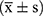Back to Journals » Journal of Pain Research » Volume 18
Effective Dosage of Ciprofol for the Induction of General Anesthesia Across Diverse Age Groups in Adults: A Single-Center, Prospective, Non-Randomized Sequential Trial
Authors Deng L, Zhang C, Tan M, Zeng W, Luo G, Li P
Received 19 October 2024
Accepted for publication 14 May 2025
Published 14 June 2025 Volume 2025:18 Pages 2983—2992
DOI https://doi.org/10.2147/JPR.S496223
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jinlei Li
Lizhen Deng,* Chunyuan Zhang,* Meiyun Tan, Wei Zeng, Guozhan Luo, Ping Li
Department of Anesthesiology, Affiliated Boai Hospital of Zhongshan, Zhongshan, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Meiyun Tan, Department of Anesthesiology, Affiliated Boai Hospital of Zhongshan, No. 6 Chenggui Road, East District, Zhongshan, Guangdong, 528400, People’s Republic of China, Tel +86-13726028414, Email [email protected]
Purpose: A new Chinese-developed intravenous anesthetic called ciprofol enhances propofol’s effectiveness against GABAA receptors by adding cyclopropyl. This study aims to determine the optimal dosage of ciprofol for inducing general anesthesia in adult patients of different ages and its correlation with Narcotrend index (NTI).
Patients and Methods: 105 patients were stratified into three age groups: 18– 40 (Group A), 41– 65 (Group B), and 66– 85 (Group C) years. Initial doses of 0.4 mg/kg (Groups A and B) and 0.3 mg/kg (Group C) ciprofol tosilate were administered, adjusted by 0.05 mg/kg based on sedation efficacy. The Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale and Dixon up-and-down method were used to calculate ED50, ED95, and NTI50.
Results: Effective dosages were: youth (ED50=0.526 mg/kg, ED95=0.610 mg/kg), middle-aged (ED50=0.366 mg/kg, ED95=0.450 mg/kg), and elderly (ED50=0.324 mg/kg, ED95=0.408 mg/kg). NTI50 were 38.068 (33.496– 44.188), 44.963 (39.311– 52.270), and 47.214 (39.792– 57.420) for the three groups, respectively.
Conclusion: Ciprofol is safe and effective for anesthesia induction across age groups, with lower doses required for elderly patients. NTI reduction was dose-dependent and slower in elderly patients.
Keywords: ciprofol, anesthesia induction, optimal dose, adult, sequential allocation
Introduction
Propofol, a classic intravenous anesthetic, is associated with limitations such as injection pain and cardiorespiratory depression.1–4 Ciprofol, a novel GABAA receptor agonist developed in China, exhibits higher receptor affinity and reduced adverse effects.5–8 Some studies have shown that the effective drug dose decreases with age, with elderly patients requiring a lower ED50 of cyclopofol compared to younger and middle-aged patients. Our study examines the dose–response relationship and NTI correlation in adults aged 18–85, categorizing participants by age to determine the precise dose range for each group.
Materials and Methods
Study Design
This study is a single-center, prospective, non-randomized clinical cohort investigation into the dose–response relationship of a specific drug. Approval for the study was granted by the Ethics Committee of Zhongshan Boai Hospital on May 23, 2023 (Ethics Committee No. ky-2023-015-05). The research study (ChiCTR2300072307, registered on 09/06/2023) has been registered in the Chinese Clinical Trial Registration Center (https://www.chictr.org.cn/bin/project/edit?pid=198928, and the public title of the study: The effective dose and NTI50 of ciprofol for general anesthesia induction in adult patients of different ages were determined by sequential method). The initial participant was enrolled in the trial on July 22, 2023, while the final participant was enrolled on April 28, 2024.
Inclusion and Exclusion Criteria
The study adhered to the principles outlined in the Helsinki Declaration. Prior to their participation, all individuals provided written informed consent. 105 inpatients scheduled for laparoscopic abdominal surgery under general anesthesia, categorized into age-specific cohorts: young (18–40 years old), middle-aged (41–65 years old), and elderly (66–85 years old), with 35 participants in each group. Participants were also required to have a BMI between 18.5 and 24.9 kg/m2, be categorized as ASA class I or II, and demonstrate normal head and neck mobility with a Mallampati classification of I or II. The exclusion criteria for participation in this trial encompass patients who refuse or are considered inappropriate for inclusion, those necessitating immediate surgical intervention, individuals with depleted blood volume and shock, a past history of general anesthesia or recent use of other sedative medications, allergies to ciprofol, non-depolarizing muscle relaxants, and opioids, as well as severe medical conditions such as myasthenia gravis, schizophrenia, severe depression affecting the neuromuscular junction, cardiovascular disorders, significant impairment of essential organs, severe anemia, coagulation abnormalities, and difficulty in airway management.
Anesthesia Procedure and Statistical Analysis
All participants are mandated to observe a fasting period of 6 hours, refrain from consuming liquids for 2 hours, and abstain from atropine usage prior to the surgical procedure. Upon entry into the operating room, standard monitoring protocols will encompass electrocardiogram (ECG), heart rate (HR), pulse oximetry (SpO2), and mean arterial pressure (MAP). An intravenous line will be inserted to deliver a continuous infusion of Ringer’s solution at a rate of 6–10 mL/kg/h. Standardized Narcotrend monitoring will be initiated to evaluate the depth of anesthesia. Subsequent to the administration of 3 mL/kg of preload fluid to all patients, anesthesia induction will be administered in a sequential trial fashion, with the target dose of ciprofol being provided. The medication is delivered intravenously within a one-minute timeframe, during which the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) is assessed for a total of five minutes. Upon reaching a MOAA/S score of 0, sufentanil at a dosage of 0.35 μg/kg and rocuronium at a dosage of 0.15 mg/kg are administered intravenously. Subsequently, manual assisted ventilation is commenced for a period of three minutes, followed by endotracheal intubation guided by a video laryngoscope. The maintenance of Narcotrend levels D1 to D2 during the procedure involves adjusting anesthesia using ciprofol at a dosage range of 0.4 to 2.4 mg/kg/h and Remifentanil at a dosage range of 0.1 to 0.3 μg/kg/min, according to the depth of anesthesia. Volume-controlled ventilation (VCV) is employed throughout the surgery to ensure PETCO2 remains within the range of 35 to 45 mmHg. Additionally, the dosage of ciprofol is halved ten minutes prior to the conclusion of the surgery. After the closure of the skin, the cessation of both ciprofol and Remifentanil occurs, and intravenous administration of sufentanil at a dosage of 0.1 μg/kg is initiated. Subsequently, patients will be extubated in the operating room and undergo a 30-minute observation period in the post-anesthesia care unit (PACU).
Each patient cohort will receive the specified dose of ciprofol via an intravenous infusion pump over a 30-second period during the induction phase. The level of consciousness, as measured by the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) score, will be assessed at 5-second intervals, with a total observation duration of 3 minutes.
In accordance with established sedation protocols and the MOAA/S scale, the dosages of ciprofol administered to successive patients will be adjusted through a sequential trial approach. The initial induction dose for the first patient will be 0.4 mg/kg (0.3 mg/kg for elderly patients), with subsequent doses increasing by increments of 0.05 mg/kg. A favorable sedation outcome, as evidenced by a MOAA/S score of 0 during induction, will be deemed a positive response (+) and will lead to a reduction of one dose increment for the following patient. If the MOAA/S score remains at 1 or above during induction, signifying sedation failure, it is categorized as a negative response (-), prompting an increase in the subsequent patient’s ciprofol induction dose by one dose gradient. This iterative process continues until at least 7 transitions have taken place. Probability regression analysis will be utilized to ascertain the optimal induction dose of ciprofol. Interventions during the examination: In cases where the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) score remains at or above 1, sedation failure is acknowledged, prompting the immediate administration of an additional half-dose with an extended administration time of 10 seconds. Should sedation continue to be inadequate, a second half-dose is administered one minute later. If sedation remains insufficient, propofol is administered at a dosage of 0.5–1mg/kg as a remedial measure until the MOAA/S score reaches 0. In cases of bradycardia, it is recommended to administer intravenous atropine at a dosage of 0.01 mg/kg, while for hypotension, intravenous ephedrine at a dosage of 0.1 mg/kg is advised, with the possibility of repeating as necessary.
This study, which is not randomized, is a clinical cohort study focusing on drug dose-response in different age groups, with strict adherence to double-blinding principles. The individuals tasked with preparing the standard solution of ciprofol and conducting sedation assessments using MOAA/S scoring are kept unaware of the study protocol. The sedation assessment findings are then communicated accurately to the Pharmacist, who proceeds to provide the anesthesiologist with the next patient’s designated medication for anesthesia administration in adherence to established guidelines.
Observation Parameters
Primary Observation Parameters
Primary observation indicators include the MOAA/S score and the determination of sedation response. A MOAA/S score of ≥1 signifies sedation failure, while a score of 0 indicates sedation success.
Secondary Observation Parameters
1. Narcotrend Values: Collect the Narcotrend values of the patient at 1 minute prior to anesthesia (T1), 30 seconds (T2), 1 minute (T3), 2 minutes (T4), 3 minutes (T5), and post-administration of ciprofol. Additionally, document the minimum Narcotrend value observed when the patient’s MOAA/S score reaches 0, along with the corresponding dose of ciprofol administered for induction.
2. MAP, HR, and SpO2 Values: The values of mean arterial pressure (MAP), heart rate (HR), and oxygen saturation (SPO2) were recorded at each time point (T1, T2, T3, T4, and T5).
3. Pain Assessment Scores.
4. Adverse Reactions in Perioperative Patients: Adverse reactions experienced by perioperative patients, such as hypotension, bradycardia, tachycardia, respiratory depression, nausea, vomiting, chills, delayed recovery, and delirium, were documented.
5. Statistical methods: Data analysis was performed using SPSS 26.0 software. Normally distributed continuous variables are expressed as mean ± standard deviation (±s). Categorical variables were analyzed with the chi-square test. Repeated Measures ANOVA and one-way ANOVA were employed for comparisons. When homogeneity of variance was met, pairwise group comparisons used the Least Significant Difference (LSD) method; when violated, the Games-Howell test was applied. Intergroup gender differences were assessed via chi-square test. A two-tailed P < 0.05 was considered statistically significant.
Identification of ED50 and ED95
The Dixon trial comprises groups of 20–40 patients each, with a 10% dropout rate factored in. A sample size of 35 patients per group was ultimately selected, yielding an initial total of 105 patients. The effective dose (ED) of ciprofol induction is established through the Dixon up-and-down method, with dose–response data analyzed via Probit regression. Data is presented as mean and 95% confidence interval [mean (1.96SD), 95% CI], with dose values as x–values and response percentage as Y. Regression coefficients are calculated using regression analysis to estimate the effective dose value on the linear Probit regression plot, thereby creating a dose–response curve for ciprofol. The sequential formula and probit regression model were used to calculate ED50, and ED95 was determined using the probit regression model.
Determination of NTI50
The NTI50 was defined as the NTI corresponding to the point at which 50% of patients achieved sleep. The lowest NTI obtained during the experiment was recorded and used as a reference point for determining NTI50 through a geometric progression analysis within each group. NTI50 could be determined by substituting the geometric progression data into the sequential formula and probit regression model. The NTI was recorded even when the MOAA/S score was 0, indicating the patient was asleep. Pearson’s linear correlations were used to examine the relationship between the total dose of remimazolam tosilate and the decrease in NTI. The sequential formula and probit regression model were used to calculate NTI50.
Results
Patient Characteristics
The timeline is presented in Figure 1. Out of 105 elderly patients assessed, 10 were excluded and 22 were not enrolled, leaving 73 patients in the trial. Demographics and baseline parameters are summarized in Table 1.
 |
Table 1 The Characteristic Data |
 |
Figure 1 The timeline of the study. Abbreviations: Y, youth; M, middle-age; E, elderly. |
Hemodynamic Changes
Ciprofol administration induced changes in MAP, HR, SpO2, and NTI across all three age groups (Figure 2). Mean arterial pressure (MAP) decreased post-administration (P<0.05), with elderly patients showing higher baseline MAP (Table 2). Heart rate (HR) transiently increased in all groups (P<0.05). Young patients exhibited higher HR than other groups both pre- and post-administration (P < 0.05), while SpO2 showed no intergroup differences (P > 0.5). Adverse events included hypotension (1 youth, 2 middle-aged, 2 elderly), coughing (1 middle-aged, 1 elderly), and no allergic reactions or other complications.
 |
Table 2 The Vital Signs and NTI Before and After Administration |
 |
Figure 3 Dixon up-and-down plots for the youth group. |
 |
Figure 4 Dixon up-and-down plots for the middle age group. |
 |
Figure 5 Dixon up-and-down plots for the elderly group. |
Narcotrend Index Changes
NTI reduction was dose-dependent, with slower declines in elderly patients (Figure 2). Post-administration NTI decreased significantly from baseline in all groups (P < 0.05). While both middle-aged and elderly groups had lower NTI than the young group (P < 0.05), their intergroup difference was not significant.
Effective Doses
A 95% confidence interval was established for the effective dose (ED) values of ciprofol induction using the probit method. Youth: ED50=0.526 mg/kg (95% CI 0.494–0.561), ED95=0.610 mg/kg (95% CI 0.571–0.703). Middle-aged: ED50=0.366 mg/kg (95% CI 0.337–0.396), ED95=0.450 mg/kg (95% CI 0.414–0.537). Elderly: ED50=0.324 mg/kg (95% CI 0.290–0.359), ED95=0.408 mg/kg (95% CI 0.370–0.498). The Dixon up-and-down plots for each group are shown in Figures 3–5. The dose–effect response curve of ciprofol induction in each group is shown in Figure 6.
 |
Figure 6 Dose–Effect response curve of ciprofol induction in each group. |
NTI50
In the study of the correlation between remimazolam tosilate induction and NTI, with NTI50 of 38.068 (33.496–44.188), 44.963 (39.311–52.270), and 47.214 (39.792–57.420) observed in the youth, middle-aged, and elderly age cohorts, respectively.
Discussion
Ciprofol, the first autonomously developed Class 1 intravenous anesthetic in China, belongs to the GABAA receptor agonist class along with propofol. By introducing a cyclopropyl group into the propofol structure, ciprofol acquires a chiral configuration that enhances its stereo effects. Its binding affinity to the GABAA receptor exceeds that of propofol by a factor of approximately 4 to 5.6,8 Qin et al (year) conducted a study utilizing mice to determine the median effective dose (ED50) of ciprofol as 1.5mg/kg, the median lethal dose (LD50) as 9.9mg/kg, and the therapeutic index (TI) as 6.6, indicating a wider safety margin compared to propofol. Furthermore, a clinical controlled trial evaluating ciprofol and propofol for gastrointestinal endoscopy revealed that the sedative/anesthetic effect achieved with 0.4–0.5mg/kg of ciprofol is comparable to 1.5–2.0mg/kg of propofol. Moreover, research has demonstrated a reduction in the occurrence of injection pain, hypotension, respiratory depression, and myoclonus with ciprofol, resulting in improved stability of vital signs.9–12 These results bolster the claim that ciprofol is a secure, efficient, and minimally harmful intravenous anesthetic. Nonetheless, existing studies do not provide sufficient data on the most effective dosage of ciprofol for inducing general anesthesia in different age demographics, especially in the elderly population. Due to the age-related decline in organ function in elderly individuals, changes in the distribution and metabolism of anesthetic drugs are observed.13 As a result, there is a need for further investigation into the assessment and selection of medication dosages and safety profiles. Thus, this study aims to investigate the optimal dosage of ciprofol for general anesthesia in different age groups.
In our study, we determined the effective dosages of ciprofol for anesthesia induction in patients belonging to different age cohorts. Specifically, we determined the ED50 values for the youth, middle-aged, and elderly groups to be 0.526 mg/kg (95% CI 0.494–0.561 mg/kg), 0.366 mg/kg (95% CI 0.337–0.396 mg/kg), and 0.324 mg/kg (95% CI 0.290–0.359 mg/kg), respectively. Furthermore, we calculated the corresponding ED95 values for these groups as 0.610 mg/kg (95% CI 0.571–0.703 mg/kg), 0.450 mg/kg (95% CI 0.414–0.537 mg/kg), and 0.408 mg/kg (95% CI 0.370–0.498 mg/kg), respectively. During the statistical analysis of data, a 95% confidence interval was calculated for the effective dosage (ED) values. The results indicate that ciprofol may be safely and effectively used for anesthesia induction in adult patients of different age groups, with the effective dosage for anesthesia induction decreasing with increasing age. Li et al14 documented that a dosage of 0.3mg/kg ciprofol can be efficaciously employed for intravenous general anesthesia in elderly patients aged 65 to 73, albeit with a marginally elevated occurrence of adverse reactions compared to the non-elderly cohort. However, our study found that the optimal dosage (ED95) of ciprofol for intravenous anesthesia induction in elderly patients is 0.408 mg/kg, slightly higher than the recommended dosage. This dosage is lower than the optimal dosage for younger patients (0.610 mg/kg). Notably, the vital signs of elderly patients in our study remained stable and comparable to those of younger and middle-aged patients.
The results of our study demonstrate a statistically significant difference in baseline blood pressure among the three patient groups (P < 0.05), with mean arterial pressure (MAP) exhibiting a positive association with aging and decreased vascular elasticity resulting in elevated blood pressure. Across all age groups, there was a reduction in mean arterial pressure levels following the intravenous injection of ciprofol within a 30-second timeframe (T2), potentially due to the vasodilatory effects of ciprofol.15 However, the decrease in blood pressure within each group was limited to 20%, and there was no statistically significant difference in ΔMAP between the groups, suggesting a state of hemodynamic stability. These findings suggest that the impact of ciprofol on blood pressure at this effective dosage may not vary significantly across different age groups. A study conducted by Wang et al revealed that the administration of ciprofol (0.4mg/kg) during anesthesia induction led to a notable reduction in cardiovascular adverse events and a decrease in variability in blood pressure and heart rate compared to propofol (2.0mg/kg).6 Nevertheless, further extensive research is necessary to ascertain the superiority of ciprofol over propofol in ensuring hemodynamic stability, particularly in elderly patients.
Compared to the youth cohort, the middle-aged and elderly cohorts exhibited a statistically significant decrease in baseline heart rate (HR) (P < 0.05), which was attributed to the age-related reduction in sympathetic nervous system excitability leading to bradycardia.16 Further analysis of HR levels within each cohort revealed a transient rise in HR after the administration of ciprofol for 30 seconds (T2), with this increase observed across all age groups (P < 0.05). The transient increase in blood pressure observed after the administration of ciprofol is thought to be associated with compensatory mechanisms involving circulatory inhibition and heightened sympathetic nervous system activity following the induction of hypotension. This temporary elevation is subsequently followed by a return to baseline blood pressure levels within one minute post-administration. Analysis of the changes in ΔSpO2 among the three patient groups during the study did not show any statistically significant differences, and there were no occurrences of hypoxemia reported among the participants throughout the trial. These findings are consistent with previous research suggesting that ciprofol exhibits reduced levels of respiratory depression compared to propofol.17 However, it is conceivable that the utilization of oxygen masks during the anesthesia induction may have impacted these results.
Analysis of the changes in NTI indicated that the initial NTI(T1) in the elderly group was significantly lower compared to the younger and middle-aged groups (Figure 2, P < 0.05). Following administration at 30 seconds (T2), there was a significant decrease in NTI across all groups (P < 0.05), reaching their lowest levels at 1 minute post-administration (T3). Subsequently, NTI exhibited a gradual increase after 3 minutes post-administration, indicating a potential correlation between ciprofol sedation depth and NTI levels. Upon comparing groups at the same time point, it was observed that the magnitude of NTI reduction following administration was significantly greater in the youth group compared to the middle-aged and elderly groups (P < 0.05). This difference implies a potentially heightened correlation between anesthetic depth and NTI alterations in the younger cohort. Alternatively, the differences in drug response between youth, middle-aged, and elderly cohorts may be explained by variations in dosage administration. Youth may receive higher doses, leading to a more rapid attainment of peak drug concentration and subsequent pronounced decrease in NTI over a shorter period. In contrast, middle-aged and elderly individuals may require lower doses, resulting in a delayed onset of action, prolonged time to achieve peak drug concentration, and a more gradual decrease in vital signs and NTI.
Li et al14 noted that elderly patients receiving a dosage of 0.3mg/kg during ciprofol anesthesia induction exhibited comparable clinical efficacy to non-elderly subjects administered a dosage of 0.4mg/kg. However, the incidence of drug-related adverse reactions in elderly patients was slightly higher than in the non-elderly group. These findings contrast with the results of our own study. Our study found that the effective dose for elderly individuals during anesthesia induction was 0.408 mg/kg, higher than the suggested dosage of 0.3 mg/kg. The youth group had an ED95 of 0.610 mg/kg and the middle-aged group had an ED95 of 0.45 mg/kg, both exceeding recommended dosages.14 However, it is crucial to note that no patients experienced severe cardiovascular adverse events during the trial, underscoring the broader therapeutic range and improved safety profile of ciprofol.
The study possesses several limitations that warrant consideration. Initially, there were distinct variations in preoperative vital signs and baseline inconsistencies observed within each group. The assessment of the hemodynamic effects of ciprofol on the three patient groups was contingent upon analyzing the fluctuation amplitude (∆) value of vital signs before and after drug administration. It is important to note that potential errors may have been introduced due to variations in baseline values. Second, the research investigated the effectiveness and optimal dosage of ciprofol anesthesia among diverse demographic cohorts; however, it did not include a controlled clinical trial comparing ciprofol with propofol. Further research is necessary to ascertain the superiority of ciprofol over propofol for anesthesia induction in elderly individuals. Third, insufficient research has been conducted on the correlation between ciprofol and neurophysiological parameters, particularly NTI or BIS, both domestically and internationally. Although the study did not conclusively establish a strong link between ciprofol sedation depth and NTI, it did suggest a potentially stronger connection between anesthesia depth and NTI changes in younger subjects. Additional research is necessary to validate this finding and comprehensively explore the association between ciprofol and NTI. Additionally, A single-center study’s limitations require validation through larger, multicenter trials to improve credibility and generalizability.
Conclusion
Ciprofol is a safe and effective agent for anesthesia induction in adults across age groups, requiring dose adjustments in elderly patients. Additionally, elderly patients show delayed onset and attenuated NTI-level reductions compared to younger patients during intravenous induction. Dosage adjustments based on age and NTI are recommended.
Acknowledgments
Supported by Zhongshan Science and Technology Bureau (Grant No. 2023B1043).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lundström S, Twycross R, Mihalyo M, et al. Propofol. J Pain Symptom Manage. 2010;40(3):466–470. doi:10.1016/j.jpainsymman.2010.07.001
2. Liu Q, Kong A-L, Chen R, et al. Propofol and arrhythmias: two sides of the coin. Acta Pharmacol Sin. 2011;32(6):817–823. doi:10.1038/aps.2011.42
3. Mashour GA, Sanders RD, Lee U. Propofol anesthesia: a leap into the void? Anesthesiology. 2022;136(3):405–407. doi:10.1097/ALN.0000000000004110
4. Marik PE. Propofol: therapeutic indications and side-effects. Curr Pharm Des. 2004;10(29):3639–3649. doi:10.2174/1381612043382846
5. Bian Y, Zhang H, Ma S, et al. Mass balance, pharmacokinetics and pharmacodynamics of intravenous HSK3486, a novel anaesthetic, administered to healthy subjects. Br J Clin Pharmacol. 2021;87(1):93–105. doi:10.1111/bcp.14363
6. Wang W, Wu L, Zhang C, et al. Is propofol injection pain really important to patients? BMC Anesthesiol. 2017;17(1):1–6.
7. Picard P, Tramer MR. Prevention of pain on injection with propofol: a quantitative systematic review. Anesth Analg. 2000;90(4):963–969. doi:10.1097/00000539-200004000-00035
8. Wei Y, Qiu G, Lei B, et al. Oral delivery of propofol with methoxymethylphosphonic acid as the delivery vehicle. J Med Chem. 2017;60(20):8580–8590.
9. Wang X, Wang X, Liu J, et al. Effects of ciprofol for the induction of general anesthesia in patients scheduled for elective surgery compared to propofol: a Phase 3, multicenter, randomized, double-blind, comparative study. Eur Rev Med Pharmacol Sci. 2022;26(5):1607–1617. doi:10.26355/eurrev_202203_28228
10. Pace NL, Styllanou MP, Warltier DC. Advances in and limitations of up-and-down methodology. Anesthesiology. 2007;107(1):144–152. doi:10.1097/01.anes.0000267514.42592.2a
11. Tan M, Zhang C, Zeng W, et al. Determining the effective dose of esketamine for mitigating pain during propofol injection by Dixon’s up-and-down method: a double-blind, prospective clinical study of drug dose response. BMC Anesthesiol. 2022;22(1):368. doi:10.1186/s12871-022-01914-z
12. Ludbrook G, Li F, Sleigh J, et al. Assessments of onset and duration of drug effects and pharmaco- kinetics by dose level of HSK3486, a new sedative-hypnotic agent, in healthy female/ male subjects: a Phase I multiarm randomized controlled clinical trial. AnesthAnalg. 2021;133(1):e16.
13. Arden JR, Holley FO, Stanski DR. Increased sensitivity to etomidate in the elderly: initial distribution versus altered brain response. Anesthesiology. 1986;65(1):19–27. doi:10.1097/00000542-198607000-00004
14. Li X, Yang D, Li Q, et al. Safety, Pharmacokinetics, and pharmacodynamics of a single bolus of the γ-aminobutyric acid (GABA) receptor potentiator HSK3486 in healthy Chinese elderly and non-elderly. Front Pharmacol. 2021;12:735700. doi:10.3389/fphar.2021.735700
15. Li J, Wang X, Liu J, et al. Comparison of ciprofol (HSK3486)versus propofol for the induction of deep sedation during gastroscopy and colonoscopy procedures: a multi-centre, noninferiority, randomized, controlled phase 3 clinical trial. Basic Clin Pharmacol Toxicol. 2022;131(2):138–148. doi:10.1111/bcpt.13761
16. Sahinovic MM, Struys M, Absalom AR. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet. 2018;57(12):1539–1558. doi:10.1007/s40262-018-0672-3
17. Nagakawa T, Yamazaki M, Hatakeyama N, et al. The mechanisms of propofol-mediated hyperpolarization of in situ rat mesenteric vascular smooth muscle. Anesth Analg. 2003;97(6):1639–1645. doi:10.1213/01.ANE.0000087043.61777.1F
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.