Back to Journals » Therapeutics and Clinical Risk Management » Volume 20
Effect of Levosimendan on Low Cardiac Output Syndrome After Pericardiectomy
Authors Fang L, Zhu P, Yu G , Lv W, Hu J
Received 16 September 2024
Accepted for publication 10 December 2024
Published 13 December 2024 Volume 2024:20 Pages 861—869
DOI https://doi.org/10.2147/TCRM.S496574
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor De Yun Wang
Likui Fang,1,2 Pengfei Zhu,2 Guocan Yu,2 Wang Lv,1 Jian Hu1
1Department of Thoracic Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, 310003, People’s Republic of China; 2Department of Thoracic Surgery, Hangzhou Red Cross Hospital, Hangzhou, 310003, People’s Republic of China
Correspondence: Jian Hu, Department of Thoracic Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, 79 Qingchun Road, Hangzhou, 310003, People’s Republic of China, Email [email protected]
Background: Low cardiac output syndrome (LCOS) after pericardiectomy is associated with high morbidity and mortality. This study aimed to assess the effect of levosimendan on postoperative LCOS in the patients with constrictive pericarditis.
Methods: Patients were retrospectively enrolled, and those receiving the treatment of levosimendan were assigned in the LEVO (+) group, and others were in the LEVO (-) group. Postoperative outcomes including durations of intubation, vasoactive agents using, ICU stay, hospital stay and mortality were compared between the two groups.
Results: A total of 32 patients were eligible for analysis, 19 of whom were in the LEVO (+) group, and 13 of whom were in the LEVO (-) group. The LEVO (+) group was associated with shorter postoperative duration of intubation (P < 0.001), vasopressor using (P = 0.006), ICU stay (P = 0.001) and hospital stay (P = 0.042), and less incidence of acute liver or kidney injury (P = 0.046). There were no significant differences in 30-day mortality and 1-year mortality between the LEVO (+) group and the LEVO (-) group. The prevalence of adverse events in the LEVO (+) group was acceptable.
Conclusion: Levosimendan could be administered in the patients with constrictive pericarditis developing LCOS after pericardiectomy to enhanced postoperative recovery.
Keywords: levosimendan, low cardiac output syndrome, constrictive pericarditis, pericardiectomy
Introduction
Constrictive pericarditis is a rare but life-threatening disease caused by inflammation and fibrosis of the pericardium, leading to diastolic heart failure eventually.1 Although the prevalence of constrictive pericarditis has not been systematically investigated, approximately 1.8% of patients with acute pericarditis would develop chronic constrictive pericarditis.2 The dominant cause of constrictive pericarditis is tuberculosis worldwide, especially in the developing countries.3,4 In the majority of cases, constrictive pericarditis is progressive and drug therapy such as diuretic is strictly palliative.5 Until now, the only definitive treatment is pericardiectomy.6,7 However, the risk of pericardiectomy remains high and postoperative low cardiac output syndrome (LCOS) is a major contributor to mortality.8–10
LCOS is a common but severe complication following cardiac surgery. This syndrome is characterized by decreased heart pump function and diminished cardiac output leading to poor tissue perfusion.11,12 The management of LCOS involves the early use of inotropes and mechanical circulatory support devices, but this syndrome remains associated with postoperative mortality that is up to 15 times as high as that in patients without this syndrome.13 Levosimendan, a calcium sensitizer and an ATP-sensitive potassium-channel opener, was introduced clinically twenty years ago and seemed to be effective in the treatment of LCOS.14 As an inotropic agent, levosimendan has raised a lot of interest in the field of perioperative management of cardiac surgery. However, the perioperative use of levosimendan in constrictive pericarditis has not been reported. Considering the pharmacologic properties of levosimendan and the results of previous studies, we designed a retrospective study to test the hypothesis that levosimendan could enhance the recovery of patients with LCOS after pericardiectomy.
Methods
Study Population
We retrospectively reviewed the records of patients with constrictive pericarditis in our department from November 2012 to March 2024. The preoperative diagnosis of constrictive pericarditis mainly depended on clinical symptoms, echocardiography, chest imaging and central venous pressure (CVP). Preoperative CVP was measured by central venous catheterization through internal jugular route with the use of ultrasound guidance. The study patients were eligible if they underwent an isolated pericardiectomy and were recognized as LCOS after surgery. We excluded patients with malignant tumors and patients who underwent other surgery during the same period. The perioperative characteristics of included patients were extracted from the hospital's electronic medical records system. The study protocol was approved by the Institutional Review Board of Hangzhou Red Cross Hospital (No. 2024092), and written patient informed consent was waived.
Treatment Procedure
All patients received general anesthesia, and then pericardiectomy by median sternotomy was performed without the use of cardiopulmonary bypass (CPB). The extent of pericardiectomy included at least the anterolateral pericardium between the two phrenic nerves, the basal pericardium over the diaphragmatic surface, the pericardium on the great arteries and the pericardium from superior vena cava-right atrium junction to inferior vena cava-right atrium junction. All patients were transferred to the intensive care unit (ICU) after surgery. Some of these patients were treated with levosimendan, with a loading dose (6–12 μg/kg over ten minutes) followed a maintenance infusion rate of 0.1 to 0.2 μg/kg/min for 24 hours.
Postoperative Outcomes
LCOS was characterized by myocardial dysfunction with a cardiac index (CI) <2.0 L/min/m2, systolic blood pressure <90 mmHg, and evidence of tissue hypoperfusion without hypovolemia.11 Other major postoperative complications included pulmonary complications, acute liver or kidney injury and deep venous thrombosis which occurred after pericardiectomy. The data on the following outcomes were also collected, including postoperative intubation, duration of using vasoactive agents, postoperative ICU stay, duration of chest drainage, postoperative hospital stay and 30-day mortality. Follow-up was performed until 1 year after pericardiectomy. The follow-up information was obtained from the hospital outpatient clinic records or collected by telephone.
Statistical Analysis
The study patients were divided into two groups according to the use of levosimendan. Patients receiving treatment for levosimendan were assigned in the LEVO (+) group, and others were in the LEVO (-) group. Categorical data were compared using the chi-square test, the corrected chi-square test or the Fisher exact test, and continuous data using the t test or Mann–Whitney U-test depending on the actual condition. Kaplan–Meier method and the Log rank test were performed to analyze the long-term survival impact of levosimendan. These analyses were conducted using SPSS software (version 27.0, IBM SPSS Inc. United States). All tests were 2-sided, and statistical significance was set at P value <0.05.
Results
Baseline Characteristics
A total of 166 patients underwent isolated pericardiectomy and 32 (19.3%) cases were recognized as LCOS after surgery. Of these, 19 (59.4%) patients received treatment for levosimendan and were allocated to the LEVO (+) group, while the remaining 13 (40.6%) patients were allocated to the LEVO (-) group.
Preoperative baseline characteristics of the two groups were compared in Table 1. The preoperative characteristics were similar between the two groups, including age, blood pressure, NYHA functional class, coexisting conditions and left ventricular ejection fraction (LVEF). We also compared intraoperative characteristics such as operative duration, blood loss, intraoperative fluid infusion rate, blood transfusion and urine output (Table 2). No significant differences were observed between the two groups.
 |
Table 1 Preoperative Baseline Characteristics of the Patients |
 |
Table 2 Intraoperative Characteristics of the Patients |
Postoperative Outcomes
The comparison of postoperative outcomes between the LEVO (-) group and the LEVO (+) group was shown in Table 3. The durations of postoperative intubation, vasopressor using and ICU stay were significantly longer in the LEVO (-) group than the LEVO (+) group (P < 0.001, P = 0.006 and 0.001, respectively). Pulmonary complications were observed in 8 (61.5%) patients in the LEVO (-) group and 4 (21.1%) patients in the LEVO (+) group, but the difference was not significant (P = 0.051), as well as the incidence of deep venous thrombosis (P = 0.406). Occurrence of acute liver or kidney injury was more frequent in the LEVO (-) group than the LEVO (+) group (61.5% vs 26.3%, P = 0.046). The postoperative duration of chest drainage did not differ significantly between the two groups, but the postoperative hospital stay was significantly longer in the LEVO (-) group (P = 0.042).
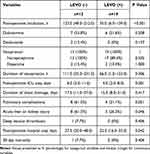 |
Table 3 Comparison of LEVO (-) and LEVO (+) Groups on Postoperative Outcomes |
In terms of 30-day mortality, 1 (7.7%) patient died due to multiple organ dysfunction in the LEVO (-) group, while no death was observed in the LEVO (+) group. However, the difference was not statistically significant. Follow-up information was successfully collected in all patients. Kaplan–Meier survival curves showed no between-group difference in mortality rates over time (P = 0.227) (Figure 1).
 |
Figure 1 Kaplan–Meier survival estimates of all-cause mortality. |
Safety Outcomes
Total adverse events were reported in 13 of 19 patients (68.4%) in the LEVO (+) group (Table 4). Hypotension during the infusion of levosimendan was the most common adverse event and was observed in 11 of 19 patients (57.9%), followed by arrhythmia in 4 of 19 patients (21.1%) and hypokalemia in 1 of 19 patients (5.3%). It should be noted that no patients had their levosimendan infusion interrupted due to adverse events and no serious adverse events caused by levosimendan were observed.
 |
Table 4 Safety Outcomes in the LEVO (+) Group |
Discussion
Due to the lack of literature on the use of levosimendan in postoperative LCOS in constrictive pericarditis, we designed this retrospective study to explore the value of levosimendan in this cohort of patients. We assigned patients with LCOS after pericardiectomy into two groups according to whether levosimendan was used or not. The administration of levosimendan was associated with significant improvement in postoperative outcomes including shorter postoperative duration of intubation, vasopressor using, ICU stay and hospital stay, and less incidence of acute liver or kidney injury (Figure 2). Although no significant difference was observed between the two groups for the incidence of pulmonary complications, there was also a trend toward a decrease with the use of levosimendan.
 |
Figure 2 The effect of levosimendan in improving postoperative outcomes in patients with LCOS after pericardiectomy. |
LCOS is a major postoperative complication of pericardiectomy, and the incidence has been reported to range from 10% to 66.1%.8,9 A great proportion of deaths linked with LCOS occurred in the previous study, ranging from 33.3% to 100%.9,10,15 In this study, postoperative LCOS occurred in 19.3% of patients underwent pericardiectomy and was associated with one case of death. The mechanism by which LCOS occurred postoperatively has been unclear and might be related to the presence of myocardial atrophy or fibrosis, especially in cases of long-standing constriction.16 LCOS could contribute to acute liver or kidney injury whose hypothesized mechanisms might include hypoperfusion and oxidative stress.17,18 The treatment of LCOS is challenging, and the goal is to provide adequate hemodynamic support and increase tissue oxygen delivery to prevent worsening organ dysfunction and failure.11 Several positive studies have shown the positive roles of levosimendan in maintaining hemodynamic stability and preserving renal function, and our study also provided additional support for these roles.19
Levosimendan could increase cardiac output and stroke volume and reduce peripheral vascular resistance, without increasing myocardial oxygen demand and with a prolonged duration of action.20 Previous randomized controlled studies have shown that levosimendan was associated with a higher rate of hemodynamic improvement, a lower rate of inotrope use and a lower incidence of periprocedural myocardial infarction than dobutamine or milrinone.21,22 In addition, several meta-analyses suggested a higher survival rate with levosimendan than with other treatment regimens among patients undergoing cardiac surgery.14,23 However, there were three multicenter, randomized, placebo-controlled, Phase 3 trials, the LEVO-CTS, CHEETAH and LICORN trials, investigating the potential benefits of levosimendan in patients undergoing cardiac surgery, showing no significant differences in mortality, renal replacement therapy, use of a mechanical cardiac assist device, or duration of catecholamine infusion between levosimendan and placebo.24–26 Similarly, we also did not observe a significant survival benefit in the LEVO (+) group in our retrospective study.
It was worth mentioning that the CHEETAH trial randomly assigned 506 patients with established postoperative LCOS to receive either levosimendan or placebo, and no significant benefit of levosimendan in primary end point was observed.24 Moreover, Sunny et al compared levosimendan, milrinone and dobutamine for the treatment of LCOS after CPB in patients receiving valve replacement surgery, and found that levosimendan was equally effective for dobutamine and better than milrinone in these patients.27 However, the study by Levin, R. L. et al randomly assigned 137 patients with LCOS after surgery to receive either levosimendan or dobutamine infusion, and reported that although both agents improved hemodynamic parameters, the effect of levosimendan was greater and occurred earlier than that of dobutamine.28 In the LEVO-CTS trial, the incidence of LCOS was significantly lower among patients assigned to receive levosimendan than placebo.25 A meta-analysis involving a total of 3198 patients also suggested preemptive levosimendan treatment was associated with a 9.1% absolute risk reduction of LCOS in patients with left ventricular dysfunction undergoing cardiac surgery.29 It should be noticed that the cardiac surgical procedure in previous studies only included coronary artery bypass grafting and valve surgeries. The role of levosimendan after pericardiectomy lacked relevant study. The results of our analysis revealing that levosimendan was associated with better short-term postoperative outcomes might provide preliminary evidence for the use of levosimendan in patients with LCOS after pericardiectomy. Our findings highlighted the need for further studies to specify patient subpopulations who might truly benefit from levosimendan.
Additionally, levosimendan has a favorable safety profile, with the common adverse events such as hypotension, arrhythmia, hypokalemia and headache, which is an important reason that the interest in levosimendan has grown.19 Due to the lack of a stable and reliable control group in our study, we only listed the adverse events in the LEVO (+) group and found that the incidences of adverse events were similar to previous studies.25,26 No adverse events leading to drug interruption were observed, and no serious adverse events due to levosimendan were recorded.
This study has several limitations. First, because of the low prevalence of constrictive pericarditis and fewer cases of LCOS after pericardiectomy, the sample size of our study was small. Therefore, the results served only as a reference and the benefits of levosimendan required to be validated by further large-scale clinical trials. Second, due to the nature of retrospective design, the analysis of adverse events of using levosimendan after pericardiectomy lacked a stable and reliable control group, leaving the safety outcomes in this cohort of patients to be confirmed by further studies. Finally, we did not systematically collect cardiac-output data before and after the use of levosimendan, which could help us understand and interpret the results of this study.
Conclusion
Levosimendan could effectively improve postoperative outcomes in the patients with LCOS after pericardiectomy, with reducing the need for prolonged intubation and vasopressor using, shortening the length of stay in the ICU and hospital, and potentially optimizing hepatic and renal function. Due to the positive role of levosimendan in our preliminary study, we could consider the postoperative use of levosimendan in these patients.
Abbreviations
LCOS, low cardiac output syndrome; CVP, central venous pressure; CPB, cardiopulmonary bypass; ICU, intensive care unit; CI, cardiac index; LEVF, left ventricular ejection fraction.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study protocol was approved by the Institutional Review Board of Hangzhou Red Cross Hospital (No. 2024092). Due to the retrospective nature of the study and without any specific intervention, the informed consent has been agreed to be waived. The data were maintained with confidentiality. The present study complied with the Declaration of Helsinki.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Syed FF, Schaff HV, Oh JK. Constrictive pericarditis--a curable diastolic heart failure. Nat Rev Cardiol. 2014;11(9):530–544. doi:10.1038/nrcardio.2014.100
2. Imazio M, Brucato A, Maestroni S, et al. Risk of constrictive pericarditis after acute pericarditis. Circulation. 2011;124(11):1270–1275. doi:10.1161/CIRCULATIONAHA.111.018580
3. Gillombardo CB, Hoit BD. Constrictive pericarditis in the new millennium. J Cardiol. 2024;83(4):219–227. doi:10.1016/j.jjcc.2023.09.003
4. Reddy P, Kane GC, Oh JK, et al. The Evolving Etiologic and Epidemiologic Portrait of Pericardial Disease. Can J Cardiol. 2023;39(8):1047–1058. doi:10.1016/j.cjca.2023.05.011
5. Welch TD. Constrictive pericarditis: diagnosis, management and clinical outcomes. Heart. 2018;104(9):725–731. doi:10.1136/heartjnl-2017-311683
6. Al-Kazaz M, Klein AL, Oh JK, et al. Pericardial Diseases and Best Practices for Pericardiectomy: JACC State-of-The-Art Review. Journal of the American College of Cardiology. 2024;84(6):561–580. doi:10.1016/j.jacc.2024.05.048
7. Yesiltas MA, Kavala AA, Turkyilmaz S, et al. Surgical treatment of constrictive pericarditis at a single center: 10 years of experience. Acta chirurgica Belgica. 2024;124(2):107–113. doi:10.1080/00015458.2023.2216377
8. Mutyaba AK, Balkaran S, Cloete R, et al. Constrictive pericarditis requiring pericardiectomy at Groote Schuur Hospital, Cape Town, South Africa: causes and perioperative outcomes in the HIV era (1990-2012). J Thorac Cardiovasc Surg. 2014;148(6):3058–3065. doi:10.1016/j.jtcvs.2014.07.065
9. Choi MS, Jeong DS, Oh JK, et al. Long-term results of radical pericardiectomy for constrictive pericarditis in Korean population. J Cardiothorac Surg. 2019;14(1):32. doi:10.1186/s13019-019-0845-7
10. Wang J, Zhang X, Liu X, et al. Predictors of low cardiac output after isolated pericardiectomy: an observational study. Perioper Med. 2022;11(1):34. doi:10.1186/s13741-022-00267-y
11. Lomivorotov VV, Efremov SM, Kirov MY, et al. Low-Cardiac-Output Syndrome After Cardiac Surgery. J Cardiothorac Vascular Anesthesia. 2017;31(1):291–308. doi:10.1053/j.jvca.2016.05.029
12. Epting CL, McBride ME, Wald EL, et al. Pathophysiology of Post-Operative Low Cardiac Output Syndrome. Curr Vasc Pharmacol. 2016;14(1):14–23. doi:10.2174/1570161113666151014123718
13. Nishimura RA, Otto CM, Bonow RO, et al. AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159–e95. doi:10.1161/CIR.0000000000000503
14. Uhlig K, Efremov L, Tongers J, et al. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev. 2020;11(11):CD009669. doi:10.1002/14651858.CD009669.pub4
15. Gillaspie EA, Stulak JM, Daly RC, et al. A 20-year experience with isolated pericardiectomy: analysis of indications and outcomes. J Thorac Cardiovasc Surg. 2016;152(2):448–458. doi:10.1016/j.jtcvs.2016.03.098
16. Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur Heart J. 2004;25(7):587–610.
17. Cheruku SR, Raphael J, Neyra JA, et al. Acute Kidney Injury after Cardiac Surgery: prediction, Prevention, and Management. Anesthesiology. 2023;139(6):880–898. doi:10.1097/ALN.0000000000004734
18. Song B, Dang H, Dong R. Analysis of risk factors of low cardiac output syndrome after congenital heart disease operation: what can we do. J Cardiothorac Surg. 2021;16(1):135. doi:10.1186/s13019-021-01518-7
19. Welker CC, Mielke JAR, Ramakrishna H. Levosimendan and Low Cardiac Output After Cardiac Surgery: analysis of Trial Data. J Cardiothorac Vascular Anesthesia. 2023;37(7):1294–1297. doi:10.1053/j.jvca.2023.03.011
20. Desai AS, Jarcho JA. Levosimendan for the Low Cardiac Output Syndrome after Cardiac Surgery. New Engl J Med. 2017;376(21):2076–2078. doi:10.1056/NEJMe1705455
21. Follath F, Cleland JG, Just H, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet. 2002;360(9328):196–202. doi:10.1016/S0140-6736(02)09455-2
22. De Hert SG, Lorsomradee S, Cromheecke S, et al. The effects of levosimendan in cardiac surgery patients with poor left ventricular function. Anesthesia Analg. 2007;104(4):766–773. doi:10.1213/01.ane.0000256863.92050.d3
23. Koster G, Wetterslev J, Gluud C, et al. Effects of levosimendan for low cardiac output syndrome in critically ill patients: systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2015;41(2):203–221. doi:10.1007/s00134-014-3604-1
24. Landoni G, Lomivorotov VV, Alvaro G, et al. Levosimendan for Hemodynamic Support after Cardiac Surgery. New Engl J Med. 2017;376(21):2021–2031. doi:10.1056/NEJMoa1616325
25. Mehta RH, Leimberger JD, van Diepen S, et al. Levosimendan in Patients with Left Ventricular Dysfunction Undergoing Cardiac Surgery. New Engl J Med. 2017;376(21):2032–2042. doi:10.1056/NEJMoa1616218
26. Cholley B, Caruba T, Grosjean S, et al. Effect of Levosimendan on Low Cardiac Output Syndrome in Patients With Low Ejection Fraction Undergoing Coronary Artery Bypass Grafting With Cardiopulmonary Bypass: the LICORN Randomized Clinical Trial. JAMA. 2017;318(6):548–556. doi:10.1001/jama.2017.9973
27. Sunny YM, Karim HM, et al. Comparison of Levosimendan, Milrinone and Dobutamine in treating Low Cardiac Output Syndrome Following Valve Replacement Surgeries with Cardiopulmonary Bypass. J Clin Diagn Res. 2016;10(12):UC05–UC8.
28. Levin RL, Degrange MA, Porcile R, et al. The calcium sensitizer levosimendan gives superior results to dobutamine in postoperative low cardiac output syndrome. Rev Esp Cardiol. 2008;61(5):471–479. doi:10.1157/13119990
29. Weber C, Esser M, Eghbalzadeh K, et al. Levosimendan Reduces Mortality and Low Cardiac Output Syndrome in Cardiac Surgery. The Thoracic and Cardiovascular Surgeon. 2020;68(5):401–409. doi:10.1055/s-0039-3400496
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

