Back to Journals » Drug Design, Development and Therapy » Volume 19
Effect of Esketamine-Based Opioid-Sparing Anesthesia Protocol on the Quality of Early Recovery After Urological Surgery: A Randomized Clinical Trial
Authors Qi Y , Li W , Ren Y , Sun J, Zhu Y , Wang L, Zhou M, Wang L
Received 9 December 2024
Accepted for publication 11 March 2025
Published 17 March 2025 Volume 2025:19 Pages 2005—2016
DOI https://doi.org/10.2147/DDDT.S511112
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Georgios Panos
Yu Qi,1,2,* Weihua Li,1,3,* Ying Ren,1,3,* Jia Sun,1,2 Yangzi Zhu,1,2 Long Wang,1,2 Meiyan Zhou,1,2 Liwei Wang1– 3
1The Xuzhou Clinical College of Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 2Department of Anesthesiology, Xuzhou Central Hospital, Xuzhou, Jiangsu, People’s Republic of China; 3College of Anesthesiology, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Meiyan Zhou; Liwei Wang, The Xuzhou Clinical College of Xuzhou Medical University, Department of Anesthesiology, Xuzhou Central Hospital, No. 199, Jiefang South Road, Xuzhou, Jiangsu, People’s Republic of China, Email [email protected] ; [email protected]
Purpose: The quality of postoperative recovery under enhanced recovery after surgery protocols has always been the focus of anesthesiologists. It has been proven that esketamine application during the perioperative period can reduce the use of opioid drugs and improve the quality of postoperative recovery. The present study explored the effect of the esketamine-based opioid-sparing anesthesia protocol on the quality of postoperative recovery in patients undergoing elective urological surgery.
Patients and Methods: A randomized, double-blind, controlled clinical trial was adopted. Patients aged 18– 65 years, with American Society of Anesthesiologists physical status grades I–III, scheduled for elective laparoscopic partial nephrectomy or unilateral nephrectomy in urological surgery, were randomly divided into the OSA group and the control group. The OSA group received 0.25 mg/kg of esketamine for anesthesia induction during the operation, and maintenance was carried out at a rate of 0.125 mg·kg⁻¹·h⁻¹. The primary outcome measure was the Quality of Recovery Scale-15 score 24 hours after the operation.
Results: The total Quality of Recovery Scale-15 score was significantly higher in the OSA than in control groups 24 hours after the operation (114 [108, 116] vs 106 [102, 109], p < 0.001). The anesthesia recovery level was better in the OSA than in control groups in the post-anesthesia care unit, demonstrated by shorter eye-opening time (19 [17 20] vs 22 [18, 22], p = 0.031) and extubation time (20 [20, 23] vs 25 [20, 25], p = 0.004). Additionally, the incidence of nausea and vomiting within 0– 48 hours after surgery was lower in the OSA than in control groups.
Conclusion: The esketamine-based opioid-sparing anesthesia protocol can improve the quality of early postoperative recovery and the level of anesthesia recovery, and accelerate rehabilitation in patients undergoing elective urological surgery.
Keywords: esketamine, opioid-sparing anesthesia, quality of recovery, postoperative pain, nephrectomy
Introduction
Laparoscopic partial nephrectomy and unilateral nephrectomy have become the most desirable patient choice in recent years due to the notable upward trend in the incidence of kidney tumors.1 However, a series of intraoperative operations, such as abdominal wall incisions, tissue damage, the continuous expansion of the abdominal wall and peritoneum caused by pneumoperitoneum, intraoperative traction and suture of the kidneys and ureters, and the continuous stimulation of the peritoneum by residual carbon dioxide after surgery, seriously affect the postoperative recovery of patients.2
With the continuous development of the concept of enhanced recovery after surgery (ERAS) protocols, improving the quality of postoperative recovery of patients has increasingly become the focus of anesthesiologists.3 In the past few decades, opioid-drug-based general anesthesia (GA) has become the mainstay of anesthesia. Although opioid drugs can produce a strong analgesic effect, their use is associated with manifold adverse events,4,5 such as immunosuppression, postoperative nausea and vomiting (PONV), hyperalgesia, respiratory depression, gastrointestinal paralysis, chronic pain, and opioid dependence. In recent years, opioid-free anesthesia (OFA) has gradually become an alternative option for clinical anesthesia and has received considerable attention and popularity.6 Although opioid-free anesthesia has now been applied in various surgeries, such as gynecological surgeries, general surgeries, spinal and joint surgeries, and major open surgeries,7–9 the effectiveness and safety of opioid-free anesthesia protocols remain controversial.10,11 Some studies found no clinically significant benefits with opioid-free anesthesia.12 Thus, opioid-sparing anesthesia (OSA) seems to be accepted by more anesthesiologists. Opioid-sparing anesthesia is defined as the complex anesthesia model established by the combined application of non-opioid analgesic drugs or regional anesthesia and other techniques to reduce the use of opioid drugs during the perioperative period, thus, decrease the risk of opioid-related adverse reactions.13
Esketamine is a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist with a high affinity for NMDA and opioid receptors.14,15 It also has mild respiratory depression, mild circulatory excitation, and relaxation effects on bronchial smooth muscle.16 Studies have shown that the use of esketamine during the perioperative period can reduce the consumption of opioid drugs and improve the postoperative recovery quality of patients.17,18
Currently, only a handful of studies have assessed the effect of esketamine-based opioid-sparing anesthesia protocol on the quality of postoperative recovery in urological surgery. We hypothesized that the esketamine-based opioid-sparing anesthesia protocol could improve the quality of postoperative recovery in patients. Therefore, the present randomized, double-blind, controlled study sought to explore the effect of the esketamine-based opioid-sparing anesthesia protocol on the quality of early postoperative recovery in patients undergoing elective laparoscopic partial nephrectomy or unilateral nephrectomy, to verify the safety and efficacy of opioid-sparing anesthesia in urology.
Methods
Study Design and Participants
This randomized, double-blind, controlled clinical trial included patients scheduled for elective urological surgery, who were randomly assigned to receive the OSA protocol (the OSA group) or the standard anesthesia protocol (the control group). The study adhered to the principles of the Declaration of Helsinki. The study was approved by the Institutional Ethics Committee of Xuzhou Central Hospital (No. XZXY-LK-20230427-059) on April 27, 2023. The trial was registered with the Chinese Clinical Trial Registry (ChiCTR2300071295) before patient enrollment. All subjects signed an informed consent form before participating in the study. This study followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines.19 The trial protocol is presented in Supplementary information 1.
This randomized controlled trial was conducted from June 2023 to September 2024. The trial ended after the follow-up of the last participant was completed. Inclusion criteria: (1) Patients aged 18–65 years old; (2) with American Society of Anesthesiologists (ASA) physical status grades I–III; (3) those undergoing elective laparoscopic partial nephrectomy or unilateral total nephrectomy under general anesthesia with tracheal intubation; and (4) those with an operation duration of 1–3 hours and willing to use patient-controlled intravenous analgesia (PCIA). Exclusion criteria: (1) Patients refusing to participate; (2) those with severe hypertension, pulmonary hypertension, or excessively high intracranial or intraocular pressure; (3) those with diseases of vital organs such as heart, liver, lung, and kidney and unable to tolerate the surgery; (4) those with a history of central nervous system diseases; (5) those allergic to esketamine or other anesthetic drugs; and (6) those with cognitive impairment or inability to communicate. A total of 172 participants were included in the final analysis.
Randomization and Blinding
A researcher who was not involved in patient recruitment, coordination, data collection, or outcome assessment generated the random sequence in a 1:1 ratio (block randomization with a block size of 4) using the SPSS 26.0 software. The random results were concealed in sequentially numbered and sealed opaque envelopes. Before anesthesia induction, a researcher who was unaware of the randomization procedure opened the envelopes and assigned patients to the opioid-sparing (OSA) group or the control group according to the random sequence. The responsible anesthesiologists were informed about the study medications but received no information identifying individual patients’ group assignments. Except for the different medications, all patients received standardized intraoperative management and monitoring. Subjects, clinicians except for anesthesiologists, and the researchers responsible for patient recruitment and outcome assessment were completely blinded to the group allocation. The outcome assessors did not access the anesthesia records and were not involved in the treatment of patients. The PCIA pump was placed in an opaque bag with all identifying labels removed to ensure blinding.
Anesthesia and Postoperative Analgesia Management
Neither group of patients received preoperative medications. Routine intraoperative monitoring was established, including pulse oximetry, electrocardiogram, invasive blood pressure monitoring, body temperature, and bispectral index (BIS). The BIS value was maintained at around 50. Furthermore, the surgical pleth index (SPI) was utilized to monitor intraoperative injury perception to assess the patient’s intraoperative analgesia level. A value of 20–50 indicates an appropriate analgesia level under GA. A value >50 indicates insufficient analgesia and requires an increase in the dose of analgesic drugs. A value <20 indicates excessive analgesia and requires a reduction in the infusion rate of analgesic drugs. Anesthesia was induced with esketamine 0.25 mg/kg, propofol 2 mg/kg, sufentanil 0.3 μg/kg, and cisatracurium besylate 0.15 mg/kg in the OSA group and propofol 2 mg/kg, sufentanil 0.4 mg./kg, and cisatracurium besylate 0.15 mg/kg in the control group. Preoxygenation was performed for 5 minutes after anesthesia induction, followed by tracheal intubation and mechanical ventilation using a lung protective ventilation strategy. Anesthesia maintenance in both groups was achieved with propofol 4–12 mg·kg⁻¹·h⁻¹ and remifentanil 0.1–1.0 μg·kg⁻¹·min⁻¹, with intermittent supplementation of cisatracurium besylate. The OSA group was continuously infused with esketamine at a rate of 0.125 mg·kg⁻¹·h⁻¹, whereas the control group received an equal dose of normal saline. During the operation, drugs were adjusted according to the hemodynamic changes of the patients to maintain the fluctuation of intraoperative mean arterial pressure (MAP) within 20% of the baseline blood pressure. A small dose of norepinephrine or ephedrine was given intravenously when MAP was lower than 20% of the baseline blood pressure, and intravenous nicardipine 0.5 mg was given intravenously or the anesthetic dose was adjusted when MAP was higher than 20% of the baseline blood pressure. Meanwhile, atropine 0.3–0.5 mg was given intravenously when the heart rate (HR) was less than 40 beats/minute, and anesthesia was deepened or a β-blocker was used when the HR was greater than 100 beats/minute. Esketamine infusion was stopped 20 minutes before the end of surgery in the OSA group. Propofol infusion was stopped in both groups when the skin was sutured and remifentanil infusion was stopped at the end of surgery.
All patients received postoperative analgesia with a PCIA device. PCIA with sufentanil (0.015 μg·kg⁻¹·h⁻¹), esketamine (0.015 mg·kg⁻¹·h⁻¹), and tropisetron (10 mg) was used for the OSA group, and that with sufentanil (0.03 μg·kg⁻¹·h⁻¹) and tropisetron (10 mg) in the control group. PCIA parameters were as follows: total volume, 100 mL; continuous infusion rate, 2 mL/h for 48 hours; patient-controlled volume, 1.5 mL; and lockout time, 15 minutes. If the Numerical Rating Scale (NRS) score was ≥4, an additional 50 mg of flurbiprofen was administered intravenously for rescue analgesia. PCIA infusion was suspended if the score was always <1 and symptoms such as respiratory depression, confusion, or unstable blood pressure occurred. PCIA was resumed once these symptoms improved.
Outcome Measurements
The primary outcome measure was the early postoperative quality of recovery, which was assessed by the Quality of Recovery Scale-15 (QoR-15)20 24 hours after surgery. QoR-15 consists of 15 items, each scored on a 10-point scale. It comprises five subscales: pain (n = 2), physical comfort (n = 5), physical independence (n = 2), psychological support (n = 2), and emotional state (n = 4). The total score ranges from 0 to 150, with 0 indicating poor recovery and 150 indicating excellent recovery. The Chinese version of the QoR-15 scale used in this study was translated and developed by West China Hospital of Sichuan University based on the English version of the QoR-15 scale, and the authorization for its use was obtained21(The QoR-15 questionnaire can be found in Supplementary figure 1). The QoR-15 questionnaire was completed by the patient when feasible or with the assistance of a surgical ward nurse who is blinded to the treatment group allocation.
Secondary outcome measures included the QoR-15 scores at 48 and 72 hours after surgery, the anesthesia recovery level in the post-anesthesia care unit (PACU), intraoperative hemodynamics, the total consumption of opioids during surgery and within 48 hours after surgery, the incidence of moderate-to-severe postoperative pain within 0–24 hours and 24–48 hours after surgery, the incidence of nausea and vomiting within 0–24 hours and 24–48 hours after surgery, and other postoperative adverse events. The anesthesia recovery level in the PACU included the time to respiratory recovery, time to eye-opening, time to extubation, Ramsay sedation score (RSS), Sedation-Agitation Scale (SAS) score, and NRS score. Moderate-to-severe postoperative pain was defined as an NRS score of ≥4. To evaluate intraoperative hemodynamics, blood pressure and heart rate were recorded before anesthesia induction (T0), after induction (T1), immediately after intubation (T2), at the time of skin incision (T3), 30 minutes after the start of surgery (T4), 60 minutes after the start of surgery (T5), 90 minutes after the start of surgery (T6), and at the end of surgery (T7).
Safety
Safety-related data were collected during the study follow-up by both the anesthesia team and investigators involved in the research. Safety was evaluated during the anesthesia by assessing intra-operative hemodynamic status, heart rhythm, and the incidence of anaphylactic reactions. Additional adverse effects potentially attributable to the study interventions were recorded during subsequent follow-up visits, with serious adverse events being documented at any point during the follow-up period.
Sample Size and Statistical Analysis
According to our pilot study, the postoperative QoR-15 score was 106.4 ± 10.2 for patients undergoing elective laparoscopic partial nephrectomy or unilateral total nephrectomy. Referring to the literature, the minimum clinically important difference (MCID) of QoR-15 was 6.22 PASS 20.0.6 software was used to calculate the sample size, with the test power defined as 80% and the test level as 0.05, and 47 cases were needed in each group. Considering a 10% dropout rate, 53 cases were finally included in each group, with a total of 106 cases.
The Shapiro–Wilk test was used to assess normality. Normally distributed continuous variables were expressed as mean ± standard deviation (SD), and homogeneity of variance was evaluated using Levene’s test. Independent samples t-test or Welch’s t-test was selected based on the homogeneity of variances. Non-normally distributed continuous variables were expressed as median (25th-75th percentiles) and compared using the Mann–Whitney U-test. Categorical variables were expressed as frequency (percentage, %) and analyzed using the χ2 test or Fisher’s exact test. For repeated measures data, repeated measures analysis of variance (RM-ANOVA) was employed if the data met the assumptions of normality (verified at each time point) and sphericity (assessed via Mauchly’s test), with Greenhouse-Geisser corrections applied when sphericity was violated. Post hoc pairwise comparisons were adjusted using the Bonferroni method to control Type I error. If the data violated RM-ANOVA assumptions (eg, non-normality or missing values), generalized estimating equations (GEE) were used with an exchangeable working correlation matrix to account for within-subject correlations, robust standard errors (Huber-White estimator) for valid inference, and fixed effects for time, group, and their interaction. All analyses were performed using SPSS 26.0 (IBM Corp., Armonk, NY, USA), and a two-tailed p < 0.05 was considered statistically significant. GraphPad Prism version 10.1((GraphPad Software, San Diego, CA, USA)) for Windows was utilized to generate graphs.
Results
A total of 172 patients were screened between June 2023 and September 2024, of whom 66 patients were excluded. The remaining 106 patients were randomly assigned to the OSA group and the control group. All randomized patients underwent their surgical procedures with designated anesthesia regimens. During the postoperative follow-up period, 2 patients in the OSA group and 3 patients in the control group were lost to follow-up. Finally, 51 patients in the OSA group and 50 in the control group were included in the analysis (Figure 1). Clinical characteristics were comparable between the two groups, with no differences observed in the baseline data (Table 1).
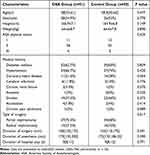 |
Table 1 Characteristics at Baseline |
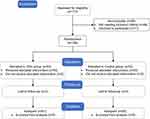 |
Figure 1 Flow diagram. |
Primary Outcome
The total score of QoR-15 was significantly higher in the OSA group than in the control group 24 hours after surgery (114 [108, 116] vs 106 [102, 109], p <0.001) and 48 hours after surgery (123 [119, 125] vs.117 [113, 121], p<0.001) (Figure 2). No significant difference in the total score of QoR-15 was found between the two groups 72 hours after surgery. Moreover, we performed a generalized estimating equations (GEE) analysis, which revealed that the OSA group demonstrated significantly higher postoperative QoR-15 scores compared to the control group (B = 0.068, p < 0.001). Furthermore, scores exhibited significant improvement over time (48h vs 24h: B = 4.749, p < 0.001; 72h vs 24h: B = 4.807, p < 0.001). Specifically,“Physical comfort” and “Emotional state” scores were significantly higher in the OSA than in control groups 24 hours and 48 hours after surgery (Table 2, Supplementary Tables 1-3).
 |
Table 2 QoR-15 Scores Between Two Groups at Different Time Points |
Secondary Outcome
Compared with the control group, the OSA group reduced the dosage of sufentanil (60 [60, 70] vs 110 [110,130], p<0.001) and remifentanil (1944 [1576, 2173] vs 2520 [1948, 2941], p<0.001) during the perioperative period (Figure 3A and B). This indicates that the OSA group significantly reduced the dosage of perioperative opioid drugs. Additionally, no disparity was found between the two groups in the dosage of propofol utilized during the operation (Supplementary figure 2).
No significant statistical difference in the incidence of moderate-to-severe pain was found between the two groups 0–24 and 24–48 hours after the operation (21.6% vs 26%, 9.8% vs 14%) (Figure 3C). Furthermore, no significant difference in the NRS scores at rest was found between the two groups 24 and 48 hours after the operation. However, NRS scores while coughing were significantly lower in the OSA than in control groups 24 hours (3 [2, 3] vs 3 [3, 4], p = 0.003) and 48 hours (1 [1, 2] vs 2 [1, 2], p = 0.023) after the operation (Supplementary figure 3).
The total incidence of PONV was significantly lower in the OSA group than in the control group (13.7% vs 32%). No statistically significant disparity was found between the two groups regarding the incidence of PONV in the PACU. Nevertheless, the incidence of PONV was lower in the OSA than in control groups 0–24 hours (11.8% vs 28%, p = 0.048) and 24–48 hours (3.9% vs 20%, p = 0.029) after the operation (Figure 3D).
Moreover, the eye-opening time (19 [17, 20] vs 22 [18, 22], p = 0.031) and extubation time (20 [20, 23] vs.25 [20, 25], p = 0.004) were significantly shortened in the PACU of the OSA group compared with the control group, indicating that the anesthesia recovery level in the OSA group was superior to that in the control group. In addition, no difference was found between the two groups regarding the respiratory recovery time, RSS score, NRS score, and incidence of agitation in the PACU (Table 3).
 |
Table 3 Comparison of Anesthesia Recovery Level Between the Two Groups in PACU |
Further repeated-measures analysis of variance was conducted on the hemodynamics at various intraoperative time points. The results showed that the overall intraoperative heart rate (HR) and mean arterial pressure (MAP) in the OSA group were higher than those in the control group, presenting a non-linear change trend of rapid decline followed by a gradual stabilization. Bonferroni post - hoc tests indicated that the MAP and HR in the OSA group were significantly higher than those in the control group immediately after induction, immediately after intubation, and at the time of skin incision (p < 0.05) (Supplementary figure 4). A follow-up was conducted to assess postoperative adverse reactions and complications in patients. No statistically significant difference in postoperative adverse reactions was found between the two groups (Table 4).
 |
Table 4 Occurrence of Postoperative Adverse Reactions in Patients |
Discussion
The current study confirmed that compared to the conventional opioid anesthesia protocol, the esketamine-based opioid-sparing anesthesia protocol can improve the quality of early postoperative recovery in patients undergoing urological surgery, although it fails to reduce the occurrence of moderate to severe pain after surgery in patients.
The analysis of the five dimensions of the QoR-15 scale showed that the OSA group was significantly superior to the control group in “Physical comfort” and “Emotional state” dimensions 24 and 48 hours after the operation, consistent with the findings of a previous study on the quality of postoperative recovery after laparoscopic rectal cancer surgery with low-dose intravenous esketamine administration during the operation.23 Previous studies have demonstrated that esketamine has anti-anxiety and anti-depressant effects.24 The level of glutamate in the brain may increase in an anxious state, overly activating the NMDA receptor, leading to excessive excitation of neurons and impaired neural plasticity. Esketamine restores the normal function of neurons by antagonizing the NMDA receptor and reducing the excitatory toxicity of glutamate.25 Moreover, esketamine can increase the levels of neurotransmitters such as dopamine in the ventral striatum and caudate nucleus, which stimulates the limbic system structure, thereby improving the emotional state and alleviating anxiety symptoms. This may also be the reason for the improvement in the “Emotional state” dimension in the OSA group in our study.24,26 In the specific questions of QoR-15, we observed that the sleep quality was significantly better in the OSA group than in the control group 24 hours after the operation, consistent with previous clinical studies.27,28 Studies have shown that esketamine can regulate the circadian rhythm, which may be associated with the improvement in sleep quality after the operation.29
Notably, while there was no difference between the two groups in the total amount of propofol used during the perioperative period, the anesthesia recovery level was significantly better in the OSA group than in the control group in the PACU, especially in terms of eye-opening time and extubation time. A previous animal study found that esketamine promoted awakening in mice after isoflurane inhalation anesthesia.30 The study suggested that esketamine may promote the excitation of glutamatergic neurons in the paraventricular nucleus of the thalamus, thereby accelerating awakening after anesthesia. Furthermore, previous studies have shown that consciousness recovery after general anesthesia is associated with enhanced connectivity in the high gamma band, and administration of sub-anesthetic doses of ketamine can enhance gamma-band brain electrical power.31,32 These reasons may cause the eye-opening time and extubation time of the OSA group in the PACU to be shorter than those of the control group, thereby showing the difference in the anesthesia recovery level. However, a recent clinical study reported that a single dose of esketamine given to gynecological surgery patients under sevoflurane anesthesia showed no significant awakening-promoting result.33 Our study found that continuous infusion of a small dose of esketamine during the operation accelerated the awakening of patients from anesthesia. One of the reasons for the discrepancy in the results is that a small dose of esketamine was continuously used during the induction of anesthesia and intraoperative maintenance stage, that is, the administration method of esketamine is different from previous studies. Another reason may be the difference in anesthesia methods, that is, the difference in the mechanisms of total intravenous anesthesia and total inhalation anesthesia. This provides a reference value for investigating whether different esketamine-assisted anesthesia regimens can accelerate patient awakening.
Additionally, no difference was found between the two groups in the incidence of moderate-to-severe pain after the operation, indicating that the esketamine-based opioid-sparing anesthesia protocol can provide adequate perioperative analgesia while reducing the use of opioid drugs during the perioperative period, consistent with a previous study. Our data also demonstrated that the opioid-sparing anesthesia protocol can maintain a relatively stable perioperative hemodynamic level and effectively reduce the incidence of postoperative adverse reactions, especially PONV. This aligns with previous reports, which may be explained by the sympathomimetic effect of esketamine and the reduction in the dosage of opioid drugs.34–36
During the postoperative follow-up in this study, no serious adverse events were found. We consider that on the one hand, this is because the dose of esketamine used in this study was less than the maximum dose in the drug instructions, and the dose we used has been proved to be safe and effective in previous studies.37 On the other hand, in the study design stage, we described in detail the treatment measures for various possible adverse events during the perioperative period to ensure the clinical safety of patients.
Nonetheless, this study still has some limitations. Firstly, the sample size calculated based on the total score of QoR-15 may not accurately distinguish the differences in each dimension of QoR-15. Further studies with larger sample sizes are needed to validate our findings. Secondly, the opioid-sparing anesthesia protocol in this study is based on esketamine, a single non-opioid analgesic drug. Future studies should combine multiple non-opioid drugs and regional block techniques to further explore the safety and feasibility of the opioid-sparing anesthesia protocol. Finally, this study explored the quality of early postoperative recovery in patients undergoing elective urological surgeries with the opioid-sparing anesthesia protocol, with surgical types being limited to laparoscopic partial nephrectomy and unilateral nephrectomy, therefore lacking diversity. Thus, surgical types should be expanded for further exploration in the future.
Conclusion
In summary, the esketamine-based OSA protocol can be safely and effectively used in urological surgeries. Compared with a conventional opioid-based general anesthesia protocol, the esketamine-based OSA protocol can improve the quality of early postoperative recovery in patients undergoing urological surgeries and accelerate their rehabilitation, although it fails to reduce the occurrence of moderate to severe pain after surgery in patients.
Data Sharing Statement
All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request. Any information we share will be deidentified.
Acknowledgment
The authors thank all patients, surgeons, and nursing staff involved in this trial.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Xuzhou Medical Young Reserve Talent Program (No. XWRCHT20220017), grant (Dr. Meiyan Zhou), Jiangsu Province’s Key Project on Elderly Health Research (NO.LKZ2023016), grant (Dr. Liwei Wang),Xuzhou Medical Key Talent Training Project (No. XWRCHT20210033),grant (Dr. Liwei Wang), Jiangsu Province’s Key Discipline / Laboratory of Medicine (No. JSDW202231), grant (Dr. Liwei Wang) and the Key Research and Development Program of Scientific and Technological Innovation in Xuzhou City (No.KC23146), grant (Dr. Liwei Wang).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Soerensen SJC, Montez‐Rath ME, Cheng I, et al. Groundwater constituents and the incidence of kidney cancer. Cancer. 2023;129(20):3309–3317. doi:10.1002/cncr.34898
2. Calpin GG, Ryan FR, McHugh FT, McGuire BB. Comparing the outcomes of open, laparoscopic and robot‐assisted partial nephrectomy: a network meta‐analysis. BJU Int. 2023;132(4):353–364. doi:10.1111/bju.16093
3. Miao C, Yu A, Yuan H, Gu M, Wang Z. Effect of enhanced recovery after surgery on postoperative recovery and quality of life in patients undergoing laparoscopic partial nephrectomy. Front Oncol. 2020;10:513874. doi:10.3389/fonc.2020.513874
4. Kim DD, Ramirez MF, Cata JP. Opioid use, misuse, and abuse: a narrative review about interventions to reduce opioid consumption and related adverse events in the perioperative setting. Minerva Anestesiol. 2022;88(4):300–307. doi:10.23736/S0375-9393.21.15937-1
5. Blum KA, Liew LY, Dutia AR, et al. Opioid-free anesthesia: a practical guide for teaching and implementation. Minerva Anestesiol. 2024;90(4):300–310. doi:10.23736/S0375-9393.23.17824-2
6. Olausson A, Svensson CJ, Andréll P, Jildenstål P, Thörn S, Wolf A. Total opioid‐free general anaesthesia can improve postoperative outcomes after surgery, without evidence of adverse effects on patient safety and pain management: a systematic review and meta‐analysis. Acta Anaesthesiol Scand. 2022;66(2):170–185. doi:10.1111/aas.13994
7. Choi H, Song JY, Oh EJ, Chae MS, Yu S, Moon YE. The effect of opioid-free anesthesia on the quality of recovery after gynecological laparoscopy: a prospective randomized controlled trial. J Pain Res. 2022;15:2197–2209. doi:10.2147/JPR.S373412
8. Ahmed SA, Abdelghany MS, Afandy ME. The effect of opioid-free anesthesia on the post-operative opioid consumption in laparoscopic bariatric surgeries: a randomized controlled double-blind study. J Opioid Manag. 2022;18(1):47–56. doi:10.5055/jom.2022.0694
9. Barakat H, Al Nawwar R, Abou Nader J, Aouad M, Yazbeck Karam V, Gholmieh L. Opioid-free versus opioid-based anesthesia in major spine surgery: a prospective, randomized, controlled clinical trial. Minerva Anestesiol. 2024;90(6):482–490. doi:10.23736/S0375-9393.24.17962-X
10. Xue FS, Su K, Cheng Y. Opioid-free anaesthesia reduces postoperative nausea and vomiting after thoracoscopic lung resection. Br J Anaesth. 2024;132(4):267–276. doi:10.1016/j.bja.2024.01.022
11. Harkouk H, Fletcher D, Beloeil H. Opioid free anaesthesia: myth or reality? Anaesth Crit Care Pain Med. 2019;38(2):111–112. doi:10.1016/j.accpm.2019.01.005
12. Chassery C, Atthar V, Marty P, et al. Opioid-free versus opioid-sparing anaesthesia in ambulatory total Hip arthroplasty: a randomised controlled trial. Br J Anaesth. 2024;132(2):352–358. doi:10.1016/j.bja.2023.10.031
13. Qiu Y, Lu X, Liu Y, Chen X, Wu J. Efficacy of the intraoperative opioid-sparing anesthesia on quality of patients’ recovery in video-assisted thoracoscopic surgery: a randomized trial. J Thorac Dis. 2022;14(7):2544–2555. doi:10.21037/jtd-22-50
14. Mion G, Himmelseher S. Esketamine: less drowsiness, more analgesia. Anesth Analg. 2024;139(1):78–91. doi:10.1213/ANE.0000000000006851
15. Zhang Y, Ye F, Zhang T, et al. Structural basis of ketamine action on human NMDA receptors. Nature. 2021;596(7871):301–305. doi:10.1038/s41586-021-03769-9
16. Jonkman K, Van Rijnsoever E, Olofsen E, et al. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120(5):1117–1127. doi:10.1016/j.bja.2018.02.021
17. Sun L, Zhao Y, Li Y, et al. Effect of continuous subanesthetic esketamine infusion on postoperative fatigue in patients undergoing laparoscopic radical resection for colorectal cancer: a randomized controlled study. Am J Cancer Res. 2023;13(6):2554–2563.
18. Liu J, Yin J, Yin J, et al. Effect of esketamine-based opioid-sparing anesthesia strategy on postoperative pain and recovery quality in patients undergoing total laparoscopic hysterectomy: a randomized controlled trail. Heliyon. 2024;10(3):e24941. doi:10.1016/j.heliyon.2024.e24941
19. Schulz KF, Altman DG, Moher D, Group CONSORT. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;
20. Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score. Anesthesiology. 2013;118(6):1332–1340. doi:10.1097/ALN.0b013e318289b84b
21. Bu XS, Zhang J, Zuo YX. Validation of the Chinese version of the quality of recovery-15 score and its comparison with the post-operative quality recovery scale. The Patient. 2016;9(3):251–259. doi:10.1007/s40271-015-0148-6
22. Myles PS, Myles DB. An updated minimal clinically important difference for the QoR-15 scale. Anesthesiology. 2021;135(5):934–935. doi:10.1097/ALN.0000000000003977
23. Xu Y, He L, Liu S, Zhang C, Ai Y. Intraoperative intravenous low-dose esketamine improves quality of early recovery after laparoscopic radical resection of colorectal cancer: a prospective, randomized controlled trial. PLoS One. 2023;18(6):e0286590. doi:10.1371/journal.pone.0286590
24. Luo T, Deng Z, Ren Q, Mu F, Zhang Y, Wang H. Effects of esketamine on postoperative negative emotions and early cognitive disorders in patients undergoing non-cardiac thoracic surgery: a randomized controlled trial. J Clin Anesth. 2024;95:111447. doi:10.1016/j.jclinane.2024.111447
25. McIntyre RS, Rosenblat JD, Nemeroff CB, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry. 2021;178(5):383–399. doi:10.1176/appi.ajp.2020.20081251
26. Bozymski KM, Crouse EL, Titus-Lay EN, Ott CA, Nofziger JL, Kirkwood CK. Esketamine: a novel option for treatment-resistant depression. Ann Pharmacother. 2020;54(6):567–576. doi:10.1177/1060028019892644
27. Qiu D, Wang XM, Yang JJ, et al. Effect of intraoperative esketamine infusion on postoperative sleep disturbance after gynecological laparoscopy: a randomized clinical trial. JAMA Network Open. 2022;5(12):e2244514. doi:10.1001/jamanetworkopen.2022.44514
28. Zhu M, Xu S, Ju X, Wang S, Yu X. Effects of the different doses of esketamine on postoperative quality of recovery in patients undergoing modified radical mastectomy: a randomized, double-blind, controlled trial. Drug Des Devel Ther. 2022;16:4291–4299. doi:10.2147/DDDT.S392784
29. Kohtala S, Alitalo O, Rosenholm M, Rozov S, Rantamäki T. Time is of the essence: coupling sleep-wake and circadian neurobiology to the antidepressant effects of ketamine. Pharmacol Ther. 2021;221:107741. doi:10.1016/j.pharmthera.2020.107741
30. Duan WY, Peng K, Qin HM, et al. Esketamine accelerates emergence from isoflurane general anaesthesia by activating the paraventricular thalamus glutamatergic neurones in mice. Br J Anaesth. 2024;132(2):334–342. doi:10.1016/j.bja.2023.10.038
31. Li D, Hambrecht-Wiedbusch VS, Mashour GA. Accelerated recovery of consciousness after general anesthesia is associated with increased functional brain connectivity in the high-gamma bandwidth. Front Syst Neurosci. 2017;11:16. doi:10.3389/fnsys.2017.00016
32. Pal D, Hambrecht-Wiedbusch VS, Silverstein BH, Mashour GA. Electroencephalographic coherence and cortical acetylcholine during ketamine-induced unconsciousness. Br J Anaesth. 2015;114(6):979–989. doi:10.1093/bja/aev095
33. Liu T, Zhang X, Li A, et al. Effects of intra-operative administration of subanesthetic s-ketamine on emergence from sevoflurane anesthesia: a randomized double-blind placebo-controlled study. BMC Anesthesiol. 2023;23(1):221. doi:10.1186/s12871-023-02170-5
34. Feng CD, Xu Y, Chen S, et al. Opioid-free anaesthesia reduces postoperative nausea and vomiting after thoracoscopic lung resection: a randomised controlled trial. Br J Anaesth. 2024;132(2):267–276. doi:10.1016/j.bja.2023.11.008
35. Song N, Yang Y, Zheng Z, et al. Effect of esketamine added to propofol sedation on desaturation and hypotension in bidirectional endoscopy: a randomized clinical trial. JAMA Network Open. 2023;6(12):e2347886. doi:10.1001/jamanetworkopen.2023.47886
36. Veith SB, Nickl R, Rössel T, Lachmann B, Koch T, Richter T. Hemodynamics and cutaneous microcirculation during induction of general anesthesia with and without esketamine. Clin Hemorheol Microcirc. 2023;84(4):385–398. doi:10.3233/CH-231711
37. Ren L, Yang J, Li Y, Wang Y. Effect of continuous infusion of different doses of esketamine on the bispectral index during sevoflurane anesthesia: a randomized controlled trial. Drug Des Devel Ther. 2024;18:1727–1741. doi:10.2147/DDDT.S45762
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Effect of Dexmedetomidine and Two Different Doses of Esketamine Combined Infusion on the Quality of Recovery in Patients Undergoing Modified Radical Mastectomy for Breast Cancer - A Randomised Controlled Study
Huang Z, Liu N, Hu S, Ju X, Xu S, Wang S
Drug Design, Development and Therapy 2023, 17:2613-2621
Published Date: 28 August 2023
Intraoperative Administration of Esketamine is Associated with Reduced Opioid Consumption After Laparoscopic Gynecological Surgery: A Randomized Controlled Trial
Huan C, Zhang T, Jiang Y, He S, Jin J
Drug Design, Development and Therapy 2025, 19:229-238
Published Date: 13 January 2025



