Back to Journals » Journal of Pain Research » Volume 18
Effectiveness of Repetitive Peripheral Magnetic Stimulation (rPMS) in Relieving Post-Needling Soreness in Patient with Upper Trapezius Myofascial Pain Syndrome: A Double-Blind, Randomized Clinical Trial
Authors Vearasilp A , Sukareechai C
Received 24 January 2025
Accepted for publication 29 April 2025
Published 20 May 2025 Volume 2025:18 Pages 2541—2548
DOI https://doi.org/10.2147/JPR.S519318
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Anja Vearasilp, Chomkajee Sukareechai
Department of Rehabilitation Medicine, Panyananthaphikkhu Chonprathan Medical Center Srinakharinwirot University, Nonthaburi, Thailand
Correspondence: Chomkajee Sukareechai, Department of Rehabilitation Medicine, Panyananthaphikkhu Chonprathan Medical Center Srinakharinwirot University, Nonthaburi, 11120, Thailand, Tel +662 502 2345 ; +668 1803 8182, Email [email protected]
Objective: To study the effectiveness of relieving post-needling soreness with repetitive peripheral magnetic stimulation (rPMS) compared with sham.
Methods: This double-blind, randomized clinical trial evaluated the effects of repetitive peripheral magnetic stimulation (rPMS) on post-needling soreness following dry-needling treatment. Participants who had active myofascial pain trigger points (MTrPs), in the upper trapezius muscle and received dry needling at the upper trapezius muscle were randomly assigned to either an rPMS group, which received targeted magnetic stimulation at the site of post-needling soreness, or a sham group, which receiving a placebo intervention simulating the rPMS procedure. The rPMS parameters were set to standard mode with normal current direction and a biphasic waveform. Specific settings included an inter-pulse interval of 10, burst pulse of 2, pulse B/A ratio of 1.0, a repetitive rate of 20 pulses per second, and a total of 20 pulse trains over a 10-minute session. Results were assessed using a standardized pain scale to quantify soreness levels at various intervals post-treatment, ultimately aiming to determine if rPMS significantly enhances recovery compared to sham stimulation of magnetic stimulation sessions at the sore area or a sham group undergoing a placebo intervention mimicking the rPMS procedure. The primary outcome was the pressure pain threshold (PPT) change, measured immediately after dry needling and post-intervention. Secondary outcomes included changes in pain intensity, assessed using the Numeric Rating Scale (NRS) immediately post-dry needling, post-intervention, and at 24- and 48-hours post-intervention, as well as neck range of motion, measured at the same intervals. This methodology provided a robust framework to compare the therapeutic effects of rPMS with a placebo intervention in managing post-needling soreness.
Results: The rPMS group demonstrated a significant increase in PPT compared to the sham group (P=0.002). The Numeric Rating Scale (NRS) also significantly improved in the rPMS group compared to the sham group (P < 0.05). No serious adverse events were reported.
Conclusion: Repetitive peripheral magnetic stimulation (rPMS) is an effective method for relieving post-needling soreness compared to sham treatment. This non-invasive modality may benefit clinical practice by enhancing patient comfort and recovery after needling interventions.
Keywords: Repetitive Peripheral Magnetic Stimulation (rPMS), post-needling soreness, pain management, dry needling, myofascial pain syndrome, randomized clinical trial
Introduction
Myofascial pain syndrome (MPS) is a common cause of musculoskeletal pain, particularly affecting the neck, shoulders, and upper back, and is frequently encountered in rehabilitation clinics.1,2 It is characterized by myofascial trigger points (MTrPs) and hyperirritable spots within taut bands of skeletal muscles that cause localized and referred pain, impairing workability and quality of life.3 MPS standard management involves correcting factors such as posture and ergonomics and directly treating MTrPs through interventions like stretching, strengthening, friction massage, and various physical modalities4–6 such as ultrasound, shock wave, and magnetic stimulation. Deep dry needling (DDN), a widely used treatment for MTrPs, involves the insertion of needles into trigger points to elicit a local twitch response, helping to release muscle tension and reduce pain.7–9 However, DDN can cause complications, including bruising, pain during needle insertion, and post-needling soreness,10 which can reduce patient compliance. A study by Aitor Martín-Pintado-Zugasti reported an incidence rate of 2.19% post-needling adverse effects associated with DDN.11
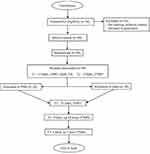 |
Figure 1 Protocol flowchart. |
 |
Figure 2 Illustrates the outcome of relieving post-needling soreness with repetitive peripheral magnetic stimulation (rPMS) compared to sham treatment, showing changes in pain scores over time. |
Repetitive magnetic stimulation was introduced in the early 1980s as transcranial magnetic stimulation (TMS) for psychiatric treatment. It later evolved into rPMS to manage pain conditions like traumatic brachial plexopathy, neuropathic pain, low back pain, spasticity, muscle strength, and dysphagia.12–16 rPMS uses electromagnetic induction to generate electrical currents in tissues without direct contact, causing neuron depolarization and muscle contractions. Its therapeutic effects include pain relief, muscle relaxation, reduced swelling, and improved circulation.15 To address this, non-invasive options like repetitive peripheral magnetic stimulation (rPMS) have been proposed for their pain-relieving effects and potential to reduce post-needling soreness. The concerns of patients regarding the side effects of DDN were reaffirmed. This study aims to compare the efficacy of rPMS with a sham control in reducing post-needling soreness following DDN in patients with MPS by a double-blind, randomized clinical trial.
Materials and Methods
Study Design Methods
A 12-month, double-blind, randomized controlled trial (RCT) was conducted at Panyananthaphikkhu Chonprathan Medical Center (PCMC) Srinakharinwirot University, Thailand. The detailed study process is illustrated in Figure 1.
This study was conducted in accordance with the ethical principle outlined in the Declaration of Helsinki. Ethical approval was obtained from the Human Research Ethics Committee of the Panyananthaphikkhu Chonprathan Medical Center Ethics Committee (approval number EC 004/66), and written informed consent was obtained from all participants prior to their inclusion in the study. The study was reviewed and approved by the TCTR Committee on 13 July 2023. The TCTR identification number is TCTR20230713013 (https://www.thaiclinicaltrials.org/show/ TCTR20230713013).
Setting and Participants
The study was conducted at the Department of Rehabilitation Medicine, PCMC Srinakharinwirot University, between 2023 and 2024, with 40 patients recruited. Eligible participants were aged 18 to 60 years, diagnosed with MPS in the upper trapezius muscle, and had at least one MTrP on one side. Participants underwent DDN and experienced post-needling soreness, with at least five local twitch responses recorded via ultrasonography. Participants were required to have a body mass index (BMI) between 19 and 25 kg/m², the ability to communicate in Thai, and provide informed consent. Participants were excluded if they had contraindications to electromagnetic wave therapy, such as metal implants, pacemakers, a history of seizures, pregnancy, or severe heart disease, or if they had undergone prior rPMS treatment. Additionally, using anti-inflammatory painkillers or muscle relaxants within 24 hours before the intervention led to exclusion. Contraindications to needling or acupuncture, including bleeding disorders, needle phobia, skin conditions, or allergies to antiseptics or wound dressings, also resulted in exclusion from the study.
Randomization and Blinding
Eligible participants were randomly assigned to either the rPMS or the sham group in a 1:1 ratio. Age and sex were used for stratified randomization with block randomization. The allocation was concealed, and both participants and assessors were blinded to the group assignments. The sample size was calculated using the formula for estimating a randomized controlled trial (RCT) with continuous data.17,18 Based on the formula for sample size calculation for randomized controlled trials with numerical outcomes, PPT was the main outcome and was used for the calculation. From the report by Ester Cerezo-Tellez,19 the mean (SD) in the intervention group and control group was 4.4 (1.3) kg/cm³ and 2.9 (1.3) kg/cm³. With a ratio of intervention and control of 1:1 and a confidence level of 95%, the required sample size in each group was 20 patients.
Study Procedures
The research assistant was key in obtaining informed consent from participants at the outpatient clinic of the Department of Rehabilitation Medicine, PCMC, Srinakharinwirot University. After the study was explained to the volunteers, they were given one week to decide whether to participate before baseline data collection began. During this period, the research assistant collected demographic data and explained how pain levels would be assessed using the Pressure Pain Threshold (PPT), Numeric Rating Scale (NRS), and neck range of motion (ROM), ensuring volunteers had the opportunity to ask any questions. Participants rated their upper trapezius pain using the NRS, while the research assistant measured PPT using a Commander™ Algometer (JTECH Medical, Salt Lake City, USA). The procedure involved pressing the 1 cm² pad of the Algometer at the muscle’s trigger point and applying increasing pressure until pain or discomfort was reported within 5 seconds. Three measurements were taken at each point, with 10-second intervals, and the mean was calculated to determine the pain pressure threshold.
Deep dry needling (DDN) achieved muscle relaxation. Following skin disinfection with alcohol, an Eccu needle (0.25 x 40 mm) was inserted into the MPTrp, inducing five muscle twitches. ultrasonography was used to record these muscle twitches, and video data was collected. Post-needling care involved applying dry cotton to stop bleeding for 10 seconds, after which plaster was placed once bleeding had ceased.
The participants were guided to receive pain relief treatment via electromagnetic stimulation. The stimulator head was covered with an opaque cloth bag. Participants were allocated as follows: the rPMS group received treatment with the rPMS parameters were set to standard mode with normal current direction and a biphasic waveform. Specific settings included an inter-pulse interval of 10, burst pulse of 2, pulse B/A ratio of 1.0, a repetitive rate of 20 pulses per second, and a total of 20 pulse trains over a 10-minute session with an amplitude sufficient to induce visible muscle contraction. The sham group received identical parameters, except the amplitude was set to 1%, ensuring no muscle contraction. Pain was reassessed using the Numerical Rating Scale (NRS) and Pressure Pain Threshold (PPT), and any adverse reactions were documented. Participants received post-DDN care instructions and were advised on symptoms that would require medical attention. Finally, the research assistant conducted follow-up assessments by phone 48 hours and one week after the intervention, inquiring about pain levels and any adverse reactions.
Intervention
rPMS Group
Participants received deep dry needling at the MTrPs in the upper trapezius muscle, followed by repetitive peripheral magnetic stimulation (rPMS) applied to the treated area.
Sham Group
Participants received deep dry needling at the MTrPs in the upper trapezius muscle, followed by a sham intervention with an inactive rPMS device.
Analysis
Statistical significance was determined by a two-sided P-value threshold of less than 0.05. Data management and preparation were carried out using Microsoft Excel 2021. Descriptive statistics and analyses for all study variables were performed using SPSS version 18.0 (SPSS Inc., Chicago, US) and Stata 17 (StataCorp, College Station, TX, USA).
Results
The study enrolled 40 patients, categorized as rPMS (20 patients; 50%) and sham (20 patients; 50%). The average age of all patients was 37.5 years, and half were male. There was no significant difference between both groups in terms of age, sex, comorbid diseases, needle injection site, post-injection parameters, and muscle twitching (Table 1). The sham group was slightly older than the rPMS group (41.6 vs 33.5; p = 0.05).
The outcomes of the study, including PPT, NRS, and neck range of motion. The primary outcome, PPT, significantly increased in the rPMS group (6.7 to 8.1; p = 0.002), while the sham group slightly increased (6.0 to 6.5; p = 0.145). The preliminary analysis confirmed that pain scores significantly decreased over time from T1 to T5 in the the rPMS group. In contrast, no significant changes in pain scores were observed in the sham group. These results are illustrated in Figures 2 and 3, which show the changes in pain scores over the time and the changes in pressure pain threshold (PPT) over time, respectively. Both groups did not have a significant change in the neck ROM between T2 and T3 (Table 2). Comparisons between the rPMS and sham groups of the three outcomes and time points were not significantly different except for the average PPT. The average PPT at T3 between the rPMS and sham groups was significantly different (8.1 vs 6.5; p = 0.031) as shown in Table 2.
 |
Table 2 The Outcome of Relieving Post-Needling Soreness With Repetitive Peripheral Magnetic Stimulation (rPMS) Compared With Sham |
Discussion
Our study demonstrates that repetitive peripheral magnetic stimulation (rPMS) significantly alleviated post-needling soreness, as evidenced by increased pressure pain threshold (PPT) and reduced pain intensity (NRS) compared to sham stimulation. The analgesic effect observed may be explained through both central and peripheral mechanisms.
Centrally, our findings align with the gate control theory proposed by Melzack and Wall,20 where 20 hz rPMS likely activated large-diameter Aβ fibers, inhibiting nociceptive input at the spinal dorsal horn. This rapid onset of pain relief may help interrupt central sensitization, particularly in the context of acute post-needling discomfort.
Peripherally, the visible muscle contractions induced by rPMS may have promoted local blood flow and reduced the accumulation of inflammatory mediators such as bradykinin and protons. These effects are consistent with Mense’s description of post-needling soreness as a combination of local tissue trauma and neural sensitization.21 Additionally, rPMS may normalize muscle spindle activity and promote reflexive muscle relaxation, contributing to pain relief.
Our findings are consistent with those of Jiravichitchai et al,22 who demonstrated rPMS efficacy in chronic low back pain. Together, these studies suggest that rPMS exerts neuromodulatory effects across a variety of pain conditions by engaging both peripheral sensory and central regulatory pathways.
Clinically, rPMS may reduce the need for pharmacologic pain management. In our study, paracetamol use was observed in only 10% of the rPMS group, compared to 20% in the sham group. Notably, no rPMS participants required stronger analgesics such as NSAIDs. These findings align with the pain-reducing potential of rPMS described in Park et al’s recent meta-analysis on acute postoperative pain.23
Although rPMS effectively reduced soreness, it did not significantly improve cervical range of motion (ROM). This suggests that ROM changes may be more attributable to dry needling-induced twitch responses than to neuromodulation alone. While rPMS may enhance muscle relaxation via motor neuron inhibition, this may be insufficient to produce measurable ROM gains in the short term.
Although the primary outcomes of this study focused on pain reduction following dry needling, we conducted brief follow-up interviews via telephone to explore possible effects on broader behavioral domains. Among the participants, four reported post-needling analgesic use: two from the rPMS group (both used paracetamol), and two from the sham group (one used etoricoxib and one used paracetamol). Interestingly, one participant in the sham group reported sleep disturbances, which prompted the use of analgesic medication. No participants in either group reported changes in mood, emotional state, or other health-related issues. While these observations were not formally measured using standardized instruments, they suggest a potential secondary benefit of rPMS in improving post-procedural comfort. Future studies should incorporate validated tools to assess sleep quality, mood, and overall quality of life to better understand the broader clinical impact of rPMS.
Additional limitations include the single-center design, modest sample size, absence of quantitative sensory testing to assess central sensitization, and lack of repeated rPMS sessions. Furthermore, age differences between groups (rPMS 33.5 vs sham 41.6 years) may have influenced pain perception despite randomization.
In clinical practice, integrating rPMS into dry needling protocols may:
1. Reduce reliance on pharmacological pain relief
2. Address both peripheral and central pain mechanisms
3. Enhance patient comfort and adherence to treatment
Future studies should:
- Optimize stimulation parameters across patient populations
- Assess cumulative effects of multiple rPMS sessions
- Include objective measures of central sensitization
- Evaluate cost-effectiveness relative to standard care
Conclusion
Repetitive peripheral magnetic stimulation (rPMS) is an effective method for relieving post-needling soreness compared to sham treatment. This non-invasive modality may benefit clinical practice by enhancing patient comfort and recovery after needling interventions.
Data Sharing Statement
The author(s) confirm that individual deidentified participant data, including demographic information, baseline characteristics, and outcome measurements, will be available from the corresponding author upon reasonable request. Study-related documents, including the study protocol and statistical analysis plan, will also be shared. Data will be accessible by contacting Dr. Chomkajee Sukareechai at [email protected]. The data will be available immediately following publication and will remain accessible for a period of 5 years.
Disclosure
The author(s) report no conflicts of interest in this work.
References
1. Simons D. Myofascial Pain and Dysfunction. The Trigger Point Manual. 1999.
2. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222.
3. Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol. 2004;14(1):95–107. doi:10.1016/j.jelekin.2003.09.018
4. Sukareechai C, Sukareechai S. Comparison of radial shockwave and dry needling therapies in the treatment of myofascial pain syndrome. Int J Ther Rehabil. 2019;26(8):1–8. doi:10.12968/ijtr.2016.0072
5. Galasso A, Urits I, An D, et al. A comprehensive review of the treatment and management of myofascial pain syndrome. Curr Pain Headache Rep. 2020;24:1–11. doi:10.1007/s11916-020-00877-5
6. Bennett R. Myofascial pain syndromes and their evaluation. Best Pract Res. 2007;21(3):427–445. doi:10.1016/j.berh.2007.02.014
7. Yehoshua I, Rimon O, Mizrahi Reuveni M, et al. Dry needling for the treatment of acute myofascial pain syndrome in general practitioners’ clinics: a cohort study. BMC Primary Care. 2022;23(1):339. doi:10.1186/s12875-022-01951-0
8. Liu L, Huang Q-M, Liu Q-G, et al. Effectiveness of dry needling for myofascial trigger points associated with neck and shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2015;96(5):944–955. doi:10.1016/j.apmr.2014.12.015
9. Gattie E, Cleland JA, Snodgrass S. The effectiveness of trigger point dry needling for musculoskeletal conditions by physical therapists: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2017;47(3):133–149. doi:10.2519/jospt.2017.7096
10. Brady S, McEvoy J, Dommerholt J, et al. Adverse events following trigger point dry needling: a prospective survey of chartered physiotherapists. J Man Manip Ther. 2014;22(3):134–140. doi:10.1179/2042618613Y.0000000044
11. Martín-Pintado-Zugasti A, Mayoral Del Moral O, Gerwin RD, et al. Post-needling soreness after myofascial trigger point dry needling: current status and future research. J Bodyw Mov Ther. 2018;22(4):941–946. doi:10.1016/j.jbmt.2018.01.003
12. Smania N, Corato E, Fiaschi A, et al. Therapeutic effects of peripheral repetitive magnetic stimulation on myofascial pain syndrome. Clin Neurophysiol. 2003;114(2):350–358. doi:10.1016/S1388-2457(02)00367-X
13. Smania N, Corato E, Fiaschi A, et al. Repetitive magnetic stimulation a novel therapeutic approach for myofascial pain syndrome. J Neurol. 2005;252:307–314. doi:10.1007/s00415-005-0642-1
14. Lo YL, Fook-Chong S, Huerto AP, et al. A randomized, placebo-controlled trial of repetitive spinal magnetic stimulation in lumbosacral spondylotic pain. Pain Med. 2011;12(7):1041–1045. doi:10.1111/j.1526-4637.2011.01143.x
15. Zarkovic D, Kazalakova K. Repetitive peripheral magnetic stimulation as pain management solution in musculoskeletal and neurological disorders—a pilot study. Int J Physiother. 2016;3:671–675. doi:10.15621/ijphy/2016/v3i6/124739
16. Kanjanapanang N, Chang K-V, Peripheral magnetic stimulation. 2018.
17. Martín-Pintado-Zugasti A, Fernández‐Carnero J, León‐Hernández JV, et al. Postneedling soreness and tenderness after different dosages of dry needling of an active myofascial trigger point in patients with neck pain: a randomized controlled trial. Pm&r. 2018;10(12):1311–1320. doi:10.1016/j.pmrj.2018.05.015
18. Machin D. Sample Size Tables for Clinical Studies. John Wiley & Sons; 2011.
19. Cerezo-Téllez E, Lacomba MT, Fuentes-Gallardo I, et al. Dry needling of the trapezius muscle in office workers with neck pain: a randomized clinical trial. J Man Manip Ther. 2016;24(4):223–232. doi:10.1179/2042618615Y.0000000004
20. Melzack R, Wall PD. Pain mechanisms: a new theory. Sur Anesthesiol. 1967;11(2):89–90. doi:10.1097/00132586-196704000-00002
21. Mense S. Muscle pain: mechanisms and clinical significance. Dtsch Arztebl Int. 2008;105(12):214. doi:10.3238/artzebl.2008.0214
22. Jiravichitchai T, Khamket C, Ruthiraphong P. Short-term efficacy of repetitive peripheral magnetic stimulation for chronic low back pain: a double-blinded randomized control Trial. J Med Assoc Thailand. 2024;107(11):894–901.
23. Park S, Park R, Westwood D, et al. Effect of peripheral magnetic stimulation on acute and chronic pain after surgery: a systematic review and meta-analysis. J Pain. 2023;24(7):1151–1162. doi:10.1016/j.jpain.2023.02.031
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



