clinical utility of clozapine. Hence, monitoring clozapine blood levels and identifying determinants that influence serum clozapine concentrations are of significant clinical importance.
Common cold represents the most prevalent infectious disease affecting humans, with a high incidence rate observed across all age groups.13 Recent evidence suggests a potential correlation between infection and clozapine concentrations. It has been reported that infections may result in a notable increase in clozapine blood levels.14,15 More recently, a significant increase in clozapine blood levels following the administration of the second dose of the COVID-19 vaccine was observed.16 Similarly, elevated serum levels of clozapine have been documented during the COVID-19 infection period.17 Additionally, a statistically significant positive correlation has been observed between clozapine concentration and C-reactive protein (CRP), a widely used inflammatory marker, during the COVID-19 infection period.17 However, there is a limited number of studies assessing the impact of the common cold, the most prevalent infectious disease, on clozapine concentrations in patients with schizophrenia.
Given the potential relationship between infection and clozapine concentration, the main aim of this study was to (1) investigate the effect of common cold on the serum clozapine concentrations; and (2) examine the association between the changes in the serum clozapine concentrations and hematologic changes caused common cold in patients with schizophrenia.
Materials and Methods
Study Design and Participants
In this study, schizophrenic patients with common cold and treated with clozapine at Hefei Fourth People’s Hospital from October 2020 to July 2022 were selected. The inclusion criteria were as follows: (1) meeting the ICD-10 diagnostic criteria of schizophrenia; (2) meeting the diagnosis of the common cold in accordance with the guidelines outlined in the Expert Consensus on Standardized Diagnosis and Treatment of the Common Cold (typical symptoms of common cold, including two or more symptoms: fever, sore throat, cough, nasal congestion, runny nose, headache, malaise, or fatigue);18 (3) administration of Ganmaoling granules to all patients for cold treatment; (4) The steady-state blood concentration of clozapine was measured; (5) The dosage of clozapine remained consistent during both the baseline and cold periods. The exclusion criteria were as follows: (1) combined use of drugs that affect clozapine blood concentration; (2) accompanied by serious heart, liver, kidney and other diseases. According to the above criteria, a total of 65 hospitalized patients were included. The ethics committee at Hefei Fourth People’s Hospital gave its approval to this procedure (HSY-IRB-PJ-CY), which was carried out in accordance with the principles outlined in the Declaration of Helsinki.
Data Extraction
The medical records of patients with schizophrenia were obtained through the doctor workstation system and related information was collected: (1) Demographic data including age, sex, height, weight, and disease diagnosis; (2) Medication situation including drug name, dosage, time, combination of drugs; (3) Laboratory examination including blood concentration of clozapine, and parameters of routine haematological and biochemical laboratory tests.
Blood Clozapine Concentration Detection
In this study, steady-state clozapine serum concentrations were measured after oral administration of clozapine for at least 1 week. Blood samples were obtained from the participants’ veins at 5:00 a.m. following an overnight fast. Collection was performed using dry tubes devoid of any additives. Subsequently, the blood samples were centrifuged immediately at 3000 rpm for 5 minutes at 4°C. The resultant supernatant was then collected and designated as the serum sample. The US waters-TQS UPLC-MS/MS mass spectrometer was used to detect the serum concentration of clozapine, and the accompanying quality control samples were tested daily. The deviation of the test results of the quality control samples was < 15%.
Blood Routine Test
In this study, the parameters of routine haematological and biochemical laboratory tests in patients with schizophrenia were tested by Siemens ADVIA 2120i blood analyzer (Siemens Health care Diagnostics Inc.) by Clinical examination and Biochemical Laboratory of Clinical Laboratory of our hospital. The parameters of routine haematological test included white blood cell count (WBC), neutrophils count (NE), number of lymphocytes, number of monocytes, number of eosinophils, number of basophils, number of red blood cells (RBC), hemoglobin concentration (HB), packed cell volume (PCV). The parameters of biochemical laboratory test included albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bile acid (TBA), total cholesterol (TC), creatinine, and uric acid.
Statistical Analysis
SPSS (version 17.0; IBM Corp., Armonk, NY, USA) was used to analyze the data. The data are expressed as mean ± standard deviation (SD), and the P value for statistical significance was set at 0.05. Paired t test was used to compare blood clozapine concentration between the cold period and the baseline period, and independent samples t-test was used to compare the gender differences in the impact of colds on the changes in the serum concentration and C/D ratios of clozapine. Pearson correlation analysis was conducted to analyze the association between the changes in serum concentration and concentration/dose (C/D) ratios of clozapine and changes in WBC and NE counts.
Results
Demographic and Clinical Characteristics of the Patients
A total of 65 schizophrenic patients with common cold receiving clozapine treatment, including 34 males and 31 females, were enrolled. The average age was (38.12 ± 13.00) years and BMI was (25.17 ± 3.14) kg/m2. The main symptoms of common cold are runny nose, nasal congestion, sneezing and itchy nose. The daily doses of clozapine ranged from 100 to 400 mg. The anti-cold medicine was Ganmaoling granules, and the daily dose was 30 g. The average number of days from the onset of cold symptoms to the measurement of clozapine concentration was (4.89 ± 4.33) days.
Comparison of Serum Clozapine Concentration and Clozapine C/D ratios between the Baseline Period and Cold Period
According to the data presented in Figure 1A–B, the serum clozapine concentration (t = −9.856, P < 0.001) and clozapine C/D ratios (t = −10.071, P < 0.001) were found to be significantly elevated in the cold period compared to the baseline period. Among the 65 patients, 8 individuals with common cold had not yet taken Ganmaoling granules at the time of blood concentration testing for clozapine. We compared the serum clozapine concentration and clozapine C/D ratios between the baseline and cold periods in these 8 patients. The serum clozapine concentration (t = −3.594, P = 0.009) and clozapine C/D ratios (t = −4.486, P = 0.003) were found to be significantly elevated in the cold period compared to the baseline period (Figure 1C–D).
We further investigated the gender differences in the impact of colds on the changes in the serum concentration and C/D ratios of clozapine. As shown in Figure 2, the changes in the serum clozapine concentration were found to be significantly elevated in female patients compared to male patients (t = −2.483, P = 0.017). No significant differences in C/D ratios of clozapine were found between groups (t = −1.313, P = 0.197).
Comparison of the Parameters of Routine Haematological and Biochemical Laboratory Tests between the Baseline Period and Cold Period
We further compared the the parameters of routine haematological and biochemical laboratory tests between the baseline period and cold period. As shown in Table 1, the WBC (t = −3.356, P = 0.001) and NE (t = −4.611, P < 0.001) in the cold period were significantly higher than those in the baseline period.
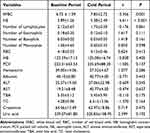 |
Table 1 Comparison of Parameters of Routine Haematological and Biochemical Laboratory Tests Between Baseline Period and Cold Period |
Association between the Changes in Serum Concentration and C/D ratios of Clozapine and changes in WBC and NE Counts
As shown in Figure 3, changes in the serum clozapine concentration were positively correlated to the changes in WBC (r = 0.303, P = 0.014) and NE (r = 0.315, P = 0.011) counts. Similarly, changes in clozapine C/D ratios were positively correlated to the changes in WBC (r = 0.275, P = 0.027) and NE (r = 0.328, P = 0.008) counts.
Discussion
The primary objective of the present study was to investigate the impact of the common cold on serum clozapine concentrations in hospitalized patients with schizophrenia. Three principal findings have emerged from the analysis. Firstly, serum clozapine concentrations and clozapine C/D ratios were significantly elevated during the cold period compared to the baseline period. Secondly, the alterations in serum clozapine concentrations were observed to be significantly higher in female patients compared to their male counterparts. Thirdly, variations in serum clozapine concentrations and clozapine C/D ratios exhibited a positive correlation with changes in WBC and NE counts.
Current evidence indicates that inflammation-induced alterations in drug metabolism are prevalent.19 A substantial body of research has examined the effects of inflammation and infection on the regulation of isoforms within the cytochrome P450 (CYP450) superfamily.20 Findings reveal that infection or inflammation can significantly reduce the clearance of CYP450 substrates, with reductions ranging from 20% to 70%. This decline is primarily ascribed to the transcriptional suppression of CYP mRNAs, which subsequently results in diminished expression and activity of CYP450 enzymes.21 Moreover, the downregulation of membrane transporters and drug metabolic enzymes mediated by inflammation, in conjunction with modifications in drug plasma protein binding, can lead to alterations in drug absorption, distribution, and clearance.22 As a result, these factors may contribute to increased serum levels of drugs and the risk of potential toxicity. Clozapine is primarily metabolized by the liver’s cytochrome P450 enzyme system, and studies on gene polymorphisms have indicated that variations in the CYP1A2 enzyme within this system significantly influence clozapine metabolism, particularly in relation to its catalytic demethylation.23,24 It is noteworthy that infection or inflammation can result in a significant reduction in CYP1A2 expression by up to 90%.25 The proposed mechanism involves a direct decrease in CYP450 gene transcription, mediated by cytokine-induced transcriptional repression, which subsequently leads to the downregulation of CYP activities and enzyme synthesis.26 Interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) have been identified as the cytokines responsible for the diminished expression of CYP1A2.27,28 In the present study, serum clozapine concentrations and clozapine C/D ratios were found to be significantly elevated during the cold period compared to the baseline period. Given that this is a retrospective study, the concentration of IL-6, TNF-α and CRP was not measured in these patients. Future studies that include the detection of pro-inflammatory cytokines may elucidate the mechanisms by which cold conditions lead to increased clozapine concentrations.
Systemic inflammation can be effectively evaluated through peripheral blood markers, including WBC and NE counts.29 In the current study, WBC and NE counts during the cold period were significantly elevated compared to the baseline period, indicating that schizophrenic patients with the common cold are experiencing systemic inflammation. Additionally, we investigated the relationship between alterations in serum concentrations and the C/D ratios of clozapine, and the changes in WBC and NE counts. The results showed that alterations in serum clozapine concentrations exhibited a positive correlation with changes in WBC and NE counts. Likewise, variations in clozapine C/D ratios were positively correlated with changes in WBC and NE counts. These findings suggest that elevated serum clozapine concentrations in patients with schizophrenia may be associated with systemic inflammatory status.
Among the 65 schizophrenic patients with the common cold, 57 (87.7%) were administered Ganmaoling granules during the measurement of clozapine concentration. Ganmaoling granules are a widely used compound preparation of traditional Chinese herbs, known for their therapeutic effects in alleviating cold symptoms and preventing and treating viral infections. Since the 1970s, this over-The-counter (OTC) medication has been extensively utilized in clinical practice for the treatment of headache, fever, nasal congestion, and sore throat, both in China and North America.30 Certain chemicals, including 3-O-caffeoylquinic acid, acetaminophen, and caffeine, are regarded as the primary active ingredients in this formulation.30 These compounds exhibit various physiological activities, such as antipyretic, antibacterial, antiviral, and other protective effects. Previous research has demonstrated that nearly 90% of caffeine undergoes transformation and metabolism via the CYP1A2 enzyme.31 Furthermore, caffeine has been reported to inhibit CYP1A2 isoforms, leading to elevated blood concentrations of clozapine.32 Therefore, it is reasonable to hypothesize that the increased serum concentration of clozapine observed in patients with schizophrenia may be associated with their use of Ganmaoling granules. Although serum clozapine concentrations and clozapine C/D ratios were also significantly elevated during the cold period compared to the baseline period in 8 patients who had not yet taken Ganmaoling granules when their blood clozapine levels were measured, further research is warranted to investigate the potential impact of Ganmaoling granules on clozapine concentrations.
Several studies have documented gender-associated variations in clozapine levels, with clozapine concentrations being lower in male patients compared to female patients.33,34 These differences can be attributed to factors such as hormonal balance, body composition, the function of transporters, and the activities of clozapine-metabolizing enzymes CYP1A2 and CYP3A4.35,36 For instance, the primary metabolizing enzyme of clozapine, CYP1A2, exhibits lower activity in females than in males, which is believed to contribute to the higher blood concentrations of olanzapine and clozapine observed in females.37 In the current study, we conducted a further examination of the gender differences regarding the effects of colds on the serum concentration and C/D ratios of clozapine. Our findings indicate that serum clozapine concentrations were significantly higher in female patients compared to their male counterparts. Consequently, clinical practitioners should be vigilant regarding the increased serum levels of clozapine and the potential toxic side effects associated with these elevations in patients experiencing a common cold, particularly among female patients.
This study is subject to several limitations. Firstly, it is a single-center study with a limited sample size, which may introduce sampling bias. Secondly, only eight patients with the common cold had not yet taken Ganmaoling at the time of testing blood concentrations of clozapine. A larger sample size is required to assess the effect of the common cold itself, independent of Ganmaoling administration, on clozapine concentrations. Thirdly, the study is unable to establish a causal relationship between changes in serum clozapine concentrations and alterations in WBC and NE counts. Fourthly, fever may influence the activity of the CYP1A2 enzyme, consequently altering the concentration of clozapine. Given that the patient’s body temperature was not documented during the period of the common cold, it is not feasible to determine whether the patient experienced a febrile state. This omission should be acknowledged as a limitation of the study.
In conclusion, the current study demonstrates an elevation in serum clozapine concentrations in patients with schizophrenia during episodes of the common cold, potentially associated with increased WBC and NE counts. Further multicenter studies are warranted to validate the observed increase in serum clozapine concentrations in this patient population during common cold periods. Additionally, longitudinal investigations are required to elucidate the causal relationship between changes in serum clozapine concentrations and alterations in systemic inflammation markers, including WBC and NE counts.
Funding
This study was provided by the Research Fund Project of Anhui Medical University (Project No: 2021xkj113), Research Fund Project of Hefei Fourth People’s Hospital (Project No: HFSY202109), Hefei Seventh cycle Key Medical Specialty, and Anhui Province Medical and Health Key Specialty Construction Project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yi-Hang C, Chia-Yueh H, Mong-Liang L, Chun-Hsin C. Augmentation strategies for clozapine-resistant patients with schizophrenia. Curr Pharm Des. 2020;26(2): 218–227.
2. Juliet R, Urs M. Epigenetic modifications in schizophrenia and related disorders: molecular scars of environmental exposures and source of phenotypic variability. Biol Psychiatry. 2020;89(3): 215–226.
3. Carsten H, Anne Emilie S, John JM, Merete N. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry. 2017; 4(4):295–301.
4. Davy V, Brendon S, Alex JM, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. 2015;14(3):339–347.
5. Martha W, Benjamin D. The epidemiology of diabetes in psychotic disorders. Lancet Psychiatry. 2015;2(5):431–451.
6. Christoph UC, Marco S, Nicola V, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16(2):163–180.
7. Maximilian H, Adriani N, Johannes S-T, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951.
8. Correll CU, Ofer A, Benedicto C-F, et al. A guideline and checklist for initiating and managing clozapine treatment in patients with treatment-resistant schizophrenia. CNS Drugs. 2022;36(7):659–679.
9. Yerye Gibrán M-L, José Jaime -M-M, Blanca Estela P-A, et al. Integrative genomic-epigenomic analysis of clozapine-treated patients with refractory psychosis. Pharmaceuticals. 2021;14(2):118.
10. Yuji Y, Kohei K, Shinji S, et al. The relationship between plasma clozapine concentration and clinical outcome: a cross-sectional study. Acta Psychiatr Scand. 2020;143(3):227–237.
11. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51:9–62.
12. Aleksandar M, James HM. Clozapine-induced agranulocytosis. Ann Hematol. 2020;99(11):2477–2482.
13. Eccles R. Is the common cold a clinical entity or a cultural concept? Rhinology. 2013;51(1):3–8. doi:10.4193/Rhino12.123
14. Yuan-Yuan Z, Xie-Hai Z, Feng S, Jun L. Infection is associated with elevated serum concentrations of antipsychotic drugs. Int Clin Psychopharmacol. 2021;36(5):264–267.
15. Jonathan GL, Sarah N, Christopher RT, Jessica LG. Infection and inflammation leading to clozapine toxicity and intensive care: a case series. Ann Pharmacother. 2014;48(6):801–805.
16. Selene RTV, Timo M, Jan PAMB, Dan C, Peter FJS. Clozapine and COVID-19 vaccination: effects on blood levels and leukocytes. An observational cohort study. Acta Psychiatr Scand. 2022;146(2):168–178.
17. Shujuan P, Wei L, Li S, et al. Relationship between C-reactive protein and antipsychotics levels in schizophrenic patients infected with COVID-19. J Psychiatr Res. 2024;170:297–301.
18. Xu X, Zhang W, Wu X, et al. The effectiveness and safety of chaiqin qingning capsule in upper respiratory tract infections with fever: a prospective, double-blinded, randomized, multicenter controlled trial. Complementary Ther Med. 2022;68:102840. doi:10.1016/j.ctim.2022.102840
19. Ann-Cathrine Dalgård D, Erkka J, Christina M, Tore BS. Clinical and molecular perspectives on inflammation-mediated regulation of drug metabolism and transport. Clin Pharmacol Ther. 2021;112(2):277–290.
20. Alison EA, Terrilyn AR, Edward TM. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol. 2006;46:123–149.
21. Françoise S-L, Elodie G-V, Stephanie C, Romain G. Inflammation is a major regulator of drug metabolizing enzymes and transporters: consequences for the personalization of drug treatment. Pharmacol Ther. 2020;215:107627.
22. Edward TM, Kerry BG, Micheline P-M, et al. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos. 2008;36(2):205–216.
23. Estela S, Emilio F-E, Julia C, Cristina BG, María Pilar R. Impact of pharmacogenetic testing on clozapine treatment efficacy in patients with treatment-resistant schizophrenia. Biomedicines. 2024;12(3):597.
24. Massimo B, Uma J, Seán OH, et al. Validation of population pharmacokinetic models for clozapine dosage prediction. Ther Drug Monit. 2024;46(2):217–226.
25. Scott RC, Nicola SW, Gajin K, et al. Elevated clozapine levels associated with infection: a systematic review. Schizophr Res. 2017;192:50–56.
26. Kenneth WR. Cytochrome P450 regulation and drug biotransformation during inflammation and infection. Curr Drug Metab. 2004;5(3):235–243.
27. Abou Farha K, van Vliet A, Knegtering H, Bruggeman R. The value of desmethylclozapine and serum crp in clozapine toxicity: a case report. Case Reports in Psychiatry. 2012;2012:592784. doi:10.1155/2012/592784
28. Raaska K, Raitasuo V, Arstila M, Neuvonen P. Bacterial pneumonia can increase serum concentration of clozapine. Eur J Clin Pharmacol. 2002;58(5):321–322. doi:10.1007/s00228-002-0486-x
29. Habibe I, Fatih İ. Effect of ozone therapy on neutrophil/lymphocyte, platelet/lymphocyte ratios, and disease activity in ankylosing spondylitis: a self-controlled randomized study. Med Gas Res. 2022;13(2):53–58.
30. Qiong L, Xiaolan Y, Yingyi Z, Hua L, Fenyun S. Chemical fingerprint of ganmaoling granule by double-wavelength ultra high performance liquid chromatography and ultra high performance liquid chromatography with quadrupole time-of-flight mass spectrometry. J Sep Sci. 2015;38(11):1850–1857.
31. Jieping W, Luthfia D, Yundong P, Chien-Wen H, Yanmin S, Giancarlo C. Does ergogenic effect of caffeine supplementation depend on CYP1A2 genotypes? A systematic review with meta-analysis. J Sport Health Sci. 2024;13(4):499–508.
32. Taylor D. Pharmacokinetic interactions involving clozapine. Br J Psychiatry. 1997;171:109–112.
33. Yi-lang T, Peixian M, Feng-Min L, et al. Gender, age, smoking behaviour and plasma clozapine concentrations in 193 Chinese inpatients with schizophrenia. Br J Clin Pharmacol. 2007;64(1):49–56.
34. Suzanne R, Julie B, Stephen John O, Samora H, Robert H, Robert James F. A population pharmacokinetic model to guide clozapine dose selection, based on age, sex, ethnicity, body weight and smoking status. Br J Clin Pharmacol. 2023;90(1):135–145.
35. Gail DA. Gender differences in pharmacological response. Int Rev Neurobiol. 2008;83:1.
36. Marissa JS, Emma CS, Rhonda JR. Sex-specific differences in CYP450 isoforms in humans. Expert Opin Drug Metab Toxicol. 2008;4(4):413–424.
37. Elizabeth F, Joanne L, Shinnyi C, Collin D, Ming L. Sex differences in aripiprazole sensitization from adolescence to adulthood. Pharmacol Biochem Behav. 2017;156:39–47.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




