Back to Journals » Journal of Pain Research » Volume 18
Effectiveness and Safety of Platelet-Rich Plasma Combined with Pulsed Radiofrequency to Treat Patients with Infraorbital Neuralgia: A Propensity Score-Matched Analysis of a Multi-Center, Prospective Cohort Protocol
Authors Liu L, Yang D, Duan B, Luo F
Received 7 November 2024
Accepted for publication 19 March 2025
Published 26 March 2025 Volume 2025:18 Pages 1643—1656
DOI https://doi.org/10.2147/JPR.S505263
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Alaa Abd-Elsayed
Lu Liu,1,* Dong Yang,2,* Baolin Duan,3,* Fang Luo4
1Department of Day Surgery Center, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Pain Management, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China.; Key Laboratory of Anesthesiology and Resuscitation (Huazhong University of Science and Technology), Ministry of Education, Wuhan, People’s Republic of China; 3Department of Pain Management, Qinghai Provincial People’s Hospital, Qinghai, People’s Republic of China; 4Department of Pain Management, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fang Luo, Department of Pain Management, Beijing Tiantan Hospital, Capital Medical University, No. 119 South Fourth Ring West Road, Fengtai District, Beijing, 100050, People’s Republic of China, Email [email protected]
Background: Infraorbital neuralgia (IONa) is a rare but devastating type of facial pain, with a lack of current consensus on its proper management. Preliminary studies have established the efficacy of pulsed radiofrequency (PRF) in the treatment of IONa. Platelet-rich plasma (PRP) is a non-destructive technique that alleviates neuropathic pain. Till date, the efficacy of PRP combined with PRF in the treatment of IONa has not been evaluated yet.
Objective: To evaluate the efficacy and safety of PRP combined with PRF in treating refractory IONa in patients unwilling to undergo destructive therapies.
Study Design: A multicenter, prospective, observational, propensity score matching (PSM), and assessor-blinded study.
Setting: Department of pain management in Beijing, Wuhan and Qingdao, China.
Methods: A total of 240 refractory IONa patients will be allocated to either PRP combined with PRF therapy (PRP+PRF) group or PRF therapy alone (PRF) group at their own volition. Statistical analysis will be performed using Pearson’s chi-squared or Fisher’s exact test for categorical variables, Student’s t-test and Mann–Whitney U-test for continuous variables. Logistic regression will be used to evaluate the pain - relief efficacy.
Results: The primary outcome will be the 12-months response rate. The secondary outcome will include NRS score, total daily dose of carbamazepine, the 12-item Short-Form Health Survey (SF-12) scores, patient satisfaction scores (PSS) and adverse events (AEs).
Limitation: This is an observational, open-labeled study with a relatively short term follow-up. The composition of PRP and various cell ratios, which are optimal for IONa has not been published yet.
Conclusion: This will be a multi-center trial with a relatively large sample size, demonstrating the potential benefits of PRP combined with PRF therapy in IONa patients. Further, randomized controlled trial (RCT) will be necessary to confirm the efficacy of this combined therapy.
Keywords: infraorbital neuralgia, platelet-rich plasma, pulsed radiofrequency, treatment protocol
Introduction
Infraorbital neuralgia (IONa), a rare but devastating type of facial pain, is characterized by paroxysms of severe, lancinating, electric shock - like bouts of pain restricted to the distribution of infraorbital nerve, which is an isolated branch of the maxillary nerve (second branch of the trigeminal nerve).1 IONa can be defined as the neuralgia of the terminal branch of the trigeminal nerve, as per the consensus statement from the Spanish Society of Neurology’s Headache Study Group;2 however, this definition provides little insight into the pathogenesis of this neuropathic condition. Although the etiology of IONa and the potential mechanisms are still poorly understood, fracture of the orbital rim, maxillary sinusitis, maxillary antrostomy, nerve tumors, viral infection or other unknown reasons are thought to be causative factors.3,4 Previous researches indicate that cases of terminal branch neuralgia of the trigeminal nerve are rare,1 and that specific incidence of terminal branch neuralgia including IONa has not been reported yet. Despite being rare, however, this agonizing disorder generally entails repeated, severe, long-term pain that could lead to various psychological disorders, seriously impacting patient’s quality of life.5
There is no current consensus in the management of IONa, since the rare incidence of IONa allows the enrollment of even fewer patients for randomized controlled trials (RCT) for relevant therapies.3,6,7 Patients with IONa usually receive the same pharmacotherapy as trigeminal neuralgia. Patients who respond poorly or are intolerable to medical management can be supplemented with neural blockade using local anesthetics.7,8 However, despite the high short-term effect of this procedure, the duration of pain relief is rather short. Nerve blocks with local anesthetics and corticosteroid also only had a temporary effect on IONa.1 And patients must undergo repeated treatments due to relapse. Apparently, repeated injections could enhance surgical complications and corticosteroid-related side effects.9 Neuro-destructive approaches such as percutaneous infraorbital nerve ablation or open infraorbital neurectomy are usually reserved for refractory IONa with failed conservative treatments.10 However, both thermal effect of percutaneous infraorbital nerve ablation and open infraorbital neurectomy inevitably lead to numbness in the area innervated by infraorbital nerve.11–13 Hence, it is necessary to establish a non-destructive and effective treatment of IONa.
Pulsed radiofrequency (PRF), a minimally invasive percutaneous treatment technique, has been proven to be partially effective in IONa patients unresponsive to conservative therapy.14 Our previous studies in patients with IONa demonstrate that the effectivity of 42°C PRF reached 60% during a 1-year follow-up. Although proper enhancement of PRF output voltage could further improve treatment efficacy, the therapeutic effect remains limited and has a significant long-term recurrence rate.15 Another research by Jia et al suggests that the effective rate of a combination of 42°C PRF and 60°C continuous radiofrequency (CRF) treatment was 72.7% at two years. Unfortunately, the recurrence of pain and uncomfortable facial numbness were present in some of these patients after the aforementioned combined therapies.16 The improvement of therapeutic efficacy of 42°C PRF for IONa has become a pressing challenge.
Platelet-rich plasma (PRP) is a safe autologous blood product providing a supraphysiological concentration of platelets, leukocytes, growth factors and other bioactive proteins such as cytokines and chemokines.17 Activated platelets release many anti-inflammatory mediators that can reduce inflammatory reaction, promote nerve axon regeneration and reinnervate the original target tissues, providing prolonged neuropathic pain relief,18 in cases with postherpetic neuralgia,19 diabetic neuropathic pain.20,21 Doss et al reported a single case of refractory TN treated with PRP. After injections of PRP around the distal branches of the maxillary nerve, the patient was able to achieve normal social and work life with a complete resolution of their symptoms with no clinical features to suggest peripheral nerve damage for 6 months.22 A pilot study comprising 29 patients with idiopathic trigeminal neuralgia showed a significant reduction in the mean visual analogue scale (VAS) score from 9.1 at baseline to 0.0 at six months after PRP injection around the branches of the trigeminal nerve administered five times at seven days intervals.23 By now, there are a few literatures reporting the effectiveness of PRP treatment for IONa. Thus, the analgesic potential of PRP to IONa can be speculated, since infraorbital nerve is a branch of the trigeminal nerve (Maxillary nerve, CN V2).
As PRF and PRP are both non-destructive techniques, whether combination therapy can achieve better therapeutic effects than simple PRF therapy warrants a discussion. Till date, there have been a few case reports reporting that PRF combined with PRP has a better potential to control chronic pain such as osteoarthritis,24,25 supraspinatus injury,26 etc. compared to PRF alone. However, there is no evidence to support the clinical benefits of PRF combined with PRP for IONa treatment yet. We have designed this prospective study aimed to observe whether PRP injections combined with PRF treatment could be a more effective treatment option compared to utilizing only PRF for refractory IONa.
Objectives
This study aims to compare the efficacy and safety of PRP combined with PRF against PRF alone in IONa patients seeking minimally invasive treatment strategy, who respond poorly to or are intolerant of carbamazepine and refuse destructive therapies.
Methods
Study Design
This is a multicenter, prospective, observational, propensity score matching (PSM), cohort and assessor-blinded study.
Study Setting
Patient recruitment will begin on July 1st, 2024, and will end in July 1st, 2026, at the department of pain management in the following participating centers: Beijing Tiantan Hospital, Capital Medical University; Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan; Qinghai Provincial People’s Hospital, Qinghai. The study will complete on July 1st, 2027, with a follow-up period of 12 months. This time frame will be adjusted according to the actual progress. The research teams responsible for the study at each study centers will receive a specific training regarding the details of this study protocol to confirm smooth operations. All the procedures will be performed by pain physicians with over 5 years of experience and more than 30 PRF treatment for IONa before participating in this study.
Study Protocol
This study protocol was approved by the ethics committee of Beijing Tiantan Hospital (KY2023-263-03-03) and has been registered at the clinicaltrials.gov website (ID:NCT06492231). This protocol was written in compliance with the Declaration of Helsinki.27
Informed Consents
Patients will be informed in detail the benefits and potential risks of this study by well-trained physicians and will have sufficient time to consider whether to participant in this study or not. Eligible patients will sign written informed consents and will be allowed to withdraw from study or discontinue participation without any restrictions, throughout the whole study.
Study Population
According to the screening criteria, two hundred and forty patients will be enrolled in this study. Based on patient preference, participants will be divided into 2 groups: PRP+PRF group (PRP injection combined with PRF treatment) and PRF alone group (only undergoing PRF). The flow chart of patient recruitment is illustrated and summarized in Figure 1.
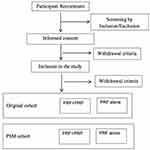 |
Figure 1 Flow chart of the patient recruitment. |
Pre-Operative Assessment
Prior to enrollment, patients’ clinical data, such as age (years), gender (male or female), Body Mass Index (BMI, kg/m2), education level, participating center, duration of disease (months), pain laterality (left/right), pain characteristics, the total daily dose of carbamazepine (milligrams per day), numeric rating scale (NRS) scores of pain (0 representing no pain and 10 representing the most severe pain imaginable), comorbidities including hypertension, diabetes mellitus, coronary heart disease and cerebrovascular disease, smoking history, patients’ quality of life (QOL, evaluated by 12-item questionnaire, SF-12 questionnaire, scores below 50 points considered poorer health status) will be collected and recorded in the Case Report Form (CRF).28
Subject Eligibility
After informed consents, researchers will fill out subject eligibility form based on the inclusion and the exclusion criteria. If patients do not meet all of the inclusion criteria or meet any of the exclusion criteria, they will not be included in the study, and will be marked as excluded.
Inclusion criteria
- Age between 18 to 75 years;
- In accordance with the diagnostic criteria of code 8B82.0 for IONa as stipulated in the 11th edition of the International Classification of Diseases (ICD-11);29
- Unresponsive to or intolerant of carbamazepine;
- Baseline NRS score ≥ 4;
- Willingness to adhere to the study protocol, including follow-up visits;
- No significant comorbidities (eg, uncontrolled diabetes, severe cardiovascular disease, or severe cerebrovascular disease) that could interfere with treatment outcomes;
- Signed informed consent.
Exclusion criteria
- Platelet count <105*109/L, ongoing anticoagulation or antiplatelet therapy, coagulation disorders or bleeding disorders;
- Severe cardiopulmonary or hepatorenal dysfunction;
- Infection at puncture site;
- Neuralgia secondary to tissue damage around the infraorbital foramen from causes such as maxillary sinusitis or tumor;
- History of neuro-destructive treatments such as radiofrequency thermocoagulation, chemical ablation, infraorbital neurectomy, infraorbital nerve avulsion, etc.;
- History of mental disorders;
- History of narcotic drug abuse;
- Unable to cooperate.
Withdrawal criteria
- Lost to follow-up during the study;
- Puncture failure or incomplete therapy;
- Receiving other treatment regimens during the study period;
- Unable to continue participation due to emerging severe comorbidities or special physiological changes during the study;
- Withdrawal from study on a voluntary basis.
Study Interventions
PRF Procedure
The procedure will be performed by experienced pain physicians at their respective participating centers, and will follow the same protocol. All the enrolled patients will be placed in the supine position with the neck slightly extended on the computed tomography (CT) scanner bed. Heart rate, electrocardiogram and pulse oximetry will be monitored continuously. Blood pressure will be measured every five minutes. The negative electrode of a Pain Management Generator PMG-230 (Baylis Medical Inc., Montreal, Canada) will be placed on the upper back of the patients. Puncture point will be identified at the surface projection point of the infraorbital foramen on the affected side, which is located at the intersection of the line connecting the external canthus and the midpoint of the upper lip and the vertical line through the ipsilateral pupil. After disinfection, local anesthesia will be performed with 1–2 mL of 1% lidocaine. Then, a 10-cm-long insulated PRF trocar needle with a 5-mm bare needle tip (PMF-21-100-5, Baylis Medical Inc) will be inserted until reaching the infraorbital foramen under the guidance of thin-slice CT (2 mm/layer, Somaton, Siemens Company, Munich, Germany). The radiofrequency electrode needle (PMK-21-100; Baylis Medical Inc) will be inserted into the trocar after removal of the stylet and confirmation of no bleeding or air when aspirated using a syringe. The sensory threshold will be determined through 50 hz, 0.1–0.2V electrical stimulation to induce a prickling pain sensation in the area of innervation of the infraorbital nerve. The depth and direction of the needle will be fine-tuned based on patient sensation to ensure accuracy. The standard PRF mode will be initially set at 42°C, and then the PRF output voltage will be gradually increased to the highest voltage that the patient can tolerate, and treatment will be continued for 360 seconds.16
PRP Procedure
The sampling kit used for PRP preparation will be II-60mL®. This sampling kit is commercialized by WEGO Ltd. Both the sampling and injection kits will be paid for by the patients. The same company will also provide the cellular centrifugation system named WG-YLJ-II®, which is double-spin LP-PRP. Under strict aseptic condition, PRP preparation will be performed at 22–26°C. Twenty mL of blood will be withdrawn from the median cubital vein (22-gauge, one-inch needle) and collected in a centrifuge test tube, gently mixed with acid citrate dextrose (ACD) as an anticoagulant in the ratio of 10:1.5. The tube will be labeled with identification data (name and age), and initially, the whole blood will undergo centrifugation at 260 g for 10 minutes and separate into 3 layers. In the second stage, the bottom layer (red blood cells) will be discarded, and the upper and middle layers will be aspirated and recentrifuged at 360 g for 15 minutes. Lastly, the remaining 2 mL of liquid-form leukocyte-poor PRP will be obtained after discarding the supernatant.21 Then, the PRF electrode needle will be removed and 2 mL PRP will be injected into the same puncture site through the trocar needle. Patients will be instructed to avoid nonsteroidal anti-inflammatory drugs (NSAIDs) 24 hours before and after injection, since both PRP and NSAIDs potentially affect the inflammatory cascade and may interact with and reduce the efficacy of PRP.30
Grouping and Additional Interventions
All interventions will comply with clinical practice guidelines. Initially, all IONa patients will continue to receive the same drug treatment as before. In addition to taking the lowest tolerated dose of carbamazepine, patients in the PRF alone group will only undergo PRF, and patients in the PRF+PRP group will also undergo PRF followed by PRP treatment. Intra-operative data such as vital signs, immediate complications or side effects related to the procedure will also be recorded in the CRF in detail. After treatment, the physician will decide whether to continue carbamazepine or not, and adjust the dosage of carbamazepine on the basis of pain severity and treatment efficacy. If the interventional treatment is effective, the total daily dose of carbamazepine will be gradually reduced by 100 mg every 7 to 14 days after pain relief.31 The tapering will be paused if pain recurs or severe withdrawal effects occur. If infraorbital foramen puncture fails, or treatment is discontinued for some reason, or if treatment is ineffective, or if patient is not satisfied with the therapeutic effect after one month, the patients can choose other neuropathic drugs, or switch to radiofrequency thermocoagulation, neurotomy or other more invasive treatments.
Follow-up
Once a patient is enrolled, the outcome assessor will make all reasonable efforts to complete the 12-months follow-up. Regular outpatient and telephone follow-ups will be performed by a designated well-trained outcome assessor at 1 day, 1 week, 1 month, 3 months, 6 months, 9 months and 12 months after the procedure to evaluate response rates, the total daily dose of carbamazepine, NRS score of pain, SF-12 QOL scores, patient satisfaction scores (PSS, 0 point indicates unsatisfactory, while 10 points indicate the most satisfactory). Adverse events (AEs, including infection, bleeding, dizziness, facial numbness, facial swelling, facial ecchymoma, eye injury) will be recorded by the operating physician in the CRFs at 30 minutes, 1 day, 1 week, 1 month, 3 months, 6 months, 9 months and 12 months after treatment. The detailed treatment options for patients who do not respond to the study protocol and switch to alternative therapies will also be recorded. All enrolled patients will be asked to record total daily dose of carbamazepine as either increased, decreased, or no change from the previous visit at the time of each follow-up. In order to maximize follow-up rate, a detailed standardized training process, including collecting detailed contact information, reminding and arranging visits/appointments, explaining treatment scheme at any time, providing gratitude will be formulated.
Data Collection and Storage
Data collection will be conducted by reviewing electronic medical records, patient visit reviews, CRFs or questionnaires. Pre-operative data, intra-operative data will be retrieved from CRFs which are filled in by the physician who performs the procedure. Follow-up data including time to take effect (the day on which that patients’ NRS reduction was >50%), analgesic effect, SF-12 scores and satisfaction assessment, operation-related AEs will be achieved via electronic medical records or phone calls by outcome assessors. Treatment compliance rate, treatment adherence rate, as well as treatment dropout rate will be recorded at the end of the study. Information about the identification of participants will only present as code numbers to maintain anonymity at all times, except in case of medical emergency or legal requirement. The final database will be stored in a research folder and will only be accessed and analyzed by an independent statistician.
Study Outcome
Primary Outcome
Primary outcome will be the 12-month response rate (cases responding to treatment/total number of cases*100%) after two different treatment protocols. The criterion of response will be a postoperative NRS reduction of >50%.
Secondary Outcome
Secondary outcome will include response rate, the total daily dose of carbamazepine, NRS scores, SF-12 QOL scores, PSS at 1 day, 1 week, 1 month, 3 months, 6 months, 9 months and 12 months after operation; operation-related AEs such as infection, bleeding, dizziness, facial numbness, facial swelling, facial ecchymoma, eye injury will be recorded at 30 minutes, 1 day, 1 week, 1 month, 3 months, 6 months, 9 months and 12 months after treatment. The time of recurrence and subsequent treatment are also collected. Recurrence is defined as an NRS score greater than 50% of that after treatment, without the addition of oral medications.
Propensity-Score Matching (PSM)
To reduce potential confounding, a 1:1 propensity score matching analysis will be performed.32 The propensity score will be estimated using binary logistic regression analysis, with treatment assignment (PRP combined with PRF vs PRF alone) as the dependent variable and the following covariates: age, gender, BMI, education level, participant center, duration of disease, pain laterality, pain characteristic, history of smoking, comorbidities (hypertension, diabetes mellitus, coronary heart disease and cerebrovascular disease), adherence and NRS scores. Nearest neighbor matching without replacement will be performed, with a caliper width set at 0.02 of the propensity score. Patients undergoing PRP combined with PRF will be individually matched to patients undergoing PRF alone based on their propensity scores. Patients without a suitable match will be excluded from analysis. Balance between groups will be assessed using standardized mean differences, with a threshold of <0.1 indicating adequate balance. Sensitivity analyses will be conducted to evaluate the robustness of the results to variations in the matching approach and potential unmeasured confounding.
Sample Size and Statistical Analysis
The main purpose of this study will be to observe the effectiveness of PRF combined with PRP, evaluated 12 months after the procedure. Our team had previously reported the 12-months effective rate of high-voltage PRF under CT guidance to treat trigeminal neuralgia.16 Thus, we speculate that the 12-months effective rate of high-voltage PRF treatment for IONa is also 73.1%. Based on our clinical experience, the effective rate of PRF with PRP was 91.5%. Power Analysis and Sample Size (PASS) V.15.0 software (NCSS Corporation, Kaysville, UT, USA) was used to compute the sample size. The results indicate that a study with a power of 80% and two-sided statistical significance level (alpha) of 0.05 would require 64 participants in each group. However, considering a 10% loss to follow-up and an additional 40% loss after propensity score matching,33 the total sample size of this study will be 237 patients. And we are planning to enroll 120 patients in each group, considering the convenience of sample collection and statistics.
All analyses will be performed with SPSS 26.0 (IBM-SPSS Inc, Armonk, NY). The response rate will be compared with the modified intent-to-treat analysis (mITT). Per protocol (PP) analysis will be conducted as a supplementary method to maintain compliance plan. Participants will be excluded in case of violation, suspension or withdrawal from designated treatment. Normal distribution will be checked by the Kolmogorov–Smirnov test. Continuous variables will be expressed as mean ± standard deviation (SD) when normally distributed and compared by Student’s t-test, or as median and interquartile range (IQR) when non-normally distributed and compared by Mann–Whitney U-test. Categorical variables will be presented as numbers with percentages and compared by Pearson’s χ2 or Fisher’s exact tests (when the expected values were <5). A repeated measures analysis of variance on ranks will be performed for the repeated data. Bonferroni correction will be used to correct multiple comparisons. Logistic regression will be used to assess pain efficacy after adjusting for several confounders, and conditional logistic regression will be used in PSM cohort. Confounding factors will be selected with a significant difference in the univariable regression model. An interim analysis will be conducted to evaluate treatment efficacy and safety after completing the initial 120 patients. A Cohen’s d value of 0.2 will be defined as a “small effect size”, and a change in the outcome score ≥ minimum clinically important difference (MCID, calculated as: d×SD) will be considered clinically meaningful. A 95% confidence level will be used to delineate confidence interval (CI). All the variables will be presented in the results section with p values and 95% CI (Upper-Lower) limits. Two-sided p-value < 0.05 will be considered statistically significant.
Allocation and blinding
Since this is an observational, controlled, open-label study, patients will be allocated based on their willingness and will also be aware of treatment allocation to ensure well-being and safety. For maintaining objectivity, the outcome assessors and data statisticians will be blinded to study arm allocation and will not be involved in enrollment and treatment processes. To avoid bias, an independent reviewer will be included in the study, who will not be involved in the direct application of intervention. This will ensure that their evaluations will base solely on objective data and not be influenced by their involvement in the procedure.
Safety Assessment
During the study period, details of any AEs or adverse device effects reported will be documented by the operating physician in the CRFs. The record will include the reason, time of initiation, duration, severity, relationship with intervention, treatment and prognosis. The trial will be closely inspected by the Institutional Review Board (IRB). Once an AE occurs, it will be treated and reported to the IRB by the research team as soon as possible. Severe AEs resulting in prolonged hospitalization or death will also be reported to the IRB and competent authorities within 24 hours and will be terminated by the IRB, if necessary. Study interventions-related AEs will be treated free of charge until recovery.
Data Monitoring Committee (DMC)
DMC will compose of experts in methodology, statistics and ethics. All members will be required to sign a conflict of interest statement. This experiment will be monitored by the DMC, which will be committed to ensuring the collection and evaluation of all data and adverse event reports. DMC will audit all data collection after including 30%, 60% and 100% of participants. In addition, DMC will obtain mid-term results after 50% of patients are enrolled. Besides IRB, AEs will also report to DMC for evaluating the correlation between events and study interventions.
Results
Time Schedule
The timeline and detailed schedule of enrollment, interventions and assessments will be presented in Table 1.
 |
Table 1 The Schedule of Enrollment and Assessment |
Pre- and intra-operative data
Baseline information and operative parameters during operation will be compared between the PRP+PRF group and the PRF alone group before and after PSM (Table 2).
 |
Table 2 Baseline Characteristics and Intraoperative Parameters Before and After PSM Between Two Groups |
Clinical Outcomes
Changes in pain intensity and quality of life between the two groups before and after PSM will be presented in Table 3.
 |
Table 3 Clinical Outcomes Between Two Groups Before and After PSM |
Adverse Events
The incidence of AEs including perioperative complications and AEs after procedures will be illustrated in Table 4.
 |
Table 4 Adverse Events and Complications Between Two Groups Before and After PSM (n, %) |
Logistic Regression
The results of logistic regression of two treatment protocols on pain efficacy adjusting for potential confounders will be shown in Table 5.
 |
Table 5 Multivariable Logistic Regression of Two Treatment Protocols on Pain Efficacy Before and After PSM |
Discussion
It is well-known that PRF alleviates neuropathic pain by generating electric field effect via high-frequency current, which facilitates nerve repair. Meanwhile, PRP exerts analgesic effects by promoting tissue repair and suppressing inflammation, courtesy of its abundant growth factors. Therefore, we aim to conduct this study to verify that combined application of PRF and PRP can achieve a more potent analgesic effect compared to PRF alone. This is a multicentric, prospective, observational study with a relatively large sample size, which could increase the statistical power of the main results. To the best of our knowledge, this study will be the first to compare the effects of combination therapy of PRP and PRF on IONa. For reduction of bias due to nonrandomized treatment selection, we will apply PSM to control for potential confounding by indication. Since the rare incidence of IONa, multiple centers are required to improve enrollment speed for performing PSM which needs to enroll more patients. In this study, PP analyses as well as mITT analyses will be performed, which indicates that the results have high reliability. Therefore, this study will provide novel directions in the efficacy and safety of PRP combined with PRF for IONa.
Limitation
Our study still has some limitations. Firstly, the study will evaluate the effectiveness and safety of combined PRP and PRF for only 1 year; a longer follow-up duration is needed to test the long-term outcome of this novel combined therapy. Secondly, the influences of PRP application vary greatly depending on the number of platelets, the ratio of inactivated vs activated platelets and the number of erythrocytes and white blood cells, etc.34 Further studies are required to determine which composition of PRP and various cell ratios are optimal for different types of IONa. Thirdly, the high cost of CT scanning and exposure to radiation will widely limit clinical accessibility of this study. Moreover, CT scanning does not provide real-time results. Ultrasound guidance seems to be a dynamic, safer and more economical method for future research. Finally, this is an observational clinical study. Although we will use PSM to enhance the study design by equalizing the observed covariates, it is crucial to recognize that PSM is only capable of balancing those covariates that are observable. Unmeasured confounding factors may still lead to biased results. Double-blind randomized controlled trials are needed to provide a higher level of evidence.
Author Contributions
LL, DY and BLD contributed equally to this work and should be considered co-first authors. All authors made a significant contribution to the work reported, whether that is in the conception, study design, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the Capital’s Funds for Health Improvement and Research (No.2020-2-2046), the Capital Medical University Research and Cultivation Fund (No. PYZ22115), and the National Key Research and Development Program of China (No. 2022YFC3602203). The sponsors had no role in the trial design, trial conduct, data handling, or writing and publication of the manuscript.
Disclosure
The authors declare that they have no competing interests for this work.
References
1. Lopez Mesonero L, Pedraza Hueso MI, Herrero Velazquez S, Guerrero Peral AL. Infraorbital neuralgia: a diagnostic possibility in patients with zygomatic arch pain. Neurologia. 2014;29:381–382. doi:10.1016/j.nrl.2013.01.003
2. Latorre G, González-García N, García-Ull J, et al. Diagnosis and treatment of trigeminal neuralgia: consensus statement from the Spanish society of neurology’s headache study group. Neurologia. 2023;26:S2173–5808(23)00027–5. doi:10.1016/j.nrleng.2023.04.005
3. Luo F, Lu J, Shen Y, et al. Effectiveness and safety of pulsed radiofrequency treatment guided by computed tomography for refractory neuralgia of infraorbital nerve: a pilot study. Pain Physician. 2015;18:E795–804.
4. Agrawal S, Kambalimath D. Trigeminal neuralgia involving supraorbital and infraorbital nerves. Natl J Maxillofac Surg. 2010;1:179–182. doi:10.4103/0975-5950.79226
5. Beigi B, Beigi M, Niyadurupola N, et al. Infraorbital nerve decompression for infraorbital neuralgia/causalgia following blowout orbital fractures: a case series. Craniomaxillofac Trauma Reconstr. 2017;10(1):22–28. doi:10.1055/s-0036-1592095
6. De Vries N, Smelt WL. Local anaesthetic block therapy of posttraumatic neuralgia of the infraorbital nerve. Rhinology. 1990;28:103–106.
7. Cok OY, Deniz S, Eker HE, Oguzkurt L, Aribogan A. Management of isolated infraorbital neuralgia by ultrasound-guided infraorbital nerve block with combination of steroid and local anesthetic. J Clin Anesth. 2017;37:146–148. doi:10.1016/j.jclinane.2016.12.007
8. Cuadrado M-L, Aledo‐Serrano Á. Symptomatic lacrimal neuralgia after ophthalmic surgery. Headache J Head Face Pain. 2015;55:323–325. doi:10.1111/head.12467
9. Sun Z, Liu L, Liu H, Luo F. Effectiveness and safety of radiofrequency thermocoagulation treatment guided by computed tomography for infraorbital neuralgia following failed conservative treatment: a retrospective study. J Pain Res. 2023;16:1005–1015. doi:10.2147/JPR.S395420 PMID: 36974307; PMCID: PMC10039627.
10. Siddiqui M, Siddiqui S, Ranasinghe J, Furgang F. Pain management: trigeminal neuralgia. Hosp Physician. 2003;39(1):64–70.
11. Ren H, Shen Y, Luo F. Treatment of supraorbital neuralgia using ultrasound-guided radiofrequency thermocoagulation of the supraorbital nerve: a retrospective study. J Pain Res. 2020;13:251–259. doi:10.2147/JPR.S228720 PMID: 32099449; PMCID: PMC6996227.
12. Lin B, Lu X, Zhai X, Cai Z. Use of sensory and motor action potentials to identify the position of trigeminal nerve divisions for radiofrequency thermocoagulation. J Neurosurg. 2014;121(6):1497–1503. doi:10.3171/2014.8.JNS132484
13. Wan Q, Zhang D, Cao X, et al. CT-guided selective percutaneous radiofrequency thermocoagulation via the foramen rotundum for isolated maxillary nerve idiopathic trigeminal neuralgia. J Neurosurg. 2018;128(1):211–214. doi:10.3171/2016.9.JNS152520
14. Ojango C, Raguso M, Fiori R, Masala S. Pulse-dose radiofrequency treatment in pain management-initial experience. Skeletal Radiol. 2018;47(5):609–618. doi:10.1007/s00256-017-2854-8
15. Lan M, Zipu J, Ying S, Hao R, Fang L. Efficacy and safety of CT-guided percutaneous pulsed radiofrequency treatment of the gasserian ganglion in patients with medically intractable idiopathic trigeminal neuralgia. J Pain Res. 2018;11:2877–2885. doi:10.2147/jpr.S179228
16. Jia Y, Cheng H, Shrestha N, et al. Effectiveness and safety of high-voltage pulsed radiofrequency to treat patients with primary trigeminal neuralgia: a multicenter, randomized, double-blind, controlled study. J Headache Pain. 2023;24(1):91. doi:10.1186/s10194-023-01629-7
17. Keene DJ, Alsousou J, Harrison P, et al. PATH-2 Trial group. Platelet-rich plasma injection for acute Achilles tendon rupture: two-year follow-up of the PATH-2 randomized, placebo-controlled, superiority trial. Bone Joint J. 2022;104-B(11):1256–1265. doi:10.1302/0301-620X.104B11.BJJ-2022-0653.R1
18. Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21(20):7794. doi:10.3390/ijms21207794
19. Zhou Z, Hu X, Yan F, et al. Observation on the effect of platelet-rich plasma combined with drugs in the treatment of herpes zoster neuralgia. Int J Neurosci. 2024;134(6):628–634. doi:10.1080/00207454.2022.213838
20. Roman SJ, Broyer Z. Use of plasma rich in growth factors for symptoms of diabetic neuropathy. Endocrinol Diabetes Metab Case Rep. 2023;2023(2):22–0396. doi:10.1530/EDM-22-0396
21. Hassanien M, Elawamy A, Kamel EZ, et al. Perineural platelet-rich plasma for diabetic neuropathic pain, could it make a difference? Pain Med. 2020;21(4):757–765. doi:10.1093/pm/pnz140
22. Doss AX. Trigeminal neuralgia treatment: a case report on short-term follow up after ultrasound guided autologous platelet rich plasma injections. WebmedCentral NEUROL. 2012;3(5):WMC003381. doi:10.9754/journal.wmc.2012.003381
23. Stamatoski A, Fidoski J. Novel perineural approach of platelet-rich plasma application in idiopathic trigeminal neuralgia treatment: a six-month follow-up pilot study[J]. Int J Oral Max Surg. 2017;46:374.
24. Giaccari LG, Coppolino F, Aurilio C, et al. Pulsed radiofrequency and platelet rich plasma in degenerative joint arthritis: two case reports and literature analyses. Life. 2023;13(6):1334. doi:10.3390/life13061334
25. Jin H, Zuo H, Xu R, Ji Y, Wang Z. A case report of ultrasound-guided knee nerve pulse radiofrequency combined with platelet-rich plasma in the treatment of knee osteoarthritis. Medicine. 2021;100(51):e27878. doi:10.1097/MD.0000000000027878
26. Jin H, Gao Y, Ji Y, et al. Case report: pulsed radiofrequency surgery combined with platelet-rich plasma injection in the treatment of supraspinatus injury. Medicine. 2021;100(51):e27797. doi:10.1097/MD.0000000000027797
27. Bibbins-Domingo K, Brubaker L, Curfman G. The 2024 revision to the declaration of Helsinki, modern ethics for medical research. JAMA. 2025;333(1):30–1doi:10.1001/jama.2024.22530
28. Jacques N, Karoutsos S, Marais L, Nathan-Denizot N. Quality of life after trigeminal nerve block in refractory trigeminal neuralgia: a retrospective cohort study and literature review. J Int Med Res. 2022;50(10):3000605221132027. doi:10.1177/03000605221132027
29. World Health Organization. International statistical classification of diseases and related health problems. World Health Organization. 2018.
30. Filardo G, Kon E, Roffi A, et al. Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2459–2474. doi:10.1007/s00167-013-2743-1
31. Bendtsen L, Zakrzewska JM, Heinskou TB, et al. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol. 2020;19(9):784–796. doi:10.1016/S1474-4422(20)30233-7
32. Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi:10.7326/0003-4819-127-8_Part_2-199710151-00064
33. Hoepelman RJ, Ochen Y, Beeres FJP, et al. Let’s agree to disagree on operative versus nonoperative (LADON) treatment for proximal humerus fractures: study protocol for an international multicenter prospective cohort study. PLoS One. 2022;17(2):e0264477. doi:10.1371/journal.pone.0264477
34. BennellKL, PatersonKL, MetcalfBR, et al.Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the restore randomized clinical trial. JAMA. 2021;326(20):2021–2030. doi:10.1001/jama.2021.19415
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

