Back to Journals » Infection and Drug Resistance » Volume 18
Effectiveness of Omadacycline in a Patient with Chlamydia psittaci and KPC-Producing Gram-Negative Bacteria Infection
Authors Yang Y, Li C, Fan X, Long W , Hu Y, Wang Y, Qu J
Received 7 November 2024
Accepted for publication 25 January 2025
Published 17 February 2025 Volume 2025:18 Pages 903—908
DOI https://doi.org/10.2147/IDR.S505311
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yong Yang,1 Cheng Li,2 Xianshuang Fan,3 Wenming Long,4 Yuwei Hu,5 Yiming Wang,6 Jian Qu6,7
1Department of Pharmacy, Affiliated Changsha Hospital of Hunan Normal University, Changsha, 410200, People’s Republic of China; 2Intensive Care Unit, Affiliated Changsha Hospital of Hunan Normal University, Changsha, 410200, People’s Republic of China; 3Department of General Medicine, the Fourth Hospital of Changsha, Changsha, 410200, People’s Republic of China; 4Department of Pharmacy, The Second People’s Hospital of Huaihua City (The Central Hospital of Huaihua City), Huaihua, 418400, People’s Republic of China; 5Department of Pharmacy, Longshan County People’s Hospital, Xiangxi Autonomous Prefecture, 416800, People’s Republic of China; 6Department of Pharmacy, The Second Xiangya Hospital, Central South University, Changsha, 410011, People’s Republic of China; 7Hunan Key Laboratory of the Research and Development of Novel Pharmaceutical Preparations, Changsha Medical University, Changsha, 410219, People’s Republic of China
Correspondence: Jian Qu, Hunan Key Laboratory of the Research and Development of Novel Pharmaceutical Preparations, Changsha Medical University, 1501 Leifeng Avenue, Xiangjiang New Area, Changsha, 410219, People’s Republic of China, Tel +86-15973190614, Fax +86-0731-85292128, Email [email protected]
Abstract: Chlamydia psittaci is one of the primary pathogens responsible for community-acquired atypical pneumonia. If not treated promptly, it can progress to severe pneumonia and may lead to multiple organ dysfunction as well as secondary infections with multi-drug-resistant (MDR) bacteria. Omadacycline, a novel aminomethylcycline antibiotic derived from tetracycline, exhibits high activity against various bacterial strains. This case report describes a patient who developed severe pneumonia caused by Chlamydia psittaci in conjunction with a MDR bacterial infection. Despite initial treatment with moxifloxacin and doxycycline, the patient experienced treatment failure. The patient’s condition deteriorated, presenting complications such as progressive infection, leukopenia, liver dysfunction, electrolyte imbalances, and respiratory alkalosis. Following the adjustment to omadacycline therapy for 48 hours, all complications were rapidly alleviated, leading to successful treatment of the patient.
Keywords: omadacycline, Chlamydia psittaci, multiple drug resistance, Kleber pneumoniae, Acinetobacter baumannii
Introduction
Chlamydia psittaci is a Gram-negative, obligate intracellular bacterium that causes zoonotic1 infections. Human infections typically occur through inhalation of aerosolized bacteria from avian sources harboring Chlamydia psittaci. This pathogen accounts for approximately 1% of community-acquired pneumonia (CAP) cases.2 The incubation period averages 5 to 14 days but can extend up to 39 days. Common clinical symptoms include fever, headache, and a dry cough.1 The severity of the disease varies among individuals, leading to hospitalization in over 50% of patients due to severe progression.3 Mortality rates vary based on the timing of intervention, with all-cause mortality in hospitalized patients estimated at 7.1% to 8.6%.1,3 Chlamydia psittaci lacks specific clinical signs, making early diagnosis challenging. Global misdiagnosis rates are estimated to be as high as 50% to 80%. However, advancements in metagenomic sequencing (mNGS) have significantly improved the sensitivity and convenience of clinical diagnosis.1,3 Tetracyclines, macrolides, and fluoroquinolones remain the primary antibiotics used for treating psittacosis. Prolonged infection can lead to increased antimicrobial resistance.4 mNGS detection in bronchoalveolar lavage fluid has shown that nearly all patients with Chlamydia psittaci infections also have concurrent infections with other microorganisms.5 Omadacycline is a novel aminomethylcycline antibiotic approved for the treatment of CAP, has demonstrated potential efficacy against MDR Gram-negative bacteria.6 Several case reports have indicated the significant effectiveness of omadacycline in treating Chlamydia psittaci infections.5,7–9 This case presents a patient with severe pneumonia due to Chlamydia psittaci complicated by KPC-producing Klebsiella pneumoniae and Acinetobacter baumannii infections. After five days of treatment with moxifloxacin, doxycycline, and meropenem, the patient experienced no significant relief from symptom of infection. Following a change to omadacycline treatment for two days, the patient’ s infectious symptoms markedly improved.
Case Description
This case report describes a 39-year-old male patient with no significant medical history who raises parrots. Recently, multiple instances of parrot mortality have been observed at his breeding facility. His wife was the first family member to exhibit fever symptoms. The patient developed a dry cough 15 days ago, accompanied by generalized fatigue, dizziness, and dyspnea, with intermittent fever peaking at 39.5°C. Despite treatment with cephalosporins at a local hospital, his condition did not improve. On December 22, 2023, the patient was admitted to our hospital with persistent symptoms of “cough, fever, and headache”.
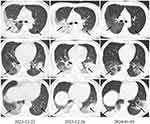
Upon admission, the patient presented with a temperature of 39.5°C but remained conscious. Based on the imaging findings (Figure 1), the patient was diagnosed with non-severe community-acquired pneumonia and received intravenous moxifloxacin at a dose of 0.4 g once daily for antimicrobial therapy. The hospital’s laboratory department conducted clinical examination indicators including inflammatory markers, liver and kidney function tests, and targeted metagenomic sequencing (tNGS) according to standard operating procedures.
Six hours post-admission, the patient exhibited drowsiness, persistent dyspnea, chills, shivering, and fatigue, with a temperature of 38.0°C and an SpO2 of 80%. The oxygenation index < 200 mmHg. Laboratory tests revealed significantly elevated levels of C-reactive protein (CRP) and interleukin-6 (IL-6), along with a reduced white blood cell count (WBC). The Mycoplasma pneumoniae IgG antibody level was 81.07 AU/mL. Cardiac enzymes levels were elevated, with creatine kinase at 292.0 U/L and lactate dehydrogenase at 394.00 U/L. Aminotransferase levels were also elevated, while renal function remained within normal limits. Detailed laboratory results are summarized in Table 1. Due to the rapid progression of the condition, the patient was transferred to the intensive care unit (ICU). Considering the possibility of psittacosis caused by Chlamydia psittaci, the antibiotic treatment regimen was adjusted to include moxifloxacin 0.4 g IV daily and doxycycline 0.1 g IV every 12 hours.
 |
Table 1 Time Change of Clinical Indicators |
By the fifth day of hospitalization, the patient showed improvement in shortness of breath and headache, although cough and fever persisted, with a temperature of 38.2°C and lethargy remained. Bronchoalveolar lavage fluid tNGS indicated Chlamydia psittaci, Klebsiella pneumoniae, Acinetobacter baumannii and blaKPC resistance gene. Chest CT compared to December 22, 2023, indicated progression of infectious lesions in the right lung and left lower lobe (Figure 1). Given the unsatisfactory treatment response and the possibility of multi-drug resistant Gram-negative bacterial infection, the treatment regimen was adjusted to include omadacycline 0.1 g IV daily (0.2 g IV on the first day) in combination with meropenem 1 g IV every 8 hours for targeted infection treatment.
On the sixth day of hospitalization, the patient remained afebrile with occasional coughing and a temperature of 36.6°C. Laboratory results indicated a WBC count of 2.06 × 109/L, while other indices showed no significant abnormalities. The patient’s symptoms of shortness of breath and fever had markedly improved. Subsequently, the patient was transferred out of the ICU to continue receiving omadacycline treatment for an additional 9 days, as detailed in Table 1. A chest CT scan performed on January 3, 2024, demonstrated significant improvement in the infection (Figure 1). The patient was discharged for home treatment on January 6, 2024. Follow-up on January 11, 2024, revealed no significant abnormalities.
Discussion
Psittacosis is characterized as an atypical pneumonia that can progress to severe pneumonia if not diagnosed and treated promptly. Compared to other Chlamydia species, Chlamydia psittaci may cause prolonged inflammatory responses.4 In this case, the patient was hospitalized 15 days after symptom onset, with examinations revealing varying degrees of damage to cardiac enzymes (CK), liver, and hematologic parameters.
The first-line treatment for Chlamydia psittaci infection includes doxycycline and tetracycline, which are typically administered for 10 to 14 days, with a maximum duration of 21 days. Effective treatment typically results in symptom resolution within 24 to 48 hours, inadequate treatment may lead to relapse or exacerbation.1 Resistance to fluoroquinolones has been documented, with minimum inhibitory concentrations (MIC) of doxycycline and fluoroquinolones being 0.05–0.2 μg/L and 0.25 μg/L, respectively, indicating a clear advantage of tetracyclines over fluoroquinolones in treating Chlamydia psittaci.10 However, there have been cases of treatment failure with doxycycline, either as monotherapy or in combination with moxifloxacin.5,7 The existence of doxycycline heteroresistance among different genotypes of Chlamydia psittaci and varying efficacy levels of doxycycline under hypoxic conditions may explain the treatment failure in this case11.
Omadacycline achieves higher concentrations in lung tissue compared to eravacycline and tigecycline.6 Previous cases of Chlamydia psittaci infections have indicated that some cases have succumbed to MDR bacterial co-infections.5,7,8 The limited antimicrobial options for treating highly virulent and transmissible strains, such as carbapenem-resistant Klebsiella pneumoniae and Acinetobacter baumannii, make omadacycline a viable alternative for these MDR infections.12,13 Omadacycline, derived from minocycline, overcomes the efflux pumps and ribosomal protection protein resistance mechanisms found in tetracycline antibiotics, including tigecycline.14 Although further evaluation is required to the steady-state concentrations for achieving therapeutic efficacy in patients with Klebsiella pneumoniae infections,15 combination therapy may be a potential strategy to enhance the efficacy of omadacycline in treating Klebsiella pneumoniae pneumonia. In this case, following the adjustment to omadacycline, the patient exhibited rapid improvement in infection symptoms, indicating that omadacycline is a viable alternative for treating psittacosis in the context of MDR bacterial infections.
Conclusion
Omadacycline exhibits broader antibacterial activity and mechanisms against resistance. This case indicates that an omadacycline-based combination regimen may serve as a novel treatment strategy for psittacosis, particularly in the context of MDR bacterial infections.
Ethical Approval
Written informed consent was provided by the patient to allow the case details and any accompanying images to be published, and this report was approved by the Ethics Committees of the Affiliated Changsha Hospital of Hunan Normal University (No. CSSDSYY-YXLL-SC-2024-03-75, date: 2024/5/9). The Affiliated Changsha Hospital of Hunan Normal University approved the publication of this report.
Acknowledgments
The authors wish to thank the patient for his support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study is supported from the Degree & Postgraduate Education Reform Project of Central South University (2023JGB123, 2024JGB061) and the Fundamental Research Funds for the Central Universities of Central South University (No. 2022XQLH154).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dembek ZF, Mothershead JL, Owens AN, et al. Psittacosis: an underappreciated and often undiagnosed disease. Pathogens. 2023;12(9):1165. doi:10.3390/pathogens12091165
2. Hogerwerf L, B DEG, Baan B, et al. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2017;145(15):3096–3105. doi:10.1017/S0950268817002060
3. Liu K, Wu L, Chen G, et al. Clinical characteristics of Chlamydia psittaci infection diagnosed by metagenomic next-generation sequencing: a retrospective multi-center study in Fujian, China. Infect Drug Resist. 2024;17:697–708. doi:10.2147/IDR.S443953
4. Goellner S, Schubert E, Liebler-Tenorio E, et al. Transcriptional response patterns of chlamydophila psittaci in different in vitro models of persistent infection. Infect Immun. 2006;74(8):4801–4808. doi:10.1128/IAI.01487-05
5. Wang DX, Xiao LX, Deng XY, et al. Omadacycline for the treatment of severe pneumonia caused by Chlamydia psittaci complicated with acute respiratory distress syndrome during the COVID-19 pandemic. Front Med. 2023;10:1207534. doi:10.3389/fmed.2023.1207534
6. Zhanel GG, Esquivel J, Zelenitsky S, et al. Omadacycline: a novel oral and intravenous aminomethylcycline antibiotic agent. Drugs. 2020;80(3):285–313. doi:10.1007/s40265-020-01257-4
7. Fang C, Xu L, Tan J, et al. Omadacycline for the treatment of severe Chlamydia psittaci pneumonia complicated with multiple organ failure: a case report. Infect Drug Resist. 2022;15:5831–5838. doi:10.2147/IDR.S384296
8. Wang J, Dong S, Fang M, et al. Omadacycline for the treatment of severe Chlamydia psittaci pneumonia complicated with guillain-barre syndrome. Infect Drug Resist. 2024;17:81–87. doi:10.2147/IDR.S437100
9. Chen Y, Tong J, Wang J. Case report of severe Chlamydia psittaci pneumonia treated with omadacycline. Int Med Case Rep J. 2024;17:659–663. doi:10.2147/IMCRJ.S473469
10. Beeckman DS, Vanrompay DC. Zoonotic chlamydophila psittaci infections from a clinical perspective. Clin Microbiol Infect. 2009;15(1):11–17. doi:10.1111/j.1469-0691.2008.02669.x
11. Borel N, Leonard C, Slade J, et al. Chlamydial antibiotic resistance and treatment failure in veterinary and human medicine. Curr Clin Microbiol Rep. 2016;3(1):10–18. doi:10.1007/s40588-016-0028-4
12. Halim J, Carr RA, Fliorent R, et al. Combinations of antibiotics effective against extensively- and pandrug-resistant Acinetobacter baumannii patient isolates. Microorganisms. 2024;12(7):1353. doi:10.3390/microorganisms12071353
13. Csiki-Fejer E, Traczewski M, Procop GW, et al. Multicenter clinical performance evaluation of omadacycline susceptibility testing of enterobacterales on VITEK 2 systems. J Clin Microbiol. 2023;61(6):e0017423. doi:10.1128/jcm.00174-23
14. LaPlante KL, Dhand A, Wright K, et al. Re-establishing the utility of tetracycline-class antibiotics for current challenges with antibiotic resistance. Ann Med. 2022;54(1):1686–1700. doi:10.1080/07853890.2022.2085881
15. Zhang A, Sun Y, Zuo M, et al. Physiologically based pharmacokinetic model for predicting omadacycline pharmacokinetics and pharmacodynamics in healthy and hepatic impairment populations. Clin Ther. 2024;46(8):629–635. doi:10.1016/j.clinthera.2024.06.014
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Omadacycline for the Treatment of Severe Chlamydia psittaci Pneumonia Complicated with Multiple Organ Failure: A Case Report
Fang C, Xu L, Tan J, Tan H, Lin J, Zhao Z
Infection and Drug Resistance 2022, 15:5831-5838
Published Date: 4 October 2022