Back to Journals » Journal of Pain Research » Volume 18
Effects of Intrathecal Sufentanil at Different Doses on Postoperative Pain Relief and Opioid Consumption in Elderly Patients Undergoing Lower Limb Orthopedic Surgery: A Randomized Controlled Trial
Authors Li Y, Gu Y , Liu W, Liu X, Wang F , Tian B , Zhou W , Ye Q
Received 17 December 2024
Accepted for publication 3 May 2025
Published 15 May 2025 Volume 2025:18 Pages 2439—2451
DOI https://doi.org/10.2147/JPR.S512653
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Karina Gritsenko
Yan Li,1,* Yinghua Gu,1,* Wenxun Liu,1 Xin Liu,1 Fa Wang,1 Biyun Tian,1 Wei Zhou,2 Qingshan Ye1
1Department of Anesthesiology, People’s Hospital of Ningxia Hui Autonomous Region, Ningxia Medical University, Yinchuan, Ningxia Hui Autonomous Region, 750001,People’s Republic of China; 2Department of Respiratory Medicine, People’s Hospital of Ningxia Hui Autonomous Region, Ningxia Medical University, Yinchuan, Ningxia Hui Autonomous Region, 750001,People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qingshan Ye Department of Anesthesiology,People’s Hospital of Ningxia Hui Autonomous Region,Ningxia Medical University, Yinchuan, Ningxia Hui Autonomous Region, 750001, People’s Republic of China, Email [email protected] Wei Zhou, Department of Respiratory Medicine, People’s Hospital of Ningxia Hui Autonomous Region,Ningxia Medical University, Yinchuan, Ningxia Hui Autonomous Region, 750001, People’s Republic of China, Email [email protected]
Introduction: Fine-needle isobaric spinal anesthesia is preferred for elderly patients undergoing lower limb fracture surgery. However, single-agent local anesthetics are limited by short block duration, hemodynamic instability, and inadequate analgesia. Intrathecal sufentanil, as an adjunct, enhances analgesia, prolongs block duration, and promotes recovery. Yet, the dose-dependent effects of intrathecal sufentanil remain understudied. This study evaluates different doses of intrathecal sufentanil in this population, aiming to optimize dosing through evidence-based strategies.
Methods: We randomly allocated 231 elderly patients into three groups: Group B (bupivacaine only), Group BS1 (bupivacaine + 5 μg sufentanil), and Group BS2 (bupivacaine + 10 μg sufentanil). We assessed baseline data, sensory and motor block characteristics, NRS scores, rescue opioid consumption, and complications.
Results: BS1 and BS2 had delayed motor block onset (median = 3 min) compared to Group B (median = 2 min), but significantly longer motor block duration (BS2: 279.5 min, P = 0.001; BS1: 268.0 min, P = 0.022 vs Group B: 223.0 min). On postoperative day 2, BS1 and BS2 showed lower NRS scores and less analgesic use than Group B. Nausea was most common in BS1, while pruritus increased in BS2. Hypoxemia was highest in BS2 on postoperative day 1.
Conclusion: Bupivacaine and sufentanil combined is a safe and effective regimen, prolonging analgesia and reducing postoperative pain and opioid use. BS2 (bupivacaine + 10 μg sufentanil) provided the best pain relief, ideal for high pain control needs, but higher pruritus and hypoxemia in BS2 suggest careful dosage adjustment based on patient tolerance.
Keywords: spinal anesthesia, sufentanil, lower limb fracture, elderly patients, analgesia
Introduction
With the increasing aging population, the incidence of fractures among the elderly continues to rise, making perioperative safety and pain management a significant challenge for anesthesiologists.1 Fine-needle isobaric spinal anesthesia, due to its simplicity, effective analgesia, and low complication rate, has become the preferred choice for lower limb surgery in elderly patients.
Bupivacaine is one of the most commonly used local anesthetics in spinal anesthesia, valued for its high efficacy, controllable onset, and moderate duration of action (1.5–2 hours). However, its dose-dependent adverse effects, such as hypotension, vomiting, respiratory depression, and shivering, limit its use as a standalone agent.2 Further studies have shown that combining bupivacaine with opioids can achieve optimal analgesic effects while reducing the side effects associated with bupivacaine alone.3 Currently, the addition of lipophilic opioids to spinal anesthetics has been proven safe and effective,4 particularly in reducing intraoperative hemodynamic fluctuations and prolonging analgesia.
Studies indicate that the combination of bupivacaine and sufentanil in elderly patients undergoing hip fracture surgery can reduce the incidence of intraoperative hypotension.5 However, existing research primarily focuses on intraoperative anesthetic efficacy and hemodynamic parameters,6–8 with limited attention to the relationship between intrathecal opioid dosage and postoperative analgesic effects. Opioids are the first-line rescue analgesics for acute pain management after lower limb fracture surgery in elderly patients.9 Nevertheless, this population often presents with multiple comorbidities, physical frailty, and cognitive impairment, making postoperative intravenous opioid rescue prone to unpredictable analgesic effects, increased adverse drug reactions, and a higher risk of long-term dependency.10,11 Inadequate postoperative analgesia not only increases the risk of delirium development or exacerbation but also contributes to respiratory, renal, and cardiac dysfunction or failure.12,13 Therefore, it is crucial to explore spinal anesthetic combinations that provide sufficient analgesia through a single injection while reducing postoperative opioid consumption.
Currently, research on the combination of local anesthetics and sufentanil in elderly patients undergoing lower limb fracture surgery, particularly studies on different sufentanil doses, remains limited. Thus, this study is designed as a prospective randomized controlled trial to compare the effects of different doses of sufentanil combined with bupivacaine on postoperative NRS scores and the use of rescue opioid analgesics. Secondary outcomes include intraoperative anesthetic efficacy and the incidence of related adverse reactions, aiming to optimize dosing through evidence-based strategies.
Materials and Methods
This study is a prospective randomized controlled trial conducted at the Anesthesiology Research Center of the Ningxia Hui Autonomous Region People’s Hospital. The study protocol has been approved by the Ethics Committee of Ningxia Hui Autonomous Region People’s Hospital(【2022】-LL-080, registration date:2022/3/30). All subjects knew and signed the informed consent form (ChiCTR2200058362, registration date:2022/04/07).The research had completed registration with China Clinical Trials Registry (Registration number:ChiCTR2200058362, registration date:2022/04/07).The study protocol followed by all experiments with human subjects were compliant with the Declaration of Helsinki. Written informed consent was obtained from every single subject, who willingly participated in this study.This study was conducted in accordance with the Consolidated Standards of Reporting Trials Checklist. This manuscript adheres to the applicable EQUATOR guideline.
Inclusion and Exclusion Criteria
Elderly patients aged 60–90 years undergoing lower limb fracture surgery under spinal anesthesia, classified as ASA physical status I–III, were included. Patients were excluded if they had contraindications to spinal anesthesia (e g, coagulation disorders, history of spinal trauma, lumbar disease unsuitable for spinal anesthesia, infection at the puncture site), history of prolonged bed rest, schizophrenia, epilepsy, Parkinson’s disease, or myasthenia gravis; severe renal impairment (requiring renal replacement therapy), severe liver impairment (Child-Pugh Class C), severe heart failure (NYHA Class III or higher), or refused to participate.
Study Protocol
A total of 231 Eligible patients were randomized to Group B, BS1, or BS2 prior to surgery using a central web-based randomization tool (http://www.randomizer.at). Randomization was stratified by center and conducted with a block size of 4. To ensure blinding, the randomization process was performed by trial personnel who were not involved in the treatment of participants.An anesthesiologist not involved in the study prepared the drugs and performed the puncture, while evaluators recorded intraoperative observations. The participants, researchers, and evaluators were all blinded to the group assignments.
- Group B: 15 mg bupivacaine(H20056442,Zhaohui Pharmaceutical Co., Ltd,China) + 1 mL cerebrospinal fluid.
- Group BS1: 15 mg bupivacaine + 5 μg sufentanil(AB40500211, Yichang Renfu Pharmaceutical Co., Ltd.,China) + 0.5 mL cerebrospinal fluid.
- Group BS2: 15 mg bupivacaine + 10 μg sufentanil.
- The bupivacaine used in this study has a specification of 5mL, 37.5mg, with each group’s dose standardized to 15mg (2mL). Cerebrospinal fluid was gently withdrawn in a fixed volume using the barbotage method according to group requirements. All study drugs were prepared in a standardized volume of 3mL to ensure consistency.
Anesthesia Method
Each participant signed informed consent before analgesia an 18-gauge catheter (18G, 381347,BD,USA) was inserted into the left forearm to establish an intravenous line, and 500 mL of Lactated Ringer’s solution (4 mL·kg⁻¹·h⁻¹) was administered, with the infusion rate adjusted as necessary to prevent post-analgesia hypotension. All patients received intraoperative monitoring with ECG, non-invasive blood pressure, and pulse oximetry, with blood pressure measured every 3 minutes. Oxygen was supplied via a face mask at a rate of 5 L/min. All patients will be anesthetized according to a standard technique. The subarachnoid block was performed as follows: After positioning the patient in the lateral decubitus position, a 25-gauge Quincke needle from the Disposable Anesthesia Puncture Kit (AS-E/SII-01, HaiSheng Pharmaceutical Co., Ltd., China) was inserted into L3–L4 or L4–L5, using a midline or paramedian approach. After confirming the free flow of cerebrospinal fluid through the needle,A total of 3 mL of the study drug was then injected in a cephalad direction over approximately 15 seconds. Spinal needle was carefully withdrawn, and the puncture site was covered with a sterile dressing. The patient was instantly placed in a supine position and provided with oxygen as previously. A forced-air warming blanket was applied to the upper body to maintain normothermia during surgery.
After intrathecal drug injection and before the start of surgery, the intraoperative analgesia was assessed and recorded by an observer who was blinded to the group allocation, every 2~3 minutes. After the surgery began, the assessments were changed to every 15 minutes until the end of the procedure. A block level of Th12 was required before the start of surgery. The sensory block to cold stimulus was assessed using thermal stimuli (ethanol drop), and the block height was recorded.
Patients who developed hypotension (systolic BP < 90 mmHg or > 30% decrease from baseline) were treated with intravenous ephedrine or phenylephrine. When the heart rate (HR) fell below 50 beats/min or decreased by more than 30% from baseline,atropine (0.5mg) was administered. Nausea and vomiting were treated by 10 mg of IV metoclopramide, and when necessary 5 mg of IV ephedrine. Naloxone 40 mg IV was administered for severe pruritus and respiratory depression (respiratory rate, 8 breaths/min). If the efficacy of intrathecal anesthesia was inadequate to meet surgical requirements, initial administration of hydromorphone, dezocine might be considered. If this failed to achieve satisfactory results, general anesthesia could be administered after thorough evaluation and communication with both the surgeon and the patient. Patients with insufficient block for general anesthesia were excluded from the statistical analysis.
Postoperative Analgesia
All patients used either a Patient-Controlled Intravenous Analgesia (PCIA) pump. Analgesia pumps are started 10–15 minutes before the surgery ends, while monitoring vital signs.PCIA: 1.5–2 μg/kg sufentanil in 100 mL at 2 mL/h.Postoperative pain was monitored, and breakthrough pain (NRS ≥ 4) was treated with dezocine or loxoprofen (compound opioid analgesic). Nonsteroidal anti-inflammatory drugs were used for patients with low body weight or poor general condition. For NRS scores of 1–3, nonsteroidal anti-inflammatory drugs were administered as needed.
Outcome Assessment
The primary outcomes were NRS scores and rescue analgesic usage rates within three days post-surgery. NRS scores ranged from 0 (no pain) to 10 (severe pain). Postoperative pain quality was Postoperative pain will be assessed every 4 hours using the 0–10 NRS scale, with the daily average representing the NRS score until the first request for supplemental analgesia. Opioid use will be recorded.Secondary outcomes include intraoperative and postoperative anesthetic effects and adverse reactions: intraoperative assessments will follow the aforementioned methods, and postoperative evaluations will be done every 4 hours.The highest sensory block level is defined as the highest level reached by the anesthetic, measured at 2-minute intervals prior to the initiation of surgery.The time to reach the highest sensory block is defined as the time from drug administration to when the patient reaches the highest sensory block level.The duration of sensory block is defined as the time from anesthetic injection to when the patient first experiences pain at the surgical site. The onset time of lower limb motor block is defined as the time from anesthetic injection to when the patient is unable to lift their thigh off the bed.The duration of motor block is the time from injection to the moment when the patient is able to lift the lower limb off the bed for more than 5 seconds.Adverse reactions included hypotension, bradycardia, tachycardia (HR > 120 bpm or > 30% increase from baseline), respiratory depression (respiratory rate < 10/min), hypoxemia (SpO2 < 90%), shivering, nausea, vomiting, itching, headache, back pain, or any other complications.
Statistical Analysis
The sample size was determined through simulation based on previous preliminary experiments in knee replacement surgery, in which the sensory blockade duration of bupivacaine combined with 5µg and 10µg sufentanil for spinal anesthesia was 323.15±28.6 minutes and 339.97±28.1 minutes, respectively. A total of 213 patients were required for a power of 90% and an alpha error of 0.05; 267 patients were selected to account for a 20% data loss. Statistical analysis was performed using SPSS version 26.0. Continuous variables were described using median (interquartile range) and mean ± standard deviation. Categorical variables were described using frequencies and percentages. Normally distributed data were compared between groups using ANOVA and post hoc Tukey’s tests. Non-parametric variables were analyzed using Mann–Whitney U-tests for two-group comparisons and Kruskal–Wallis tests for three-group comparisons. The Spearman’s rank correlation coefficient was used to analyze the correlation between variables. Chi-square or Fisher’s exact tests were used for categorical variables. P < 0.05 was considered statistically significant.
Results
A total of 267 elderly patients who underwent lower limb fracture surgery at Ningxia Hui Autonomous Region People’s Hospital from June 2023 to January 2024 were screened for this study. Among them, 20 patients were excluded for not meeting the inclusion criteria: 16 had contraindications to intrathecal anesthesia (e g, coagulation disorders or history of spinal trauma), 1 was uncooperative, 1 had a history of Parkinson’s disease, and 2 refused to participate. Consequently, 247 patients were enrolled in the randomized study. However, 16 patients were excluded due to not accepting the anesthesiologist’s intervention (Figure 1). Ultimately, 231 patients were included in the analysis.
 |
Figure 1 CONSORT flow diagram. CONSORT indicates Consolidated Standards of Reporting Trials. B group: Bupivacaine; BS1 group: Bupivacaine + 5 µg Sufentanil; BS2 group: Bupivacaine + 10 µg Sufentanil. |
Comparison of Baseline Characteristics Among the Three Groups
There were no statistically significant differences among the three groups in terms of age, gender, comorbidities, weight, ALT, AST, CREA, and BUN (P > 0.05, Table 1).
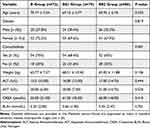 |
Table 1 Comparison of Baseline Characteristics Among the Three Groups |
Comparison of Anesthetic Effects Among the Three Groups
There were no significant differences among the three groups in the highest sensory block level, time to reach it, or duration of sensory block. The onset of lower limb motor block was earlier in Group B (median = 2 min) than in Group BS1 and BS2 (both medians = 3 min, P < 0.001). Conversely, the duration of motor block was longer in Group BS1 (median = 268.00 min, P = 0.022) and Group BS2 (median = 279.50 min, P = 0.001) compared to Group B (median = 223.00 min). (Table 2)
 |
Table 2 Comparison of Anesthetic Effects and Duration Among the Three Groups |
Comparison of Postoperative NRS Scores and Rescue Opioid Analgesic Use Among the Three Groups
Figure 2 shows the distribution of pain scores among patients on postoperative days 1 to 3. On the first postoperative day, the highest proportion of patients with pain scores of 7–9 was in Group B (44.4%), while Group BS2 had the highest proportion with scores of 4–6 (37.5%). On the second postoperative day, Group B had the largest proportion of patients with scores of 4–6 (66.7%), followed by Group BS2 (55%) and Group BS1 (49.4%). Group BS2 had the lowest proportion of patients with scores of 7–9 (5%) compared to Group B (11.1%) and Group BS1 (11.4%). On the third postoperative day, pain levels showed a decreasing trend across all groups, with Group BS2 having the highest proportion of patients with scores of 1–3 (83.8%).
Additionally, as shown in Table 3, there were no significant differences in preoperative NRS scores among the three groups. Postoperative NRS scores on postoperative day 1 and 3 were lower in Group BS2 compared to Groups B and BS1, but these differences were not statistically significant. On postoperative day 2, NRS scores were significantly lower in Group BS1 (median = 4) and Group BS2 (median = 4) compared to Group B (median = 5, P < 0.05).Analgesic usage on postoperative day 1 showed no significant differences among the groups. However, on postoperative day 2, usage rates were significantly lower in Group BS1 (82.3%) and Group BS2 (73.8%) compared to Group B (94.4%, P < 0.05). By day 3, Group BS2 had a significantly lower usage rate compared to Group B (68.8% vs 83.3%, P = 0.036).
 |
Table 3 Comparison of Postoperative NRS Scores and Rescue Opioid Analgesic Use Among the Three Groups |
Comparison of Complication Rates Among the Three Groups
Intraoperative complications varied among the groups. Nausea was most common in Group BS1 (11.39%, P = 0.003), while pruritus was significantly higher in Group BS2 (8.75%, P = 0.014). There were no significant differences in hypotension, bradycardia, respiratory depression, or vomiting.On postoperative day 1, hypoxemia was most frequent in Group BS2 (8.75%, P = 0.014), and nausea was highest in Group BS1 (27.85%, P = 0.01). No significant differences were observed in respiratory depression, shivering, vomiting, or headache (Table 4).
 |
Table 4 Comparison of Post-Anesthesia Complications Among the Three Groups |
Distribution of Surgery Types and Correlation with NRS Scores
As shown in Table 5, the surgical type distribution was similar across the three groups (Group B, Group BS1, and Group BS2), with no significant differences (P > 0.05), indicating balanced baseline characteristics without selection bias. Table 6 presents the correlation analysis using the Spearman coefficient, which showed rs=1.000 and P = 0.170. Despite the high correlation coefficient, the P-value indicates no significant association between surgical types and NRS scores. These findings suggest that surgical type does not significantly influence postoperative NRS scores and may not be a primary factor in pain outcomes for this population.
 |
Table 5 Surgery Type Distribution |
 |
Table 6 Correlation Analysis |
Discussion
This study evaluated whether different doses of intrathecal sufentanil could enhance the analgesic effect of bupivacaine, reduce postoperative NRS scores, decrease the need for rescue opioid analgesics, and minimize related side effects. Additionally, the dose-dependent effects of sufentanil on both analgesic efficacy and adverse reactions were explored. The main findings of this study are as follows: (1) The onset of lower limb motor block was significantly delayed in the BS1 and BS2 groups compared to the B group; (2) The duration of motor block was significantly prolonged in the BS1 and BS2 groups compared to the B group; (3) On postoperative day 2, the NRS scores in the BS1 and BS2 groups were significantly lower than in the B group; (4) On postoperative day 2, analgesic consumption in the BS1 and BS2 groups was significantly reduced compared to the B group; (5) On postoperative day 3, analgesic use in the BS2 group was significantly lower than in the B group; (6) The incidence of nausea was significantly higher in the BS1 group, while the incidence of pruritus was significantly higher in the BS2 group compared to the B group; (7) No significant dose-dependent effects were observed between the 5 μg and 10 μg doses of sufentanil. These results indicate that sufentanil, as an adjunct to bupivacaine, can significantly prolong motor block duration, improve postoperative analgesia, and reduce the demand for postoperative opioids. However, the use of sufentanil may also increase the incidence of nausea and pruritus, particularly at the higher dose (10 μg). Although no significant dose-dependent effects were observed between the 5 μg and 10 μg doses, the 10 μg dose demonstrated more sustained analgesic effects on postoperative day 3, suggesting its potential clinical advantage.
To explore the optimal dose range of sufentanil, we selected two doses (5 μg and 10 μg) in this study, based on a balance between efficacy and safety. According to studies by Cooper et al14 and Van Decar et al,15 the 95% effective dose of sufentanil is approximately 7.5–11.2 μg. Additionally, research by Jeffrey K. Lu et al16 indicates that doses of sufentanil exceeding 12.5 μg do not further enhance analgesic efficacy and may instead increase the risk of side effects such as respiratory depression. Based on our clinical experience, we chose two doses of sufentanil (5 μg and 10 μg) to evaluate the impact of different doses on analgesic efficacy, aiming to identify the optimal dose range that provides effective pain relief while minimizing side effects.
When evaluating the effects of sufentanil on anesthetic efficacy, we found that the onset of motor block was significantly longer in the BS1 and BS2 groups compared to the B group (B vs BS1: P < 0.001; B vs BS2: P < 0.001), and the duration of motor block was also significantly prolonged (B vs BS1: P = 0.022; B vs BS2: P = 0.001). This finding differs from the results reported by Wang Q. et al17 in a study on laparoscopic surgery for ectopic pregnancy, which may be attributed to differences in surgical type and patient characteristics (eg, age). Research by Farzi et al18 demonstrated that both sufentanil and fentanyl groups had longer motor block durations compared to the placebo group, which aligns with our results. The high lipophilicity of sufentanil may lead to slower distribution and metabolism in the subarachnoid space, thereby prolonging the duration of local anesthetic action.19,20 Additionally, sufentanil exerts its analgesic effects by binding to μ-opioid receptors in the spinal cord. Although these receptors are primarily concentrated in the dorsal horn, their indirect effects on the ventral horn (where motor neurons are located) may also contribute to the prolonged duration of motor block. Notably, the duration of motor block in the 10 μg sufentanil group (median = 324.44 ± 197.73 minutes) was longer than that in the 5 μg group (median = 293.09 ± 110.87 minutes), although this difference did not reach statistical significance. Previous studies have also shown that the maximum block level achieved with sufentanil is comparable to that of the control group, with no significant differences in the duration of sensory or motor blocks.21 However, from a clinical perspective, a difference of approximately 30 minutes may be meaningful, particularly in surgeries requiring prolonged motor block. This trend suggests that 10 μg sufentanil may provide more sustained motor block effects in clinical practice, although further research is needed to confirm its statistical significance and clinical applicability.
In terms of sensory block, the highest level of sensory block achieved in all three groups was T8, and there were no significant differences in the time to reach the highest sensory block level or in the duration of sensory block. These results are consistent with the findings of Jouybar et al.21 However, some studies suggest that intrathecal sufentanil may shorten the time to reach the highest sensory block level,19 which may be attributed to sufentanil acting as a γ-receptor agonist, altering ion channel activity or inhibiting neuronal hyperpolarization to influence neurotransmitter release.20 Our study did not observe this effect, which may be related to spinal structural abnormalities in elderly patients (eg, age-related scoliosis or kyphosis) and variations in drug distribution speed.
In addition to its effects on sensory and motor block, we further analyzed the impact of sufentanil on postoperative analgesia. The results showed that the NRS scores on postoperative days 1 and 3 in the BS2 group (bupivacaine combined with 10 μg sufentanil) were slightly lower than those in the B group and BS1 group, although the differences were not statistically significant. This may be related to the small sample size or individual variability. However, on postoperative day 2, the NRS scores in the BS1 and BS2 groups were significantly lower than those in the B group (B vs BS1: P = 0.018; B vs BS2: P < 0.001), indicating that the combination of sufentanil and bupivacaine provided superior analgesic effects compared to bupivacaine alone on postoperative day 2. These findings are consistent with existing literature. Previous studies have shown that intraoperative intrathecal sufentanil significantly improves postoperative analgesia following single-level lumbar discectomy, reduces the need for postoperative analgesics, and does not increase the incidence of urinary retention.22 Furthermore, Motiani et al23 confirmed that sufentanil, as an adjunct, significantly prolongs the duration of complete and effective analgesia and reduces postoperative VAS scores. Our results are highly consistent with these studies, further supporting the beneficial role of sufentanil as an adjunct to bupivacaine in enhancing postoperative pain management.
The use of analgesic medications was closely associated with NRS scores, highlighting the strong correlation between pain severity and analgesic demand. Our study further supports the efficacy of sufentanil in enhancing postoperative analgesia. On postoperative day 2, the rates of analgesic use in the BS1 and BS2 groups were significantly lower than in the B group (B vs BS1: P = 0.021; B vs BS2: P = 0.001), indicating that the combination of bupivacaine and sufentanil provided superior postoperative analgesic efficacy. Particularly on postoperative day 3, the BS2 group had the lowest rate of analgesic use (P = 0.036), which correlated with the lowest pain scores observed on that day. This finding further suggests that bupivacaine combined with a higher dose of sufentanil (10 μg) may provide more sustained analgesic effects and reduce the need for rescue analgesics. Previous studies have indicated that doses of sufentanil exceeding 12.5 μg do not significantly improve analgesia and may instead increase the risk of side effects such as respiratory depression.16 Therefore, within the tested dose range, 10 μg sufentanil appears to provide more effective analgesia than 5 μg. Although the statistical difference between these doses did not reach significance, the results suggest that a moderate increase in sufentanil dose (eg, 10 μg) may confer more prolonged analgesic effects. While the current data do not definitively determine the optimal dose, our findings indicate that 10 μg sufentanil offers clear advantages in improving postoperative pain control and reducing opioid consumption. Consequently, 10 μg may represent a reasonable dosing strategy for postoperative pain management, warranting further exploration and validation in future studies.
However, the side effects of combining sufentanil with bupivacaine in spinal anesthesia also warrant attention. Pruritus is a common side effect of intrathecal opioid administration, and its incidence is dose-dependent.19 The mechanism of pruritus may involve the activation of μ-opioid receptors within spinal cord segments.24 However, the exact dose threshold and mechanisms by which intrathecal sufentanil induces pruritus remain incompletely understood. In this study, intrathecal administration of 5 μg sufentanil did not significantly increase the incidence of pruritus, whereas the 10 μg dose significantly increased intraoperative pruritus (P = 0.014), consistent with previous studies.19,25 These findings further suggest that pruritus induced by sufentanil may be dose-dependent. Nevertheless, studies incorporating more dosing groups are needed to better delineate this relationship. Although pruritus is uncomfortable, its duration is typically short due to the high lipid solubility of sufentanil.
Regarding nausea, both intraoperative and postoperative nausea were significantly more common in the BS1 group compared to the B group, which exclusively used local anesthetics (intraoperative: P = 0.003; postoperative day 1: P = 0.01). This outcome aligns with clinical experience, where opioid administration is known to impact nausea rates. However, numerous studies and systematic reviews indicate that opioids such as fentanyl and sufentanil are, in fact, effective at reducing the incidence of nausea and vomiting when compared to local anesthetics alone.26,27 A meta-analysis of intrathecal sufentanil complications found that, compared to placebo, sufentanil did not increase the rates of respiratory depression, chills, bradycardia, or vomiting, which is consistent with our findings. However, in contrast to our results, this meta-analysis suggested that sufentanil did not increase the incidence of intraoperative nausea and even reduced the occurrence of vomiting during spinal anesthesia.28 Additionally, a systematic review on cesarean section surgeries reported that sufentanil may reduce the incidence of nausea (RR = 0.58, 95% CI 0.40 ~ 0.85).29 This phenomenon is thought to be due to the lipophilic nature of sufentanil, which may decrease visceral pain impulses and, consequently, reduce the need for analgesics, thus lowering the incidence of nausea and vomiting. Given the elderly patient population in our study, which included individuals with complex preoperative comorbidities and marked intraoperative physiological fluctuations (eg, changes in metabolism, gastrointestinal function, and hemodynamics), we speculate that these factors may have contributed to the increased incidence of nausea observed. Furthermore, the relatively small sample size may have introduced some bias into the results.
In addition, we observed the effects of sufentanil on respiratory function. On the first postoperative day, the incidence of hypoxemia was slightly higher in the BS2 group compared to the B group (P = 0.014), but this did not result in any significant adverse effects. These findings are consistent with those of J.K. Lu et al,16 who noted that high doses (>10 μg) of intrathecal sufentanil may elevate serum sufentanil levels, thereby increasing the risk of hypoxemia. Hypoxemia in such cases is typically managed through the administration of supplemental oxygen via nasal cannula, which can rapidly reverse the condition. While there is limited clinical data regarding sufentanil-induced hypoxemia and the evidence remains inconclusive, our results suggest that the use of intrathecal sufentanil did not cause any significant adverse effects on vital signs.
When assessing the side effects of sufentanil, we also analyzed the potential impact of surgical type on the study outcomes. The study showed that the distribution of surgical types was balanced across the three groups, and no significant correlation was observed between surgical type and NRS scores. This indicates that the differences in analgesic outcomes were primarily due to the anesthesia regimen, rather than the type of surgery. By controlling for the potential confounding effect of surgical type on pain scores, this strengthens the reliability of our findings.
However, this study has several limitations. First, although it was a randomized controlled trial, the doses and ratios of the drugs differed between the groups. To minimize bias, anesthesia administration and follow-up assessments were conducted by different personnel. However, strict adherence to the double-blind method was not feasible, which may have influenced the blinding process. Second, the relatively small sample size could limit the robustness of some statistical conclusions. Future studies should aim to increase the sample size and conduct multi-center, multi-regional trials to enhance the generalizability of the results. Additionally, this study was limited to elderly patients with lower limb fractures, so the generalizability of these findings to other populations should be further explored. Different patient groups (eg, younger patients or those undergoing other types of surgery) may have distinct physiological and pharmacokinetic characteristics. Therefore, future research should consider including a wider range of patient populations. Furthermore, the postoperative recovery needs of elderly patients have not been fully explored, and future studies could focus on the impact of the bupivacaine-sufentanil combination on early postoperative rehabilitation. Finally, when assessing the safety of intrathecal sufentanil, it is important not only to monitor clinical symptoms and conduct initial laboratory tests but also to incorporate molecular biological analyses to provide more comprehensive data.
Conclusion
Bupivacaine combined with sufentanil is a safe and effective anesthetic regimen that prolongs motor block duration, improves postoperative analgesia, and reduces the need for additional analgesics. The BS2 group (bupivacaine + 10 μg sufentanil) demonstrated the best outcomes in terms of postoperative pain relief and reduced analgesic requirements, making it particularly suitable for patients with high demands for pain control. However, the higher incidence of pruritus and hypoxemia in the BS2 group suggests that the dose of sufentanil should be carefully adjusted based on individual patient tolerance. Overall, this study provides valuable insights for optimizing anesthetic regimens, improving postoperative analgesia, and reducing side effects in elderly patients.
Data Sharing Statement
The data supporting the findings of this study are not publicly available because of institutional policy but are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of Ningxia Hui Autonomous Region People’s Hospital(【2022】-LL-080, registration date:2022/3/30). All subjects knew and signed the informed consent form (ChiCTR2200058362, registration date:2022/04/07).The research had completed registration with China Clinical Trials Registry (Registration number:ChiCTR2200058362, registration date:2022/04/07).The study protocol followed by all experiments with human subjects were compliant with the Declaration of Helsinki. Written informed consent was obtained from every single subject, who willingly participated in this study.This study was conducted in accordance with the Consolidated Standards of Reporting Trials Checklist. This manuscript adheres to the applicable EQUATOR guideline.
Acknowledgments
The authors would like to thank all the reviewers who participated in the review for this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the grants from The central government guides local science and technology development fund projects (Grant Number: 2022FRD05014).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Mun F, Ringenbach K, Baer B, et al. Factors influencing geriatric orthopaedic trauma mortality. Injury. 2022;53(3):919–924. doi:10.1016/j.injury.2022.01.005
2. Critchley LA. Hypotension, subarachnoid block and the elderly patient. Anaesthesia. 1996;51(12):1139–1143. doi:10.1111/j.1365-2044.1996.tb15051.x
3. Weiniger CF, Heesen M, Knigin D, Deutsch F, Hilber N, Avidan A. Association between hyperbaric bupivacaine dose and maternal hypotension: retrospective database study of 8226 women undergoing cesarean delivery under spinal anesthesia. Anesth Analg. 2021;133(4):967–975. doi:10.1213/ANE.0000000000005518
4. Bogra J, Arora N, Srivastava P. Synergistic effect of intrathecal fentanyl and bupivacaine in spinal anesthesia for cesarean section. BMC Anesthesiol. 2005;5(1):5. doi:10.1186/1471-2253-5-5
5. Olofsson C, Nygårds EB, Bjersten AB, Hessling A. Low-dose bupivacaine with sufentanil prevents hypotension after spinal anesthesia for hip repair in elderly patients. Acta Anaesthesiol Scand. 2004;48(10):1240–1244. doi:10.1111/j.1399-6576.2004.00504.x
6. Neves JF, Monteiro GA, Almeida JR, et al. Association of fentanyl or sufentanil and 0.5% isobaric bupivacaine in spinal anesthesia: a comparative study. Revista brasileira de anestesiologia. 2002;52(5):535–541. doi:10.1590/s0034-70942002000500003
7. Sanatkar M, Sadeghi M, Esmaeili N, et al. The hemodynamic effects of spinal block with low dose of bupivacaine and sufentanil in patients with low myocardial ejection fraction. Acta Med Iran. 2013;51(7):438–443.
8. Gupta S, Sampley S, Kathuria S, et al. Intrathecal sufentanil or fentanyl as adjuvants to low dose bupivacaine in endoscopic urological procedures. J Anaesthesiol Clin Pharmacol. 2013;29(4):509–515. doi:10.4103/0970-9185.119158
9. Griffioen MA, O’Brien G. Analgesics administered for pain during hospitalization following lower extremity fracture: a review of the literature. J Trauma Nurs. 2018;25(6):360–365. doi:10.1097/JTN.0000000000000402
10. Baboli KM, Liu H, Poggio JL. Opioid-free postoperative analgesia: is it feasible? Curr Probl Surg. 2020;57(7):100795. doi:10.1016/j.cpsurg.2020.100795
11. Lim BG, Lee IO. Anesthetic management of geriatric patients. Korean J Anesthesiol. 2020;73(1):8–29. doi:10.4097/kja.19391
12. Duggleby W, Lander J. Cognitive status and postoperative pain: older adults. J Pain Symptom Manage. 1994;9(1):19–27. doi:10.1016/0885-3924(94)90142-2
13. Cousins M. Acute and postoperative pain. In: Wall PD, Melzack R, editors. Textbook of Pain. Edinburgh: Churchill Livingstone; 1994:284–305.
14. Arkoosh VA, Torjman MC, Montgomery OC, Leighton BL, Norris MC. Does intrathecal sufentanil depress the ventilatory response to carbon dioxide in the parturient? Anesthesiology. 1994;81(3A):A1148. doi:10.1097/00000542-199409001-01147
15. Decar TV, Callicot R, Jones R, Herman N. Determination of a dose-response curve for intrathecal sufentanil in labor. Anesthesiology. 1994;81(3A):A1149. doi:10.1097/00000542-199409001-01148
16. Lu JK, Schafer PG, Gardner TL, et al. The dose-response pharmacology of intrathecal sufentanil in female volunteers. Anesth Analg. 1997;85(2):372–379. doi:10.1097/00000539-199708000-00023
17. Wang Q, She SZ, Zhang YF, Lao JX, Jin YL. Effect of intrathecal administration of sufentanil at different doses on bupivacaine spinal anesthesia in gynecologic laparoscopy. J Southern Med Univ. 2008;28(8):1474–1476. Chinese.
18. Farzi F, Mirmansouri A, Naderi Nabi B, et al. Comparing the effect of adding fentanyl, sufentanil, and placebo with intrathecal bupivacaine on duration of analgesia and complications of spinal anesthesia in patients undergoing cesarean section. Anesthesiol Pain Med. 2017;7(5):e12738.
19. Braga Ade F, Braga FS, Potério GM, Pereira RI, Reis E, Cremonesi E. Sufentanil added to hyperbaric bupivacaine for subarachnoid block in caesarean section. Eur J Anaesthesiol. 2003;20(8):631–635. doi:10.1097/00003643-200308000-00007
20. Campbell DC, Camann WR, Datta S. The addition of bupivacaine to intrathecal sufentanil for labor analgesia. Anesth Analg. 1995;81(2):305–309. doi:10.1097/00000539-199508000-00017
21. Jouybar R, Saravi ZF, Dehghani N, et al. Comparative efficacy of 3 adjuvant medications used in combination with intrathecal bupivacaine for caesarian section anesthesia: a randomized, double-blind clinical trial. Curr Ther Res Clin Exp. 2022;97:100688. doi:10.1016/j.curtheres.2022.100688
22. Abrishamkar S, Karimi M, Safavi M, Honarmand A, Safavi A. Effects of intraoperative-intrathecal sufentanil injection on postoperative pain management after single level lumbar discectomy. Middle East J Anaesthesiol. 2010;20(6):839–844.
23. Motiani P, Chaudhary S, Bahl N, Sethi AK. Intrathecal sufentanil versus fentanyl for lower limb surgeries - a randomized controlled trial. J Anaesthesiol Clin Pharmacol. 2010;26(4):507–513. doi:10.4103/0970-9185.74597
24. Schmelz M. Opioidinduzierter pruritus. Mechanismen und therapeutische ansätze [Opioid-induced pruritus. Mechanisms and treatment regimens]. Anaesthesist. 2009;58(1):61–65. German. doi:10.1007/s00101-008-1478-8
25. Roberts GW, Bekker TB, Carlsen HH, Moffatt CH, Slattery PJ, McClure AF. Postoperative nausea and vomiting are strongly influenced by postoperative opioid use in a dose-related manner. Anesth Analg. 2005;101(5):1343–1348. doi:10.1213/01.ANE.0000180204.64588.EC
26. Hamber EA, Viscomi CM. Intrathecal lipophilic opioids as adjuncts to surgical spinal anesthesia. Reg Anesth Pain Med. 1999;24(3):255–263. doi:10.1097/00115550-199924030-00015
27. Uppal V, Retter S, Casey M, Sancheti S, Matheson K, McKeen DM. Efficacy of intrathecal fentanyl for cesarean delivery: a systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. Anesth Analg. 2020;130(1):111–125. doi:10.1213/ANE.0000000000003975
28. Kjellberg F, Tramèr MR. Pharmacological control of opioid-induced pruritus: a quantitative systematic review of randomized trials. Eur J Anaesthesiol. 2001;18(6):346–357. doi:10.1097/00003643-200106000-00002
29. Hu J, Zhang C, Yan J, Wang R, Wang Y, Xu M. Sufentanil and bupivacaine combination versus bupivacaine alone for spinal anesthesia during cesarean delivery: a meta-analysis of randomized trials. PLoS One. 2016;11(3):e0152605. doi:10.1371/journal.pone.0152605
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


