Back to Journals » Drug Design, Development and Therapy » Volume 19
Effects of Preoperative Magnesium Sulphate Infusion on Emergence Agitation and Postoperative Quality of Recovery in Patients Undergoing Thoracoscopic Lobectomy
Authors Pu Y, Geng X , Wang M , Lv G, Hu Z, Fang C, Zhang X, Li W, Fan X, Chen X
Received 16 November 2024
Accepted for publication 4 May 2025
Published 29 May 2025 Volume 2025:19 Pages 4517—4525
DOI https://doi.org/10.2147/DDDT.S503714
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Yutian Pu,1– 3,* Xingyu Geng,1– 3,* Maosan Wang,1– 3 Gaochao Lv,1– 3 Ziwei Hu,1– 3 Can Fang,1– 3 Xinyue Zhang,1– 3 Wanting Li,1– 3 Xiaoxuan Fan,1– 3 Xiuxia Chen1– 3
1Department of Anesthesiology, the Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 2NMPA Key Laboratory for Research and Evaluation of Narcotic and Psychotropic Drugs, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 3Jiangsu Province Key Laboratory of Anesthesiology, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiuxia Chen, Department of Anesthesiology, the Affiliated Hospital of Xuzhou Medical University, No. 99 huaihai West Road, Xuzhou, Jiangsu, 221002, People’s Republic of China, Tel +86 18052268332, Email [email protected]
Background: Emergence agitation(EA) is common in the early phase of recovery from general anesthesia in adults, which can potentially cause unpredictable harm to both patients and medical staff. This study aimed to examine the effects of preoperative magnesium sulphate infusion on emergence agitation and postoperative quality of recovery in patients undergoing thoracoscopic lobectomy.
Patients and Methods: 84 patients undergoing thoracoscopic lobectomy were randomly assigned to either the magnesium sulphate group (group M) or the control group (group C). Group M received a 50 mg/ kg intravenous bolus of magnesium sulphate 20 minutes before induction, whereas group C was administered an equivalent volume of saline. The Riker Sedation-Agitation Scale (SAS) and the 40-item Quality of Recovery questionnaire (QoR-40) were used to evaluate emergence agitation and postoperative quality of recovery, respectively.
Results: In comparison to group C, group M demonstrated a significantly lower incidence of EA (9.5% vs 42.9%; OR, 0.14; 95% CI, 0.04– 0.47; P < 0.001) and dangerous agitation (0% vs 14.3%; OR, 2.17; 95% CI, 1.71– 2.75; P =0.011), along with a reduction in the maximal SAS score (P < 0.05). Group M exhibited higher global QoR-40 scores than group C on postoperative day 1 (POD 1)(168.3± 13.8 vs 155.6± 16.5, P< 0.001). Additionally, group M displayed lower Numerical rating scale (NRS) pain scores both at rest and during coughing in PACU and on POD 1 (P < 0.001). There were no significant statistically differences between the two groups in terms of time to extubation, incidence of delayed recovery and residual sedation (P > 0.05).
Conclusion: Preoperative magnesium sulphate infusion effectively decreased the incidence and severity of EA in patients undergoing thoracoscopic lobectomy. Furthermore, it alleviated postoperative pain and improved postoperative quality of recovery, without an increase in adverse events.
Keywords: magnesium sulphate, preoperative infusion, emergence agitation, recovery quality, thoracoscopic lobectomy
Introduction
Emergence agitation (EA) is a common complication of general anesthesia. It is manifested as acute cognitive dysfunction characterised by mental and physical over-excitement, often accompanied by a sudden increase in blood pressure and heart rate.1 It can result in various undesirable consequences, such as bleeding or cracking of the surgical site, respiratory and circulatory complications, and injuries to the patient and medical staff.2,3 These outcomes not only hinder the patient’s recovery but also exacerbate the strain on medical resources. The incidence of EA in adults fluctuates between 0.25% and 21.3% because of demographic features, type of surgery, or other factors.1–4
In recent years, lung cancer has become the leading cause of incidence and mortality among malignant tumours in China.5 Thoracoscopic lobectomy is currently the standard procedure for lung cancer treatment. The incidence of EA after lung surgeries is estimated to range from 35% to 41%, considerably higher than the overall level.6,7 Contributing factors include irritation caused by the double-lumen endotracheal tube and thoracic drainage tube, acute postoperative pain, anxiety and other negative emotions.8,9 Thus, in the management of perioperative thoracic anesthesia, it is crucial to prevent emergence agitation, optimise postoperative analgesic regimens and enhance postoperative recovery.10
Magnesium sulphate is a non-selective N-methyl-D-aspartate receptor antagonist that exerts analgesic, sedative, anti-inflammatory, and neuroprotective effects.11,12 Its perioperative application has been shown to alleviate postoperative pain, support hemodynamic stability and improve recovery outcomes.13 In addition, a meta-analysis demonstrated its efficacy in decreasing the incidence and severity of emergence agitation in children. Nevertheless, the application of magnesium sulphate in EA is highly constrained in adults. As a result, the impact of magnesium sulphate on EA after thoracoscopic lobectomy remains uncertain. This study investigated the effects of magnesium sulphate on emergence agitation and postoperative quality of recovery in thoracoscopic lobectomy patients, offering clinical drug use insights.
Material and Methods
Study Design
This research employed a prospective, double-blind, randomized controlled trial design. It received approval from the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (Ethics Approval Number: XYFY2024-KL103-01) and was registered in the Chinese Clinical Trial Registry (Registration Number: ChiCTR2400086519). It was conducted at Affiliated Hospital of Xuzhou Medical University between July 2024 and October 2024. This trial adhered to the principles outlined in the Consolidated Standards of Reporting Trials (CONSORT) guidelines and the Declaration of Helsinki. Eligible patients provided informed consent prior to participation.
Participants
Eighty-four patients slated for thoracoscopic lobectomy under general anesthesia were assessed for eligibility. Participants aged 18–65 years with a Body Mass Index (BMI) of 18–30 kg/m² and classified as American Society of Anesthesiologists (ASA) physical status I–III were included in this trial. Patients meeting any of the following criteria were excluded from participation in this study: history of psychiatric disorders or cognitive dysfunction, severe hepatic or renal diseases, bradycardia or atrioventricular block, preoperative electrolyte disorder, neuromuscular disorders, history of drug abuse, allergy to the drugs involved in this study.
Randomization and Blinding
All patients were randomly assigned to the magnesium sulphate group (group M) or the control group (group C) based on computer-generated random numbers. We used magnesium sulphate solution (MgSO4 500 mg/mL) containing 500 mg of magnesium in 1 mL of magnesium solution. A total dose of magnesium sulphate of 50 mg/kg was diluted to 20 mL. A 50 mg/ kg bolus of magnesium sulphate was infused intravenously 20 minutes before induction in group M, while an equivalent volume of saline was administered in group C, with an infusion time of over 15 minutes. Intraoperative blood magnesium concentration was determined 10 minutes following the completion of infusion by drawing venous blood from the arm contralateral to the infused one. The investigator, surgeons, anesthetists, nurses, statisticians and patients were all blinded to group allocation.
Procedures
Upon entering the operating room, patients received standard monitors, including electrocardiogram (ECG), heart rate (HR), pulse oxygen saturation (SpO2), mean arterial pressure (MAP), and bispectral index (BIS). General anesthesia was induced by sequential intravenous infusion of midazolam 0.05 mg/kg, etomidate 0.3 mg/kg, sufentanil 0.5μg/kg, and rocuronium 0.9 mg/kg. Combined intravenous-inhalational anesthesia was used for maintenance, with sevoflurane 1%–3% by inhalation, remifentanil 0.1–0.2 μg/kg/min and propofol 2–4 mg/kg/h by intravenous pumping. Intravenous remifentanil and propofol were adjusted to maintain BIS between 40 and 60. Respiratory rate was modified to regulate end-tidal carbon dioxide levels within 35 to 45 mmHg. The intercostal nerve was blocked by surgeons under direct thoracoscopic view with 0.75% ropivacaine 10 mL and 2% lidocaine 5 mL. To prevent hyperalgesia and postoperative nausea and vomiting (PONV), flurbiprofen axetil 50mg and tropisetron 2mg were injected intravenously while suturing the skin incision.
Sevoflurane was discontinued upon the resumption of two-lung ventilation. Remifentanil and propofol administration ceased upon surgery completion. Patients were transferred to Post anesthesia Care Unit (PACU). All patients received a standardized Patient Controlled Intravenous Analgesia regimen, consisting of 2 μg/mL sufentanil diluted in normal saline to a total volume of 100mL, for a duration of two day. If Numerical rating scale (NRS) pain score persisted at a level ≥4 after one press of analgesic pump, rescue flurbiprofen axetil was administered at a dose of 50 mg. Information on the drugs used in this trial is provided in the Supplementary.
Data Collection and Outcome Assessment
The primary outcome of this trial was the incidence of EA. Emergence was defined as the interval from procedure completion to 5 min after extubation. The Riker Sedation-Agitation Scale (SAS) was employed to evaluate EA (1=no arousal or response to physical stimuli; 2=arousal to physical stimuli but unable to communicate or follow instructions; 3=sedated but arousal to slight body shaking or verbal stimuli.; 4=calm and easily aroused, able to follow instructions; 5=agitated but able to regain composure after verbal dissuasion; 6=very agitated, requiring frequent verbal commands to remain calm; 7=dangerous agitation, struggling violently, attempting to remove catheters or attacking medical staff).14 All patients were assessed, and the maximum SAS score achieved by each patient was recorded. Emergence agitation was defined as the maximum SAS score observed during emergence ≥5. Intravenous propofol 1 mg/kg was administered for treatment in case of dangerous agitation.
PACU data and recovery profiles were collected as secondary outcomes. PACU data encompassed the incidence of agitation at 10 (T1), 15 (T2), 30 (T3) minutes after extubation, NRS pain score in PACU, time to extubation, the incidence of delayed recovery, residual sedation, and rescue flurbiprofen axetil administration. Recovery profiles included the 40-item Quality of Recovery questionnaire (QoR-40) score and NRS pain score on postoperative day 1 (POD 1) as well as the incidence of remedial analgesia and adverse events. Time to chest tube removal and length of hospital stay were also collected.
QoR-40 was used to assess postoperative quality of recovery. QoR-40 is made up of five dimensions: physical comfort, psychological support, emotional state, pain and physical independence. A higher score denotes a better quality of postoperative recovery. QoR-40 scores were recorded on preoperative day 1 and POD 1. Time to extubation referred to the duration between procedure completion to endotracheal tube removal. Residual sedation was defined as SAS score ≤3 after extubation.15 Remedial analgesia was defined as NRS pain score ≥4 and additional analgesics in the ward. Respiratory depression was defined as SpO2≤90% in the condition of breathing air.
Statistical Analysis
Statistical analysis was performed using SPSS (Version 25.0, IBM SPSS, Chicago, IL, USA).
Shapiro–Wilk test assessed normality, while Levene test evaluated variance homogeneity. Normally distributed measures were expressed as mean ± standard deviation, with independent samples t-tests employed for comparisons between groups. Non-normally distributed measures were expressed as median and interquartile range (IQR), with Mann–Whitney U-test employed for comparing groups. Categorical variables were expressed as numbers (%), with chi-square or Fisher’s exact test employed for between-group comparisons. P < 0.05 was considered as statistically significant.
The calculation of sample size was performed on the basis of the primary outcome, which was the incidence of EA. According to preliminary trial results, the incidence of EA was 10% in group M and 40% in group C. With PASS version 15.0 used for calculation, 66 subjects were required in our study (α=0.05, 1-β=0.8). In consideration of 20% dropout rate, each group needed 42 subjects.
Results
A total of 84 patients were enrolled in this trial. They were randomly allocated to group M (n=42) or group C (n=42). There were no exclusions, so all patients were included in the final analysis (Figure 1). The two groups had no statistical differences in baseline characteristics (Table 1).
 |
Table 1 Patient Characteristics |
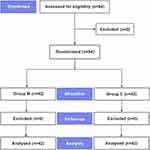 |
Figure 1 Flowchart for the study. |
Preoperative magnesium sulphate infusion notably reduced the occurrence of emergence agitation and agitation at T1 (P<0.001; P<0.001, respectively, Table 2). Group M experienced a significantly lower incidence of dangerous agitation compared to group C (P=0.011). Nevertheless, the incidence of agitation at T2 and T3 was similar between the two groups (P=0.078; P>0.999, respectively, Table 2). Moreover, the maximal SAS score was lower in group M than in group C (P=0.021, Table 3).
 |
Table 2 Incidence of Agitation |
 |
Table 3 Perioperative Data |
In comparison to Group C, Group M exhibited a reduction in the consumption of remifentanil (P=0.019, Table 3). There were no statistical differences in time to extubation as well as the incidence of delayed recovery and residual sedation between the two groups (P=0.233; P=1.000; P=1.000, respectively). Group M had a lower incidence of rescue flurbiprofen axetil administration and remedial analgesia (P=0.014; P=0.027, respectively). Group M exhibited lower NRS pain scores both at rest and when coughing in PACU and on POD 1. Group M reported a lower incidence of PONV (P=0.009). No statistical differences were observed regarding the incidence of shivering, hypotension, bradycardia, and respiratory depression (P=0.480; P=1.000; P=1.000; P=0.242, respectively). Time to chest tube removal and length of hospital stay were not different between the two groups (P=0.729; P=0.547, respectively). Blood magnesium concentration on preoperative day 1 was similar in both groups (P=0.315). During surgery and on POD 1, blood magnesium concentration was higher in group M than in group C (P<0.001; P<0.001, respectively, Table 3), yet remained within the safe range.
There were no statistical differences between the two groups in QoR-40 global and dimensional scores on preoperative day 1 (P=0.508, Table 4). Compared with group C, group M displayed higher global scores on POD 1 (P<0.001). Compared with group C, group M scored higher in the dimension of physical comfort, emotional state, and pain (P<0.001; P<0.001; P=0.004, respectively, Table 4).
 |
Table 4 Qor-40 Score |
Discussion
This study indicated that magnesium sulphate infusion was an effective intervention for reducing the incidence of both emergence agitation and dangerous agitation. Moreover, this approach was demonstrated to increase global QoR-40 scores on POD 1 and improve early postoperative quality of recovery.
Previous studies have substantiated the effectiveness of dexmedetomidine in the prevention of EA in adults. In a meta-analysis, dexmedetomidine, as an adjunct anesthetic to sevoflurane, was found to be the most effective in preventing EA, compared with propofol, midazolam, clonidine, ketamine, sufentanil, and fentanyl.16 However, the use of dexmedetomidine is frequently accompanied by adverse effects such as delayed recovery, hypotension and bradycardia. This restricts its extensive application, particularly in elderly individuals and critically ill patients.17,18
In recent years, studies have begun to explore alternative prophylactic options to dexmedetomidine. Salman et al found that in obese adults undergoing nasal surgery, magnesium sulphate and dexmedetomidine exhibited comparable efficacy in the prevention of EA. There was no statistically significant difference between intraoperative magnesium sulphate infusion and dexmedetomidine infusion in the prevention of emergence agitation.Furthermore, the former demonstrated a superior safety profile: it did not prolong reaction time and extubation time, did not increase the incidence of perioperative hypotension, and no significant heart rate depression was observed.19 These findings were consistent with our results, in which magnesium sulphate rarely caused delayed recovery and residual sedation, further validating the potential benefits of magnesium sulphate in maintaining the quality of anaesthetic recovery.
Currently, most studies have concentrated on the influence of magnesium sulphate on EA in children. Conversely, its potential for preventing EA in adults has yet to be thoroughly investigated. Additionally, the majority of trials employed a combination of loading and maintenance doses, with only a minority using a single injection. Preemptive analgesia contributes to reducing the level of postoperative pain, providing a more comfortable and stable recovery condition, and enhancing postoperative recovery.20,21 Aditya et al found that in patients undergoing hysterectomy, infusion of magnesium sulphate 50 mg/kg prior to induction was associated with a reduction in postoperative pain and a lower requirement for opioid, compared to 30 mg/kg.22 Hatice et al administered magnesium sulphate 50 mg/kg before anesthesia to patients undergoing mastectomy. As a result, they found no statistical differences in MAP, HR, and SpO2 after induction, during surgery, and after extubation, compared with blank control group. Preemptive magnesium sulphate did not result in any discernible impact on respiratory and hemodynamic parameters, with no increase in the incidence of hypotension.23 Accordingly, we chose to administer magnesium sulphate 50 mg/kg intravenously before induction.
This study found that magnesium sulphate was effective in reducing the incidence of EA, with a notable decline from 42.9% to 9.5%. It was in accordance with those reported by Elsersy et al and Su et al24,25 A key distinction was that this study employed preemptive analgesia, substantiating the efficacy of a single injection of magnesium sulphate before induction in the prevention of EA. This may offer a promising avenue for the use of magnesium sulphate. Also, magnesium sulphate diminished the maximum SAS score and the prevalence of dangerous agitation. It resulted in a significant decrease in the incidence of dangerous agitation, from 14.3% to 0%. Dangerous agitation is correlated with an elevated risk of complications and a poorer prognosis. Therefore, efforts to reduce dangerous agitation can be regarded as a means of enhancing postoperative recovery.1
In this study, compared with group C, the incidence of agitation at T1 was found to be reduced in Group M, while no such reduction was observed at T2 and T3. It may be attributed to the fact that most agitation tended to occur just before extubation. Following this acute phase, agitation appeared to subside gradually.26 The incidence of agitation at T2 and T3 was so low that the effect of magnesium sulphate was not significant. Our result was different from that of Elsersy et al who observed a reduction across all measured time points.24 It can be attribute to variations in surgical type, patient characteristics, and assessment methods.
The mechanism by which magnesium sulphate reduces the incidence of EA is unclear. It may be related to its cerebral protective effects. Magnesium sulphate exerts multiple regulatory effects on the central nervous system, competitively inhibiting NMDA receptors and calcium channels, and reducing the toxicity of neural excitation.27 Animal studies show that intravenous injection of magnesium sulphate can inhibit the increase in brain lactate concentration, improve the function of cerebral blood flow autoregulation, and reduce the neurotoxicity of brain injury.28
Imaging studies further revealed that after magnesium sulphate intervention, the power of delta wave in electroencephalogram increased and electroencephalogram changes caused by cerebral ischaemia was ameliorated.29 This suggests that magnesium sulphate may exert a beneficial effect on cerebral circulation and metabolism as well as the rhythm of electrical activity in the brain to reduce the incidence of EA.
Only a few trials have explored the effect of magnesium sulphate infusion on postoperative quality of recovery. Magnesium sulphate infusion in patients undergoing septoplasty reduced postoperative pain intensity and improved early postoperative recovery.30 This was in line with our results, which demonstrated a significant improvement in postoperative recovery quality, as evidenced by an increase in global QoR-40 scores from 155.6 to 168.3. The study of Myles et al determined that a 6.3-point change in global QoR-40 score represents a minimal clinically important difference, highlighting the significant impact of magnesium sulphate on postoperative recovery.31
This study showed that scores of physical comfort, emotional state, and pain were improved in group M, aligning with the findings of Xu et al.11 Group M reported lower NRS pain scores both at rest and when coughing in PACU and on POD 1. Therefore, it was not surprising that Group M was superior to Group C in pain dimension. These results implied that multimodal analgesia assisted with magnesium sulphate had the potential to mitigate postoperative pain and facilitate postoperative recovery.
It has been established that magnesium sulphate possesses the capacity to diminish opioid consumption and the prevalence of PONV.32 Group M exhibited a reduced incidence of PONV, as well as a lower rate of remedial analgesia and opioid consumption. Magnesium sulphate is able to inhibit pain signal transduction and produce the synergistic analgesic effect, thereby reducing opioid consumption and the adverse effects they cause.33 This may explain why magnesium sulphate improved the level of physical comfort and emotional state, suggesting the potential non-analgesic effects of magnesium sulphate.
None of the patients in group M experienced any adverse effects related to magnesium sulphate infusion, such as dyspnoea and neuromuscular paralysis.However, there are several limitations in this study. First, it was a single-centre trial and the sample size was relatively modest. Second, we performed postoperative observation for only 24 hours, without longer follow-up. Third, this study exclusively included patients undergoing thoracoscopic lobectomy, necessitating further investigation into its applicability to other populations.
Conclusion
Preoperative infusion of magnesium sulphate effectively decreased the incidence and severity of EA in patients undergoing thoracoscopic lobectomy. Furthermore, it alleviated postoperative pain and improved postoperative quality of recovery, without an increase in adverse events.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
No specific grants from public, commercial or not-for-profit funding bodies were received for this study.The authors would like to thank all study participants for their efforts.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lee SJ, Sung TY. Emergence agitation: current knowledge and unresolved questions. Korean J Anesthesiol. 2020;73(6):471–485. doi:10.4097/kja.20097
2. Munk L, Andersen G, Møller AM. Post-anaesthetic emergence delirium in adults: incidence, predictors and consequences. Acta Anaesthesiol Scand. 2016;60(8):1059–1066. doi:10.1111/aas.12717
3. Fields A, Huang J, Schroeder D, et al. Agitation in adults in the post-anaesthesia care unit after general anaesthesia. Br J Anaesth. 2018;121(5):1052–1058. doi:10.1016/j.bja.2018.07.017
4. Card E, Pandharipande P, Tomes C, et al. Emergence from general anaesthesia and evolution of delirium signs in the post-anaesthesia care unit. Br J Anaesth. 2015;115(3):411–417. doi:10.1093/bja/aeu442
5. Li M, Hu M, Jiang L, Pei J, Zhu C. Trends in Cancer Incidence and Potential Associated Factors in China. JAMA Netw Open. 2024;7(10):e2440381. doi:10.1001/jamanetworkopen.2024.40381
6. Kim JA, Ahn HJ, Yang M, Lee SH, Jeong H, Seong BG. Intraoperative use of dexmedetomidine for the prevention of emergence agitation and postoperative delirium in thoracic surgery: a randomized-controlled trial. Can J Anaesth. 2019;66(4):371–379. doi:10.1007/s12630-019-01299-7
7. Li C, Shi J, Wang K, Sun B, Gao G. Risk factors for inadequate emergence after non-cardiac thoracic surgery. J Clin Anesthesiol. 2016;32(1):33–37.
8. Kang X, Lin K, Tang H, et al. Risk Factors for Emergence Agitation in Adults Undergoing Thoracoscopic Lung Surgery: a Case-Control Study of 1,950 Patients. J Cardiothorac Vasc Anesth. 2020;34(9):2403–2409. doi:10.1053/j.jvca.2020.02.046
9. Yan F, Yuan LH, He X, Yu KF. Correlation between pre-anesthesia anxiety and emergence agitation in non-small cell lung cancer surgery patients. World J Psychiatry. 2024;14(6):930–937. doi:10.5498/wjp.v14.i6.930
10. Yoon SH, Bae J, Yoon S, Na KJ, Lee HJ. Correlation Between Pain Intensity and Quality of Recovery After Video-Assisted Thoracic Surgery for Lung Cancer Resection. J Pain Res. 2023;16:3343–3352. doi:10.2147/JPR.S426570
11. Kilic K, Sakat MS, Sahin A, Ahiskalioglu EO, Altunok H. Efficacy of intravenous magnesium sulphate infusion on postoperative pain and quality of recovery for septorhinoplasty: a randomized controlled study. Acta Otolaryngol. 2023;143(11–12):979–983. doi:10.1080/00016489.2023.2289584
12. Dahake JS, Verma N, Bawiskar D. Magnesium sulphate and Its Versatility in Anesthesia: a Comprehensive Review. Cureus. 2024;16(3):e56348. doi:10.7759/cureus.56348
13. Koo CH, Koo BW, Han J, Lee HT, Lim D, Shin HJ. The effects of intraoperative magnesium sulphate administration on emergence agitation and delirium in pediatric patients: a systematic review and meta-analysis of randomized controlled trials. Paediatr Anaesth. 2022;32(4):522–530. doi:10.1111/pan.14352
14. Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27(7):1325–1329. doi:10.1097/00003246-199907000-00022
15. Kang X, Tang X, Yu Y, et al. Intraoperative dexmedetomidine infusion is associated with reduced emergence agitation and improved recovery profiles after lung surgery: a retrospective cohort study. Drug Des Devel Ther. 2019;13:871–879. doi:10.2147/DDDT.S195221
16. Wang X, Deng Q, Liu B, Yu X. Preventing Emergence Agitation Using Ancillary Drugs with Sevoflurane for Pediatric Anesthesia: a Network Meta-Analysis. Mol Neurobiol. 2017;54(9):7312–7326. doi:10.1007/s12035-016-0229-0
17. Beloeil H, Garot M, Lebuffe G, et al. Balanced Opioid-free Anesthesia with Dexmedetomidine versus Balanced Anesthesia with Remifentanil for Major or Intermediate Noncardiac Surgery. Anesthesiology. 2021;134(4):541–551. doi:10.1097/ALN.0000000000003725
18. Zhang J, Yu Y, Miao S, et al. Effects of peri-operative intravenous administration of dexmedetomidine on emergence agitation after general anesthesia in adults: a meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2019;13:2853–2864. doi:10.2147/DDDT.S207016
19. Salman O, Ali Mohamed H. Comparison between dexmedetomidine versus magnesium sulphate infusions for mitigating emergence agitation in obese adults undergoing nasal surgery. Ain Shams J Anesthes. 2024;14(1):1.
20. Szedlák B, Mitre C, Fülesdi B. Preemptive and preventive analgesia - an important element in perioperative pain management. Orv Hetil. 2018;159(17):655–660. doi:10.1556/650.2018.31045
21. Cao L, Yang T, Hou Y, Yong S, Zhou N. Efficacy and Safety of Different Preemptive Analgesia Measures in Pain Management after Laparoscopic Cholecystectomy: a Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Pain Ther. 2024;159(17):655–660.
22. Aditya R, Indriasari I, Limawan M. Comparison of the effect of magnesium sulphate 50 mg/kg with 30 mg/kg on opioid requirement and blood magnesium level after abdominal hysterectomy. Anaesth Pain Intensi. 2024;28(3):517–523. doi:10.35975/apic.v28i3.2469
23. Hatice Akbudak I, Yılmaz S, Ilhan S, Yuksel Tanrıverdi S, Erdem E. The effect of preemptive magnesium sulphate on postoperative pain in patients undergoing mastectomy: a clinical trial. Eur Rev Med Pharmacol Sci. 2023;27(17):7907–7913. doi:10.26355/eurrev_202309_33549
24. Elsersy HE, Metyas MC, Elfeky HA, Hassan AA. Intraoperative magnesium sulphate decreases agitation and pain in patients undergoing functional endoscopic surgery: a randomised double-blind study. Eur J Anaesthesiol. 2017;34(10):658–664. doi:10.1097/EJA.0000000000000642
25. Su YH, Luo DC, Pang Y. Effects of intraoperative Magnesium sulphate infusion on emergency agitation during general anesthesia in patients undergoing radical mastectomy: a randomized controlled study. BMC Anesthesiol. 2023;23(1):326. doi:10.1186/s12871-023-02288-6
26. Lee S, Sohn JY, Hwang IE, et al. Effect of a repeated verbal reminder of orientation on emergence agitation after general anaesthesia for minimally invasive abdominal surgery: a randomised controlled trial. Br J Anaesth. 2023;130(4):439–445. doi:10.1016/j.bja.2022.12.009
27. Bilotta F, Gelb AW, Stazi E, Titi L, Paoloni FP, Rosa G. Pharmacological perioperative brain neuroprotection: a qualitative review of randomized clinical trials. Br J Anaesth. 2013;110:113–120. doi:10.1093/bja/aet059
28. Lin JY, Chung SY, Lin MC, Cheng FC. Effects of magnesium sulphate on energy metabolites and glutamate in the cortex during focal cerebral ischemia and reperfusion in the gerbil monitored by a dual-probe microdialysis technique. Life Sci. 2002;71(7):803–811. doi:10.1016/S0024-3205(02)01738-1
29. Bariskaner H, Ustun ME, Ak A, Yosunkaya A, Ulusoy HB, Gurbilek M. Effects of magnesium sulphate on tissue lactate and malondialdehyde levels after cerebral ischemia. Pharmacology. 2003;68(3):162–168. doi:10.1159/000070174
30. Xu H, Hao C, Wang X, Du J, Zhang T, Zhang X. Effect of Intraoperative infusion Magnesium sulphate Infusion on Postoperative Quality of Recovery in Patients Undergoing Total Knee Arthroplasty: a Prospective, Double-Blind, Randomized Controlled Trial. Drug Des Devel Ther. 2024;18:919–929. doi:10.2147/DDDT.S444896
31. Myles PS, Myles DB, Galagher W, Chew C, MacDonald N, Dennis A. Minimal Clinically Important Difference for Three Quality of Recovery Scales. Anesthesiology. 2016;125(1):39–45. doi:10.1097/ALN.0000000000001158
32. Hua X, Chen Y, Wu Z, et al. Effects of intra-operative magnesium sulphate infusion on orthognathic surgery: a prospective and randomized controlled trial. Heliyon. 2024;10(9):e30342. doi:10.1016/j.heliyon.2024.e30342
33. Sohn HM, Kim BY, Bae YK, Seo WS, Jeon YT. Magnesium sulphate Enables Patient Immobilization during Moderate Block and Ameliorates the Pain and Analgesic Requirements in Spine Surgery, Which Can Not Be Achieved with Opioid-Only Protocol: a Randomized Double-Blind Placebo-Controlled Study. J Clin Med. 2021;10(19):4289. doi:10.3390/jcm10194289
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

