Back to Journals » Journal of Pain Research » Volume 18
Effect of Locoregional Vs General Anesthesia on Incidence of Delayed Neurocognitive Recovery in Patients Undergoing Hip Fracture Surgery: A Randomized Controlled Trial
Authors Xie S, Zhao X, Zhao Z, Gui M, Cao X, Shen X, Luo J, Chen X, Xia Y, Yu B
Received 19 February 2025
Accepted for publication 27 May 2025
Published 12 June 2025 Volume 2025:18 Pages 2947—2960
DOI https://doi.org/10.2147/JPR.S523812
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Karina Gritsenko
Shuqi Xie,1 Xi Zhao,2 Zitong Zhao,2 Min Gui,2 Xiaodan Cao,1 Xiyuan Shen,1 Junjie Luo,3 Xiaorui Chen,4 Yuxuan Xia,1 Bin Yu2
1Department of Anesthesiology, Tongji Hospital, School of Medicine, Tongji University, Shanghai, People’s Republic of China; 2Department of Anesthesiology and Pain Rehabilitation, Shanghai YangZhi Rehabilitation Hospital, School of Medicine, Tongji University, Shanghai, People’s Republic of China; 3Department of Anesthesiology, Tenth People’s Hospital of Tongji University, Shanghai, People’s Republic of China; 4Department of Anesthesiology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, People’s Republic of China
Correspondence: Bin Yu, Department of Anesthesiology and Pain Rehabilitation, Shanghai YangZhi Rehabilitation Hospital, School of Medicine, Tongji University, 2209 Guangxing Road, Shanghai, People’s Republic of China, Tel +86 13918108880, Email [email protected]
Purpose: Delayed neurocognitive recovery is common in elderly patients undergoing major surgery under general anesthesia. We conducted a randomized controlled trial to examine whether continuous femoral nerve block plus sacral plexus block reduces the rate of delayed neurocognitive recovery in patients undergoing hip surgery.
Methods: This is a single-centre, randomized controlled trial. Patients undergoing hip surgery were randomized (1:1 ratio) to undergo surgery under either continuous femoral nerve block plus sacral plexus block or general anesthesia. The primary end point was delayed neurocognitive recovery, as assessed using a battery of neuropsychological tests at 7 days after the surgery (Z score ≤– 1.96 in at least 2 tests, and/or combined Z score ≤– 1.96), in a modified intent-to-treat population. Secondary end points included postoperative complications, moderate/severe postoperative pain (visual analogue scale ≥ 4), use of opioids within 48 hours, and 6-month all-cause mortality.
Results: A total of 168 patients were enrolled from January 2018 to May 2021. One hundred and sixty were included in the analysis (81 and 79 in the nerve block and general anesthesia, respectively). The rate of delayed neurocognitive recovery was 7.4% (6/81) in the continuous femoral nerve block plus sacral plexus block group versus 21.5% (17/79) in the general anesthesia group (odds ratio: 0.34, 95% CI: 0.14– 0.83; P = 0.01). The rate of postoperative pulmonary infection was 1.2% (1/81) in the nerve block group versus 10.1% (8/79) in the general anesthesia group OR 0.12 (95% CI 0.02,0.95; P = 0. 02). No patient died within 6 months after surgery.
Conclusion: When compared with general anesthesia, continuous nerve block anesthesia might decrease the incidence of delayed neurocognitive recover in patients undergoing hip fracture surgery. The locoregional anesthesia technique for patients undergoing hip surgery offers a safer alternative that lowers the risk of complications.
Keywords: delayed neurocognitive recovery, hip surgery, nerve block
Introduction
With rapid aging of the general population, hip fracture is increasingly common.1 Hip fracture surgeries are often associated with postoperative cognitive deficit including delirium and delayed neurocognitive recovery [postoperative cognitive dysfunction (POCD) occurring within the 1st month after surgery].2 Delayed neurocognitive recovery was associated with increased mortality, risk of leaving the labor market prematurely, and dependency on social transfer payments.3 Depending on the definition, study population, and assessment tools, the estimated incidence of delayed neurocognitive recovery ranges from 7% to 75%.4
Ninety-eight percent of hip fracture surgeries are performed under general anesthesia or neuraxial anesthesia.4 Coincidentally, sevoflurane, propofol and opioid drugs may cause postoperative neurocognitive dysfunction.5–7 Furthermore, general anesthesia has been established as an independent risk factor for pneumonia development.8,9 The inflammatory response triggered by pneumonia promotes the release of pro-inflammatory cytokines, leading to increased blood–brain barrier (BBB) permeability.10,11 These systemic cytokines can subsequently penetrate the compromised BBB, inducing neuroinflammation through microglial activation and neuronal oxidative stress.12,13 This cascade ultimately elevates the risk of postoperative neurocognitive dysfunction.
A meta-analysis that included 16 trials that compared general anesthesia (n=1395) versus spinal or epidural anesthesia (n=1313) failed to show significant difference in the incidence of delayed neurocognitive recovery.14 Another meta-analysis of trials that compared general anesthesia to spinal or epidural anesthesia alone or in combination with patients undergoing hip surgeries suggested a marginal reduction of delayed neurocognitive recovery.15
With the increasing use of ultrasound, nerve block regional anesthesia is increasingly used in clinical practice. Hebl et al16 conducted an observational study and reported lower rate of delayed neurocognitive recovery in patients receiving lumbar plexus and/or femoral nerve catheters for postoperative perineural anesthesia versus not, but the diagnostic criteria for delayed neurocognitive recovery and the anesthetic and analgesic regimens used in the control group were not clearly specified.
The hip joint is innervated by the lumbar plexus and the sacral plexus. Blocking the posterior lumbar plexus (psoas compartment block) and the sacral plexus has been increasingly used for hip surgery and demonstrated sufficient pain control and muscle relaxation.17 However, retroperitoneal hematoma has been reported in patients receiving low-molecular-weight heparin.18 American Society of Regional Anesthesia (ASRA) guidelines now explicitly recommend the same precautions as neuraxial techniques be exercised for deep procedures such as posterior lumbar plexus blocks, and any catheter be removed before anticoagulants.19 Accordingly, a significant proportion of patients are not candidate for posterior lumbar plexus block. The femoral nerve block and iliac fascia block are also referred to as the anterior approach lumbar plexus block.20 Chen’s case report21 support that the anterior lumbar plexus block combined with sacral plexus block anesthesia can be used to complete hip fracture surgery. Continuous femoral nerve block plus sacral plexus block has been used in >1000 patients over a period of 8 years in our practice, with generally satisfying results (unpublished). Compared with general anesthesia, the advantages of this nerve block technique are that patients maintain spontaneous respiration throughout the surgery and require minimal use of opioids and other general anesthetics. In 2017, we started a randomized controlled trial to test the hypothesis that continuous femoral nerve block plus sacral plexus block reduces the incidence of delayed neurocognitive recovery versus general anesthesia in patients undergoing hip fracture surgery.
Materials and Methods
Trial Design
This was a single-center, parallel-group, randomized controlled trial. This study was carried out following the guidelines of the Helsinki Declaration (World Medical Association Declaration of Helsinki) and was approved by the Ethics Committee of Tongji Hospital, School of Medicine, Tongji University (IRB #2017-444) and written informed consent was obtained from all subjects participating in the trial. The trial was registered prior to patient enrollment at www.chictr.org.cn, (CHICTR-INR-17014134, Principal investigator: Bin Yu, Date of registration: 2017–12-25, link: https://www.chictr.org.cn/showproj.html?proj=24022). The first patient was enrolled in 8th January, 2018. Trial oversight was provided by a steering committee and an independent data monitoring and safety committee.
Trial Participants
Patients (60 to 90 years of age) scheduled to undergo elective surgery to repair unilateral hip fracture with hemi-hip replacement, total hip replacement, proximal femoral nail antirotation (PFNA) internal fixation for intertrochanteric fracture, or plate internal fixation for intertrochanteric fracture, were eligible. The exclusion criteria included: 1) diabetes with peripheral neuropathy; 2) pre-existing dementia; 3) allergy to local anesthetics; 4) infection at the site of nerve block; 5) preoperative ASA physical status classification >3; 6) comorbid fracture on the same side, or previous fractures and surgeries on the opposite side.
Randomization and Concealment
Patients were randomized at a 1:1 ratio using the SAS 9.2 software (SAS Institute, Cary, NC, USA) by a statistician who was not involved in the trial otherwise. Concealment was conducted using opaque, sealed envelopes.
Intervention
Upon entering the operation room, a peripheral vein was cannulated, and patients were monitored using routine protocol (intra-arterial blood pressure, pulse oximetry, continuous electrocardiography).
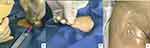
In patients assigned to continuous femoral nerve block plus sacral plexus block group (nerve block group), dexmedetomidine was given intravenously at a loading dose of 0.2–0.7 µg/kg in 10 minutes and 5-mg dezocine before proceeding to nerve block. For femoral nerve block, patients were placed in a supine position. The femoral nerve was located using ultrasound (12 MHz transducer, S-Nerve; FUJIFILM SonoSite, Bothell, WA, USA) just distal to the inguinal ligament with the nerve in the short axis view (Figures 1 and 2). Puncture was performed using a catheter-over-needle assembly (TuoRen, China, 20G catheter and 22G needle, 80 mm in length. The cannula-over-needle set comprises a hollow needle with a side hole, a needle tip with a 30° bevel, and an indwelling cannula with side holes outside the needle. Both needle and cannula were visible under ultrasound.22) at approximately 2 cm distal to the ultrasound probe. Tip of the needle was advanced under ultrasound guidance to under the fascia iliaca and guided under the fascia iliaca to near the lateral aspect of the femoral nerve. After verifying the position of the needle tip by ultrasound, 20-mL local anesthetic mixture containing 200-mg lidocaine and 50-mg ropivacaine in physiological saline was injected. Due to the “water separation” effect of the local anesthetic solution, the potential space between the iliac fascia and the surface of the iliopsoas muscle is separated by the local anesthetic solution. Fix the needle tip and simultaneously advance the outer catheter towards the cephalic end for 5–7 cm and secured to the skin with adhesive tape. Postoperative analgesia lasted for 48 hours, and consisted of background infusion of 0.2% ropivacaine at 5 mL per hour, 5 mL bolus upon button press, 10-min lockout, and maximum dose 25mL/hour.
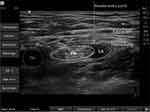 |
Figure 2 Ultrasound Image of Femoral Nerve Block. |
Sacral plexus block was conducted as previously used in our practice (Figures 3–5). Briefly, patients were placed in a lateral position with the fracture side facing upward. An ultrasound transducer (2–5 MHz curved array transducer, S-Nerve; FUJIFILM SonoSite) was placed at the midpoint of the line connecting the greater trochanter and posterior superior iliac line, and the probe was placed parallel to the inner side of the midpoint of the line. At this time, the ultrasound image showed a linear high echo (referring to the iliac bone), and the probe slides inward and downward. The iliac bone gradually appeared as a gap on the image, which was the ischial foramen. The sacral plexus nerve appears a bright elliptical image between the iliac gap. The needle was inserted out-plane along the ultrasonic probe short axis with ultimate target to the edge of ischial foramen instead of directly to the sacral plexus, and 20-mL local anesthetic mixture containing 200-mg lidocaine and 50-mg ropivacaine in physiological saline was injected above the iliac bone near the ischial foramen. This approach allowed access of the sacral plexus while avoiding blood vessels and perineal organs (eg, rectum) in the surrounding area.
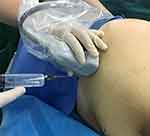 |
Figure 3 Ultrasound-Guided Sacral Plexus Block. |
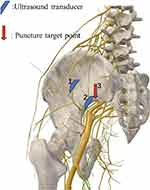 |
Figure 4 Schematic illustration of the sacral plexus block. |
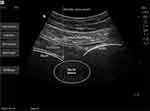 |
Figure 5 Ultrasound image of sacral plexus block. |
The femoral and sacral plexus blocks were separated by 10 minutes. Effectiveness of the blockade was evaluated 10 min after the injection of anesthetics using the Hollmen scale: 0 points, no distinction in sensation between the two limbs; 1 point, the sensation of needle prick pain is reduced compared to the contralateral limb; 2 points, the sensation of needle prick pain is dull and significantly decreased compared to the contralateral limb; 3 points, no sensation of pain from needle prick at all. A Hollmen score of ≥2 points is considered a successful block. Upon failure of the block, anesthesia was converted to general anesthesia with endotracheal intubation. Sedation was maintained at 3 or 4 Ramsay sedation score throughout the surgery by adjusting the infusion rate of dexmedetomidine. Surgery was conducted with the patients in a lateral position for total or semi arthroplasty, and supine position for PFNA internal fixation.
In patients assigned to the general anesthesia group, anesthesia was induced with propofol (1.0–2 mg/kg), rocuronium (0.3–0.9 mg/kg) and sufentanil (0.2–0.4 µg/kg). Mechanical ventilation was conducted at 8–10 mL/kg tidal volume and respiratory rate at 12 per minute. Anesthesia was maintained at 40–60 bispectral index using sevoflurane, propofol, dexmedetomidine and sufentanil. Rocuronium was given as bolus as needed. Postoperative analgesia was provided with a patient-controlled analgesia (PCA) pump during the first 48 hours (background infusion of 1.5μg sufentanil per hour, 2μg bolus injection upon button press, 10-min lockout interval, maximum dose 10µg/hour).
Intraoperative hypotension, defined as either systolic blood pressure <90 mmHg or reduction by ≥30% from baseline, was managed at the discretion of the attending anesthesiologist. Hypertension was defined as systolic blood pressure >180 mmHg or an increase by >30% from baseline that requires intervention. Bradycardia was defined as heart rate <50 beats per min or a decrease by >30% from baseline that requires intervention. Tachycardia, defined as heart rate >120 beats per min or increase by >30% from baseline that requires intervention.
Outcome Measures
The primary end point was delayed neurocognitive recovery, as assessed using a panel of neuropsychological tests at the baseline and at 1 week after surgery in the hospital ward by a trained interviewer blinded to group assignment. The results were compared to that obtained from a group of cognition-intact family members or friends of the patients with similar age and education level as reference. Five neuropsychological tests consisting of seven sub tests included:
- The Halstead-Reitan Trail Making Test (Part A), a measure of hand-eye coordination, attention, and concentration, with lower score indicating better function;
- Controlled Word Association Test, a measure of language function, with higher score indicating better function;
- Grooved Pegboard Dominant and Non-dominant Hand, a measure of manual dexterity, with lower score indicating better function;
- Digit Span (forward and backward) subtests of the Wechsler Memory Scale (Chinese edition, Hunan Medical University, Hunan, China), a measure of attention and concentration, with higher score indicating better function;
- Symbol Digit Modalities test of the Wechsler Adult Intelligence Scale-Revised (Chinese edition, Hunan Medical University), a measure of psychomotor speed, with higher score indicating better function.
The neuropsychological tests were selected because they have been used commonly and recommended in an expert consensus statement.23,24
Delayed neurocognitive recovery was defined using the International Study of Postoperative Cognitive Dysfunction 1(ISPOCD1) criteria.25 Briefly, reliable change index (RCI) was determined by subtracting the preoperative score (X1) from the postoperative score (X2), giving ΔX for each individual for a given task. The mean change for the controls ΔXc, calculated in the same way, was then subtracted from this, removing any practice effect. This score was then divided by the standard deviation (SD) for the change in test results in the reference control group (ΔXc), controlling for the expected variability: Z=(ΔX−ΔXc)/SD(ΔXc). The Z scores were then used to create a combined test score (Zcombined) using the sum of Z scores for each test divided by the SD of this summation in the reference control group. A patient was defined as having delayed neurocognitive recovery when two Z scores in individual tests or the combined Z score were –1.96 or less.
Secondary end points included postoperative complications (e.g, nausea and vomiting, pneumonia) severe/moderate postoperative pain (defined as visual analogue scale ≥4), use of opioid agents including intraoperative anaesthetics and postoperative PCA opioid consumption in 48 hours, and 6-month all-cause mortality. Postoperative pneumonia was based on at least one of the following: 1) new infiltration in chest X-ray after surgery; 2) presence of new and/or progressive and persistent respiratory symptoms indicative of pneumonia (eg, coughing and expectoration); 3) presence of fever or hypothermia; 4) physical examination showing lung consolidation signs and/or moist rale; 5) a white cell count of >10 × 109/L or <4 × 109/L; and 6) pathogen isolation from blood culture or sputum.26
Statistical Analysis
Sample size requirement was estimated based on the following assumptions: 1) the rate of delayed neurocognitive recovery at 25.8% (based on the results of an international multicentre study ISPOCD125 and 8% in the nerve block group (based on the results of our unpublished observation in 50 patients using the same neuropsychological tests and diagnostic criteria); 2) 2-sided α of 0.05, power (1 – β) of 0.80; 3) 10% loss to follow-up. The calculation yielded a total of 160 patients (80 per group).
The primary end point of delayed neurocognitive recovery was analyzed using the c2 test in a modified intent-to-treat cohort that included all patients who actually underwent surgery regardless of anesthesia protocol. Sensitivity analyses included per-protocol analysis and the worst scenario analysis (assuming delayed neurocognitive recovery in cases without assessment in the nerve block group, and assuming no delayed neurocognitive recovery in cases without assessment in the general anesthesia group). Continuous variables following normal distribution were analyzed using Student’s t-test and shown as mean ± SD; Continuous variables not following normal distribution were analyzed using Mann–Whitney U-test, and shown as median (interquartile range [IQR]). P < 0.05 (2-sided) was considered statistically significant.
Results
General Data
A total of 396 patients were screened for eligibility during a period from January 2018 to May 2021; 168 patients were enrolled (84 in each group; Figure 6). The modified intent-to-treat cohort included 160 patients (79 in the general anesthesia group, 81 in the nerve block group) (Table 1). The technical success rate was 100% for the nerve block group. No patients in the nerve block group converted to general anesthesia. The per-protocol analysis included 149 patients (73 in the general anesthesia group, 76 in the nerve block group). Type of the surgery included: hemi-hip replacement (n=36, 22.5%), total hip replacement (n=67, 41.9%) and PFNA internal fixation (n=57, 35.6%). Characteristics of the nerve block procedure are shown in Table 1. Surgery time and intraoperative blood loss did not differ between the 2 groups.
 |
Table 1 Demographic and Baseline Characteristics |
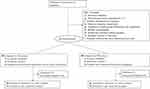 |
Figure 6 Flowchart of the study. |
Primary Outcome
In the modified ITT cohort, the rate of delayed neurocognitive recovery was 7.4% (6/81) in the nerve block group versus 21.5% (17/79) in the general anesthesia group, with an odds ratio (OR) of 0.3 (95% CI 0.1,0.8; P = 0.01) (Table 2). The combined Z score of patients who were diagnosed delayed neurocognitive recovery was −4.39±1.63 in the nerve block group versus −3.09±2.09 in the general anesthesia group (P = 0.18). Lower rate of delayed neurocognitive recovery in the nerve block group was also evident in the per-protocol analysis (7.9% versus 23.3%; OR 0.3 (95% CI, 0.1,0.8; P = 0.01) and the worst scenario analysis (9.9% versus 21.52%); OR 0.5 (95% CI, 0.2,1.0; P = 0.04). Detailed results of the neuropsychological tests are shown in Table 3. In the general anesthesia group, the neuropsychological test with the highest incidence of Z-score deterioration was Digit symbol test, used to evaluate psychomotor speed, which was 14.7% (14/75). In the nerve block group, the neuropsychological test with the highest incidence of Z-score deterioration was Digit span (backward), used to evaluate attention and concentration, which was 10.1% (8/79).
 |
Table 2 Primary and Secondary Outcomes |
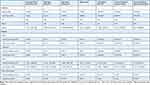 |
Table 3 Neuropsychological Test Results at Baseline and 1 week After Surgery |
Secondary Outcomes
The rate of postoperative nausea and vomiting was 8.6% (7/81) in the nerve block group versus 24.1% (19/79) in the general anesthesia group OR 0.36 (95% CI 0.16, 0.81; P = 0.01) (Table 2). The rate of postoperative pulmonary infection was 1.2% (1/81) in the nerve block group versus 10.1% (8/79) in the general anesthesia group OR 0.12 (95% CI 0.02, 0.95; P = 0. 02). The rate of moderate-to-severe postoperative pain was 11.1% (9/81) in the nerve block group versus 29.1% (23 /79) in the general anesthesia group OR 0.38 (95% CI 0.19,0.77; P = 0.004). No patient died within 6 months after surgery.
The rate of intraoperative hypotension was 30.9% (25/81) in the nerve block group versus 50.6% (40/79) in the general anesthesia group OR 0.44 (95% CI 0.2,0.8; P = 0.01). Total opioid consumption (intravenous morphine equivalent including intraoperative and postoperative PCA opioid consumption within 48 hours) was 5.0 (5.0–5.0) mg in the nerve block group versus 136.0 (128.0,148.0) mg in the general anesthesia group (P < 0.01).
Discussion
In this randomized controlled trial, we found the continuous femoral nerve block plus sacral plexus block reduced the incidence of early delayed neurocognitive recovery in patients undergoing hip surgery compared with general anesthesia. The incidence of intraoperative hypotension, postoperative nausea and vomiting, moderate/severe postoperative pain, and postoperative pneumonia were also lower in the nerve block group. The total opioids (intravenous morphine equivalent including intraoperative and postoperative PCA opioid consumption within 48 hours) were also significantly lower in the nerve block group.
The incidence of delayed neurocognitive recovery in the general anesthesia group in this trial was similar to that reported by the international multicentre study ISPOCD125 in patients (≥60 years of age) undergoing major abdominal and orthopedic surgery, thus supporting the validity, and perhaps generalizability of the findings obtained in this trial. Peripheral nerve block has been used as a part of multimodal anesthesia and postoperative pain management. In patients undergoing total knee arthroplasty (TKA), postoperative cognitive dysfunction has been reported to be attenuated by femoral nerve block with a single administration of local anesthetics,27 and by continuous lumbar or femoral block.28 The mechanisms underlying such a finding are complex and may include the following: 1) reduction of opioid use;29 2) lower rate of infection and inflammatory responses as a result of lack of mechanical ventilation and indwelling catheterization;30 3) anti-inflammatory effects of local anesthetic agents, and thus lower levels of pro-inflammatory cytokines that cross the blood-brain barrier.31,32
Peripheral nerve block for hip fracture surgery typically consists of posterior lumbar plexus block and sacral plexus block. The anesthesia and muscle relaxation obtained with such a method is usually sufficient to allow the surgery to proceed smoothly.17 However, retroperitoneal hematoma has been reported in patients receiving low-molecular-weight heparin.18 Accordingly, a significant proportion of patients are not candidate for posterior lumbar plexus block. The posterior approach of lumbar plexus block has several important limitations. First, the location is deeper and the operation requires normal coagulation function. Second, large amount of local anesthetics injected over a short period of time could spread to the spinal canal and cause hypotension. Third, postoperative analgesia with indwelling catheters is difficult. The femoral nerve block used in this trial could conceivably solve these issues. Previous studies have reported that the local anesthetic drugs can diffuse to the lumbar plexus through the fascia after femoral nerve block, thereby achieving the effect of simultaneously blocking the femoral nerve, the lateral femoral cutaneous nerve and the obturator nerve.33 The femoral nerve block is also referred to as the anterior lumbar plexus block.20
The sensory fibres of the obturator nerve that innervate the hip and knee are not consistently targeted by local anesthetic agents during the femoral nerve block.34 An anatomical study showed that upon parasacral injection, colored latex could spread to the obturator nerve and sacral nerve roots,35 thus establishing the theoretical basis for using parasacral injection for obturator and perineal blockade and for omitting separate obturator nerve block. In a previous study by Ertan et al36 the rate of obturator nerve block was 80% in inguinal paravascular block with parasacral sciatic nerve block. The results of two case reports21,37 support that femoral nerve block combined with sacral plexus block anesthesia can be used to complete hip fracture surgery.
Sacral plexus block, as described in previous studies, has been considered challenging and unreliable because of its depth.38 We modified the procedure for sacral plexus block by targeting the edge of the greater sciatic notch. The close approximation of this bony landmark to the sacral plexus allows much easier maneuvering during the procedure, and enhances the procedural reliability.
In this study, we used the cannula-over-needle set for continuous nerve block. Compared the needle-over-cannula with the Braun continuous peripheral nerve block catheter set, the needle-over-cannula set is effective, convenient, and safe in continuous femoral nerve block after total knee arthroplasty.22 The cannula-over-needle set comprises a hollow needle with a side hole, a needle tip with a 30° bevel, and an indwelling cannula with side holes outside the needle. Both needle and cannula were visible under ultrasound.22 The catheter was placed between the iliac fascia and the surface of the iliopsoas muscle. The cannula could be clearly seen with ultrasound. When injecting normal saline through the catheter, the position of the catheter tip can also be observed under ultrasound.
The dosage of the local anesthetics used in this trial is relatively large, but we did not observe signs of systemic toxicity in any patients, probably due to the 10-min interval between femoral block and sacral plexus block.
A key strength in this trial was the diagnostic criteria for delayed neurocognitive recovery based on a battery of neuropsychological tests as proposed by the ISPOCD1 study.25 These tests provide a comprehensive and accurate assessment of a variety of cognitive domains that are frequently affected after surgery and have been widely accepted as the gold standard.39
This trial has several limitations. First, this is a single-centre trial. Whether the results could be generalized requires further investigation. Second, whether decreased incidence of delayed neurocognitive recovery is associated with improved long-term outcomes remain unknown. Long-term outcomes including cognitive function, quality of life, and survival length will be investigated in the future. Third, athough anesthesia depth has been associated with delayed neurocognitive recovery,40 we were unable to compare the depth of anesthesia between the two groups of patients. Consequently, the potential confounding effects of anesthesia depth on study outcomes could not be excluded. Fourth, the lack of perioperative neuroinflammatory marker measurements in this study limits mechanistic exploration of how regional anesthesia reduces delayed neurocognitive recovery incidence. We cannot confirm whether the observed neuroprotective effects originate from reduced systemic inflammation. Future studies integrating CSF/serum biomarker profiling with advanced neuroimaging (eg, PET-MRI for microglial activation) are needed to dissect these pathways.
Conclusion
Strategies to reduce cognitive decline may allow patients to achieve improvement on long-term outcomes including cognitive function, quality of life as even short-term cognitive dysfunction has implications for quality of life 1 year later.41 Compared with general anesthesia, continuous femoral nerve block plus sacral plexus block reduced the incidence of delayed neurocognitive recovery in patients undergoing hip fracture surgery. The incidence of intraoperative hypotension, postoperative nausea and vomiting, moderate/severe postoperative pain, and postoperative pneumonia were also lower in the nerve block group. This locoregional anesthesia technique for patients undergoing hip surgery offers a safer alternative that lowers the risk of complications, especially for the elderly who are frail and already have cognitive impairment.
Data Sharing Statement
The data supporting the current study are available from the corresponding author upon reasonable request.
Funding
This work was supported by the National Natural Science Foundation of China (81974167), Science and Technology Commission of Shanghai Municipality (23Y11906500), Shanghai Rehabilitation Medical Research Center Top Priority Research Center of Shanghai (2023ZZ02027), Three-Year Action Plan for Promoting Clinical Skills and Clinical Innovation in Municipal Hospitals of Shanghai Shenkang Hospital Development Center (16CR3004A).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang C, Feng J, Wang S, et al. Incidence of and trends in hip fracture among adults in urban China: a nationwide retrospective cohort study. PLoS Med. 2020;17(8):e1003180. doi:10.1371/journal.pmed.1003180
2. Zhang Y, Shan GJ, Zhang YX, et al. Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed neurocognitive recovery in older adults. Br J Anaesth. 2018;121(3):595–604. doi:10.1016/j.bja.2018.05.059
3. Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS, Group the I. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110(3):548. doi:10.1097/ALN.0b013e318195b569
4. Boulton C, Bunning T, Johansen A, et al. National hip fracture database annual report 2017.
5. Dong Y, Zhang G, Zhang B, et al. The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol. 2009;66(5):620–631. doi:10.1001/archneurol.2009.48
6. Mardini F, Tang JX, Li JC, Arroliga MJ, Eckenhoff RG, Eckenhoff MF. Effects of propofol and surgery on neuropathology and cognition in the 3xTgAD Alzheimer transgenic mouse model. Br J Anaesth. 2017;119(3):472–480. doi:10.1093/bja/aew397
7. Zywiel MG, Prabhu A, Perruccio AV, Gandhi R. The influence of anesthesia and pain management on cognitive dysfunction after joint arthroplasty: a systematic review. Clin Orthop. 2014;472(5):1453–1466. doi:10.1007/s11999-013-3363-2
8. Tian Y, Zhu Y, Zhang K, et al. Incidence and risk factors for postoperative pneumonia following surgically treated Hip fracture in geriatric patients: a retrospective cohort study. J Orthop Surg. 2022;17(1):179. doi:10.1186/s13018-022-03071-y
9. Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113(6):1338–1350. doi:10.1097/ALN.0b013e3181fc6e0a
10. van Harten AE, Scheeren TWL, Absalom AR. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia. Anaesthesia. 2012;67(3):280–293. doi:10.1111/j.1365-2044.2011.07008.x
11. Hovens IB, Schoemaker RG, van der Zee EA, Absalom AR, Heineman E, van Leeuwen BL. Postoperative cognitive dysfunction: involvement of neuroinflammation and neuronal functioning. Brain Behav Immun. 2014;38:202–210. doi:10.1016/j.bbi.2014.02.002
12. Eckenhoff RG, Laudansky KF. Anesthesia, surgery, illness and Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:162–166. doi:10.1016/j.pnpbp.2012.06.011
13. Alam A, Hana Z, Jin Z, Suen KC, Ma D. Surgery, neuroinflammation and cognitive impairment. eBioMedicine. 2018;37:547–556. doi:10.1016/j.ebiom.2018.10.021
14. Bryson GL, Wyand A. Evidence-based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction. Can J Anesth. 2006;53(7):669–677. doi:10.1007/BF03021625
15. Mason SE, Noel-Storr A, Ritchie CW. The impact of general and regional anesthesia on the incidence of post-operative cognitive dysfunction and post-operative delirium: a systematic review with meta-analysis. J Alzheimers Dis JAD. 2010;22(Suppl 3):67–79. doi:10.3233/JAD-2010-101086
16. Hebl JR, Kopp SL, Ali MH, et al. A comprehensive anesthesia protocol that emphasizes peripheral nerve blockade for total knee and total Hip arthroplasty. J Bone Joint Surg Am. 2005;87(Suppl 2):63–70. doi:10.2106/JBJS.E.00491
17. Guay J, Parker MJ, Gajendragadkar PR, Kopp S. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev. 2016;2(2):CD000521. doi:10.1002/14651858.CD000521.pub3
18. Weller RS, Gerancher JC, Crews JC, Wade KL. Extensive retroperitoneal hematoma without neurologic deficit in two patients who underwent lumbar plexus block and were later anticoagulated. Anesthesiology. 2003;98(2):581–585. doi:10.1097/00000542-200302000-00044
19. Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American society of regional anesthesia and pain medicine evidence-based guidelines (Fourth edition). Reg Anesth Pain Med. 2018;43(3):263–309. doi:10.1097/AAP.0000000000000763
20. Capdevila X, Coimbra C, Choquet O. Approaches to the lumbar plexus: success, risks, and outcome. Reg Anesth Pain Med. 2005;30(2):150–162. doi:10.1016/j.rapm.2004.12.007
21. Chen L, Liu J, Yang J, Zhang Y, Liu Y. Combined Fascia Iliaca and Sciatic nerve block for hip surgery in the presence of severe ankylosing spondylitis: a case-based literature review. Reg Anesth Pain Med. 2016;41(2):158–163. doi:10.1097/AAP.0000000000000350
22. Yu B, Hu X, Zou T, He M, Cai G. Effects of postoperative continuous femoral nerve block analgesia with braun continuous peripheral nerve block catheter set versus novel needle-over-cannula after total knee arthroplasty. Med Sci Monit Int Med J Exp Clin Res. 2015;21:1843–1849. doi:10.12659/MSM.893617
23. Murkin JM, Newman SP, Stump DA, Blumenthal JA. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg. 1995;59(5):1289–1295. doi:10.1016/0003-4975(95)00106-u
24. Silbert B, Evered L, Scott DA, et al. Preexisting cognitive impairment is associated with postoperative cognitive dysfunction after Hip joint replacement surgery. Anesthesiology. 2015;122(6):1224–1234. doi:10.1097/ALN.0000000000000671
25. Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. international study of post-operative cognitive dysfunction. Lancet Lond Engl. 1998;351(9106):857–861. doi:10.1016/s0140-6736(97)07382-0
26. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi:10.1016/j.ajic.2008.03.002
27. YaDeau JT, Cahill JB, Zawadsky MW, et al. The effects of femoral nerve blockade in conjunction with epidural analgesia after total knee arthroplasty. Anesth Analg. 2005;101(3):891–895. doi:10.1213/01.ANE.0000159150.79908.21
28. Marino J, Russo J, Kenny M, Herenstein R, Livote E, Chelly JE. Continuous lumbar plexus block for postoperative pain control after total Hip arthroplasty. A randomized controlled trial. J Bone Joint Surg Am. 2009;91(1):29–37. doi:10.2106/JBJS.H.00079
29. Awada HN, Luna IE, Kehlet H, Wede HR, Hoevsgaard SJ, Aasvang EK. Postoperative cognitive dysfunction is rare after fast-track hip- and knee arthroplasty - but potentially related to opioid use. J Clin Anesth. 2019;57:80–86. doi:10.1016/j.jclinane.2019.03.021
30. Evered LA, Silbert BS. Postoperative cognitive dysfunction and noncardiac surgery. Anesth Analg. 2018;127(2):496–505. doi:10.1213/ANE.0000000000003514
31. Butterworth J, Hammon JW. Lidocaine for neuroprotection: more evidence of efficacy. Anesth Analg. 2002;95(5):1131–1133. doi:10.1097/00000539-200211000-00001
32. Wang D, Wu X, Li J, Xiao F, Liu X, Meng M. The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesth Analg. 2002;95(5):1134–1141, tableofcontents. doi:10.1097/00000539-200211000-00002
33. Winnie AP, Ramamurthy S, Durrani Z. The inguinal paravascular technic of lumbar plexus anesthesia: the “3-in-1 block. Anesth Analg. 1973;52(6):989–996. doi:10.1213/00000539-197311000-00036
34. Marhofer P, Nasel C, Sitzwohl C, Kapral S. Magnetic resonance imaging of the distribution of local anesthetic during the three-in-one block. Anesth Analg. 2000;90(1):119–124. doi:10.1097/00000539-200001000-00027
35. Valade N, Ripart J, Nouvellon E, et al. Does sciatic parasacral injection spread to the obturator nerve? An anatomic study. Anesth Analg. 2008;106(2):664–667, tableofcontents. doi:10.1213/ane.0b013e3181607205
36. Öztürk E, Gökyar İ, Günaydın B, Çelebi H, Babacan A, Kaya K. Comparison of parasacral and posterior sciatic nerve blocks combined with lumbar plexus block. Turk J Anaesthesiol Reanim. 2013;41(5):171–174. doi:10.5152/TJAR.2013.47
37. Coviello A, Iacovazzo C, Cirillo D, et al. Tetra-block: ultrasound femoral, lateral femoral-cutaneous, obturator, and sciatic nerve blocks in lower limb anesthesia: a case series. J Med Case Rep. 2023;17(1):270. doi:10.1186/s13256-023-04017-6
38. Ben-Ari AY, Joshi R, Uskova A, Chelly JE. Ultrasound localization of the sacral plexus using a parasacral approach. Anesth Analg. 2009;108(6):1977–1980. doi:10.1213/ane.0b013e3181a04d8e
39. Rasmussen LS, Larsen K, Houx P, et al. The assessment of postoperative cognitive function. Acta Anaesthesiol Scand. 2001;45(3):275–289. doi:10.1034/j.1399-6576.2001.045003275.x
40. Lu X, Jin X, Yang S, Xia Y. The correlation of the depth of anesthesia and postoperative cognitive impairment: a meta-analysis based on randomized controlled trials. J Clin Anesth. 2018;45:55–59. doi:10.1016/j.jclinane.2017.12.002
41. Phillips-Bute B, Mathew JP, Blumenthal JA, et al. Association of neurocognitive function and quality of life 1 year after coronary artery bypass graft (CABG) surgery. Psychosom Med. 2006;68(3):369–375. doi:10.1097/01.psy.0000221272.77984.e2
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.