Back to Journals » Drug Design, Development and Therapy » Volume 19
Effects of Prophylactic Infusion of Equivalent Doses of Norepinephrine and Phenylephrine in Preventing Spinal Anesthesia-Induced Hypotension During Cesarean Delivery on Fetal and Maternal Outcomes: A Dual-Center, Non-Inferiority Controlled Trial
Authors Mao J, Lin K, Liu X, Liu J, Liang G, Sheng Z
Received 24 December 2024
Accepted for publication 13 June 2025
Published 17 June 2025 Volume 2025:19 Pages 5143—5152
DOI https://doi.org/10.2147/DDDT.S514091
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Junqin Mao,1,* Kang Lin,2,* Xiang Liu,1 Jie Liu,1 Gang Liang,1 Zhimin Sheng1
1Department of Anesthesiology, Wenling Maternity and Child Health Care Hospital, Taizhou, Zhejiang, People’s Republic of China; 2Department of Anesthesiology, Wenling First People’s Hospital (The Affiliated Wenling Hospital of Wenzhou Medical University), Taizhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhimin Sheng, Department of Anesthesiology, Wenling Maternity and Child Health Care Hospital, No. 102, Xiabao Road, Chengdong Street, Wenling City, Taizhou, Zhejiang, 317500, People’s Republic of China, Tel +86-576-86168030, Email [email protected]
Background: Numerous studies have compared the effects of norepinephrine and phenylephrine on maternal and neonatal outcomes during cesarean delivery. However, the infusion rates are often based on clinical experience, resulting in non-equivalent doses. We aimed to compare the effects of norepinephrine and phenylephrine at equivalent doses on fetal and maternal outcomes, and assess their efficacy at the 90% effective dose (ED90).
Methods: A total of 100 parturients scheduled for cesarean delivery were randomly allocated to receive either 0.10 μg/kg/min norepinephrine (Group NE) or 0.60 μg/kg/min phenylephrine (Group PE) to prevent spinal anesthesia-induced hypotension. The primary endpoint was neonatal umbilical arterial (UA) pH and the incidence of maternal hypotension, while secondary endpoints included hemodynamic changes within the first 15 minutes, maternal adverse events, and additional neonatal measures.
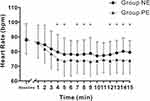
Results: Of the 95 subjects who completed the study, the UA pH in Group NE (7.296 ± 0.041) was found to be non-inferior to Group PE (7.292 ± 0.040), with a mean difference of 0.003 [95% confidence interval (CI): − 0.016 to 0.022; P = 0.009]. The incidences of hypotension (NE: 8.3% vs PE: 10.6%, P = 0.701), hypertension, nausea, and vomiting were comparable between groups. However, bradycardia incidence was significantly reduced in Group NE compared to Group PE (2.1% vs 12.8%, P = 0.046). The two groups showed no significant difference in systolic blood pressure (SBP) at most time points within the first 15 minutes, except at 7 minutes. Group NE also had a higher heart rate (HR) than Group PE in most measurements. Both groups showed similar neonatal outcomes.
Conclusion: Prophylactic infusion of 0.10 μg/kg/min norepinephrine was non-inferior to 0.60 μg/kg/min phenylephrine in terms of neonatal UA pH. These findings further support the safety of norepinephrine in obstetric anesthesia, although additional research is warranted to assess its long-term maternal and neonatal outcomes.
Keywords: cesarean delivery, norepinephrine, phenylephrine, hypotension, umbilical arterial pH, non-inferiority
Introduction
Spinal anesthesia-induced hypotension (SAIH) is a frequent complication in cesarean delivery, potentially leading to a variety of adverse effects on both mothers and neonates.1,2 The use of vasopressors is widely regarded as the most reliable and effective method for preventing and treating SAIH.3,4 Studies have shown that continuous prophylactic infusion of vasopressors provides more stable hemodynamics than single intravenous boluses.5,6 However, controversies remain regarding the optimal vasopressor and initial infusion dose.
Phenylephrine has long been the first-choice vasopressor in obstetrics because of its effective control over maternal blood pressure and favorable neonatal outcomes.7 However, its dose-dependent effects on maternal heart rate (HR) and cardiac output (CO) have raised notable concerns.8 Norepinephrine, a potent α1-adrenergic agonist and mild β1-adrenergic agonist, enhances HR and CO, making it a compelling alternative to phenylephrine.7,9
Previous studies on phenylephrine and norepinephrine for preventing SAIH during cesarean delivery have primarily focused on maternal hemodynamics,10,11 while recent research has shifted towards assessing their impact on neonatal outcomes.12,13 Currently, umbilical artery (UA) pH value analysis is frequently used as an objective indicator of neonatal outcomes.12,14
It is notable that in numerous studies, the initial vasopressor infusion doses were based on the authors’ clinical experience rather than robust evidence from the literature, which may result in inequivalent dosages and potential discrepancies in outcomes.15,16 In our previous work, a dose of 0.097 µg/kg/min was determined as the 90% effective dose (ED90) for prophylactic norepinephrine infusion in combination with a 10 mL/kg of crystalloid coloading to prevent SAIH during cesarean delivery.17 Likewise, a dose-response study determined the ED90 for phenylephrine as 0.54 µg/kg/min,18 with its relative potency ratio to norepinephrine estimated at approximately 1:6.03.19 For ease of preparation and calculation, we considered that the initial infusion rates of 0.10 µg/kg/min for norepinephrine and 0.60 µg/kg/min for phenylephrine as appropriate dosing regimens.
This study aimed to compare the maternal and fetal outcomes following prophylactic infusion of norepinephrine and phenylephrine at equivalent doses. We hypothesize that norepinephrine infusion would be non-inferior to phenylephrine infusion in maintaining neonatal acid-base status and preventing maternal hypotension.
Methods
Study Design and Patient Enrolment
This dual-center, randomized, non-inferiority controlled study was conducted at Wenling Maternity and Child Health Care Hospital (Taizhou, China) and Affiliated Xiaoshan Hospital of Hangzhou Normal University (Hangzhou, China), following approval from the Institutional Clinical Research Ethical Review Boards of both institutions (No. 2024-IRB-101 and No. 2023-IRB-015). The study followed the principles of the Declaration of Helsinki and adhered to the Consolidated Standards of Reporting Trials (CONSORT) guidelines. Written informed consent was acquired from each participant. This study was registered at the Chinese Clinical Trial Registry (accessible at www.chictr.org.cn, with the registration No. ChiCTR2400087985, and the registration date being August 8, 2024). The eligible participants were American Society of Anesthesiologists (ASA) parturients with a physical status of less than III, aged 18–40 years and had singleton pregnancies of at least 37 weeks, and scheduled for elective cesarean delivery between August 10 and December 10, 2024. Exclusion criteria were as follows: contraindications to regional anesthesia, allergy to phenylephrine or norepinephrine, height < 150 cm or > 170 cm, obesity (body mass index > 35 kg/m²), multiple gestation, diabetes mellitus, hypertensive disorders of pregnancy, cardiovascular disease, placenta previa, ruptured membranes, and significant coexisting maternal disease.
Randomization and Blinding
Using computer-generated randomization codes, parturients were allocated in a 1:1 ratio to receive an infusion of either 0.10 μg/kg/min norepinephrine (Group NE) or 0.60 μg/kg/min phenylephrine (Group PE) for intraoperative blood pressure maintenance. Randomization codes were stored in opaque envelopes, sequentially numbered to ensure concealment. The sealed envelope was opened by a nurse anesthetist with no involvement in the trial, who then prepared a 50 mL syringe labeled study drug containing norepinephrine [dose: maternal weight (kg) × 6 μg] or phenylephrine [dose: maternal weight (kg) × 36 μg], both diluted in 0.9% saline to a total volume of 50 mL. The prepared study drug was given to the anesthesiologist managing data collection, who administered it at a fixed rate of 50 mL/h. Additionally, bolus doses for treatment of hypotension (6 μg norepinephrine or 100 μg phenylephrine) were prepared in identical 10 mL syringes labeled “rescue vasopressor” by the same nurse anesthetist, ensuring allocation concealment for both the anesthesiologist and parturients.
Anesthesia Management and Intervention
Participants fasted for a minimum of 8 hours prior to the surgery, without receiving any preoperative medications. An 18-gauge intravenous catheter was inserted into the left forearm vein, and no preload fluid was administered in the waiting area. Once entering the operating room, parturients were placed in the supine position with the leftward displacement of the uterus. Subsequently, routine monitoring involved non-invasive blood pressure (NIBP), pulse oximetry, and a five-lead electrocardiogram was performed. NIBP was measured every 2 minutes following a brief period of calmness. Baseline values were derived from the average of three consecutive systolic blood pressure (SBP) and HR readings, ensuring a variation of less than 10%.
Combined spinal-epidural anesthesia was performed at the L3–4 intervertebral space with the parturient in the left lateral decubitus position, using the needle-through-needle method. Following the correct positioning of the 18–gauge epidural needle, a 27–gauge spinal needle was advanced into the subarachnoid space via the epidural needle. Upon confirming cerebrospinal fluid reflux, 3 mL (15 mg) of hyperbaric ropivacaine (comprising 2 mL of 0.75% ropivacaine, and 1 mL of 10% dextrose) was administered into the subarachnoid space at a controlled rate of 1 mL every 10 seconds, directed upward. Subsequently, the spinal needle was then removed, and an epidural catheter was inserted into the epidural space, advanced 3–4 cm toward the cephalad direction. The parturient was repositioned to the supine position, and oxygen supplementation at 5 L/min was provided through a face mask. Simultaneously with the intrathecal injection, 10 mL/kg of lactated Ringer’s solution was rapidly co-loaded over 10–15 minutes at the maximum possible rate under gravity, and then adjusted to slow infusion to maintain venous patency. Meanwhile, study drug infusions (norepinephrine at 0.10 µg/kg/min and phenylephrine at 0.60 µg/kg/min) were delivered via an infusion pump at 50 mL/h through a three-way stopcock attached to the parturient’s intravenous cannula. NIBP readings were taken every minute post-intrathecal injection and every three minutes after delivery. Sensory block was evaluated using an 18-gauge blunt epidural needle at 5 and 10 minutes after intrathecal injection with success defined as T6 or above. Unsuccessful blockade resulted in disqualification, with the next eligible participant enrolled according to the randomization code.
Upon fetal delivery, arterial and venous blood samples were collected by the obstetrician from the double–clamped segment of the umbilical cord and analyzed within 30 seconds using a blood gas analyzer by an assistant blinded to the group allocation. Additionally, Apgar scores were assessed by a pediatrician who was also unaware of the group assignment.
The study period spanned from the start of the intrathecal injection to neonatal delivery. Hypotension, defined as SBP ≤ 80% of baseline, was treated with 6 µg norepinephrine in Group NE and 100 µg phenylephrine in Group PE.20,21 If there was no response in SBP within 2 minutes after the first dose, additional vasopressor boluses were given until SBP ≥ 90% of the baseline value. Hypertension, defined as SBP ≥ 120% of baseline, was controlled by pausing the infusion and resuming it once SBP dropped below 120% of baseline. Bradycardia, defined as HR < 60 beats/min. In such cases, the infusion of vasopressors was stopped and resumed once the HR returned to ≥ 60 beats/min. If bradycardia persisted for more than 2 minutes, an intravenous dose of atropine (0.5 mg) was administered.
Data Collection and Outcome Assessment
The demographic data along with the obstetric characteristics were recorded. The primary endpoint of this study was umbilical arterial (UA) pH and the occurrence of maternal hypotension throughout the study period.
Secondary endpoints included hemodynamic parameter changes (SBP and HR) within the first 15 minutes, demographic characteristics (including age, height, weight, sensory block level, the interval from spinal anesthesia to delivery, and the total vasopressor volume administered before delivery), maternal adverse events [such as severe hypotension (defined as a decrease of ≥ 40% of baseline SBP), bradycardia, reactive hypertension, nausea and vomiting], and other measures of neonatal outcomes (including additional analysis of blood gases within the umbilical artery and vein, and Apgar scores).
Sample Size Estimation
The sample size calculation utilized non-inferiority tests for two means (differences), conducted with PASS 11.0.1 (NCSS, LLC, Kaysville, Utah). Based on a previous study, the mean UA pH was 7.33, with a standard deviation (SD) of 0.03 when phenylephrine was infused at 0.54 μg/kg/min.14 Using these data, we determined that to achieve 90% power with an alpha of 0.05 for rejecting the null hypothesis of a UA pH decrease of 0.02 units or more in the Group NE compared to the Group PE, would require a sample size of 40 subjects per group. Considering potential dropouts or incomplete data, the sample size was increased by 25%, resulting in 50 subjects per group.
Statistical Analysis
Data were analyzed using SPSS for Windows (version 22.0) and GraphPad Prism software (version 8.0.2). The Shapiro–Wilk test was applied to assess the normality of continuous variables. Normally distributed variables were evaluated with the independent-samples Student’s t-test and presented as mean ± SD, while non-normally distributed variables were assessed with the Mann–Whitney U-test and presented as median (with quartiles). Frequency data were compared using either the chi-square test or Fisher’s exact test, as appropriate, and presented as numbers (%). A p value < 0.05 was defined as statistically significant.
Results
A total of 118 participants underwent screening, with 18 excluded (7 opted not to participate, and 11 failed to meet the inclusion criteria). Subsequently, 100 participants were randomized and allocated equally to Group NE (n = 50) and Group PE (n = 50). After excluding cases of failed spinal anesthesia and unsuccessful collection of umbilical cord blood samples, the final analysis included 48 participants in Group NE and 47 in Group PE (Figure 1). Maternal data and surgical parameters are provided in Table 1. The demographic data, level of sensory block, interval from spinal anesthesia to delivery, as well as the total volume of vasopressors administered were comparable between the two groups. Intraoperative hemodynamic parameters and adverse effects are presented in Table 2. The incidences of hypotension (Group NE: 8.3%, Group PE:10.6%, P = 0.701), as well as hypertension, nausea and vomiting were comparable between groups. However, bradycardia incidence was significantly reduced in the Group NE compared to the Group PE (2.1% vs 12.8%, P = 0.046). Changes in SBP and HR within the first 15 minutes following subarachnoid block are presented in Figures 2 and 3. SBP showed no significant differences between the groups across time intervals. Only at 7 minutes after spinal anesthesia did we observe that SBP in the Group NE was higher than in the Group PE. Group NE had higher HR than Group PE in most readings.
 |
Table 1 Demographic Data, Sensory Block Level, and Surgical Parameters |
 |
Table 2 Hemodynamic Changes, and Side Effects |
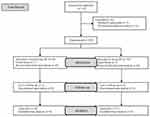 |
Figure 1 Consolidated Standards of Reporting Trials (CONSORT) flow diagram. Abbreviations: NE, norepinephrine; PE, phenylephrine. |
The values of the primary endpoint UA pH are shown in Table 3, with a mean of 7.296 ± 0.041 in the Group NE and 7.292 ± 0.040 in the Group PE. The mean difference in UA pH among the groups was 0.003 (95% CI, −0.016 to 0.022). Since the CI did not extend beyond the non-inferiority margin of −0.02, it was demonstrated that norepinephrine is not inferior to phenylephrine (non-inferiority P = 0.009) (Figure 4). Birth weight and other measures of neonatal outcome, including the umbilical blood gases and Apgar scores were also similar between the groups (Table 3).
 |
Table 3 Neonatal Outcome |
Discussion
Due to the frequent occurrence of reflex maternal bradycardia and the reduction in maternal CO associated with the use of phenylephrine, an increasing number of experts now advocate for norepinephrine as the superior vasopressor in obstetric anesthesia.7,21,22 The absence of significant suppression of UA pH values further provides strong evidence supporting the fetal safety of norepinephrine in this clinical setting.23 Our findings demonstrate that prophylactic norepinephrine infusion is non-inferior to phenylephrine in terms of UA pH outcomes; moreover, the significantly lower incidence of bradycardia in the norepinephrine group compared to the phenylephrine group (2.1% vs 12.8%; P = 0.046) highlights its potential clinical advantage. Norepinephrine may serve as a safer alternative for parturients at risk of bradycardia, particularly those with preexisting cardiac conditions, thereby reducing the need for adjunctive interventions such as atropine administration.22
Since fetal heart rate monitoring is not feasible during the operation, the impact of hypotension on the fetal condition has to be assessed using surrogate markers. UA pH was chosen as the primary endpoint due to its well-recognized role in assessing neonatal status at birth, reflecting both the respiratory and metabolic aspects of fetal acidaemia.12,14 The occurrence of respiratory fetal acidaemia is predominantly attributed to carbon dioxide build-up, which results from acute placental hypoperfusion. It is worthy of attention that the use of intraoperative vasopressors and spinal anesthesia are both associated with risk factors for this condition. Base excess (BE) may be considered a more appropriate indicator to reflect the metabolic component of fetal acidosis. However, as a calculated parameter strongly correlated with pH, the added prognostic value of UA BE compared to direct UA pH measurement remains a subject of debate.24
Prophylactic continuous infusion of vasopressors remains the widely recommended strategy for preventing SAIH in obstetric practice.5,6 In this study, the initial infusion doses of norepinephrine (0.10 μg/kg/min) and phenylephrine (0.60 μg/kg/min) were selected based on our previous dose-response study and a study by Fei Xiao et al, which established the ED90 for SAIH prevention during cesarean delivery as 0.097 µg/kg/min for norepinephrine and 0.54 µg/kg/min for phenylephrine.17,18 However, these ED90 values were derived from probability regression rather than direct observation of a 10% hypotension incidence at specific infusion rates; moreover, their wide confidence intervals also require further clinical validation. Our results demonstrated that the incidences of hypotension in Group NE and Group PE were 8.3% and 10.6%, respectively, which were remarkably close to the estimated ED90 values. Therefore, we conclude that an initial infusion of norepinephrine at 0.10 μg/kg/min and phenylephrine at 0.60 μg/kg/min is appropriate for clinical practice.
A similar study was conducted by Liu et al,14 however, their approach differed from ours in that they selected initial infusion doses of 0.08 μg/kg/min for norepinephrine and 0.54 μg/kg/min for phenylephrine based on previous dose-response studies.18,25 Although their results indicated no significant difference in UA pH between the two groups, the incidences of hypotension reached 38% for norepinephrine and 12% for phenylephrine. These findings suggest that the previously estimated ED90 values for norepinephrine and phenylephrine may have been underestimated. Several factors may account for these discrepancies. First, different studies utilized varying specific gravities and doses of drugs for spinal anesthesia, which may have influenced the outcomes. Second, the confidence intervals derived from probit regression analyses were relatively wide, potentially leading to deviations in the median ED90 values. Third, differences in fluid co-loading protocols could have contributed to the variation. While most clinical practices adopt a co-loading dose of 10 mL/kg of crystalloid,25,26 the study by Fu et al reported a maximum co–loading volume of up to 1.5 liters.27
The results of this study indicated that norepinephrine was non-inferior to phenylephrine regarding its impact on fetal UA pH outcomes, which was consistent with the findings of a recent study.12 The most plausible explanation is that the combination of prophylactic vasopressor infusion and fluid co-loading effectively prevents SAIH, thereby reducing the fetal adverse effects associated with hypotension. SAIH is known to negatively impact neonatal outcomes, with both the severity and cumulative duration of hypotensive episodes strongly associated with fetal acidemia and lower Apgar scores.2,28 Therefore, prompt intervention in respect of the duration and severity of hypotension, rather than merely concentrating on its incidence rate, may hold greater clinical significance. In this study, the occurrence rate of hypotension in both groups was approximately 10%. Moreover, NIBP was measured every minute from the start of spinal anesthesia until delivery, and any detected hypotension was immediately managed with intravenous administration of a 6 μg bolus of norepinephrine or a 100 μg bolus of phenylephrine. Consequently, neither group experienced any adverse neonatal outcomes.
Our study has several limitations. First, the UA pH value can be significantly influenced by various factors, including the timing of sampling, the duration of umbilical cord clamping at both ends, the time of analysis, and the presence of umbilical cord entanglement.29 Therefore, incorporating the cerebroplacental ratio or fetal cardiac output may further aid in the early assessment of fetal hypoxia and the prediction of outcomes. Second, we defined the non-inferiority margin using a small effect size of 0.02 pH units. If a smaller effect size of 0.01 pH units had been used, it might detect even slight potentially detrimental effects of norepinephrine. However, this would have required a larger sample size, significantly increasing the workload. Third, we conducted the trial across two clinical centers, recognizing that factors such as variations in medical resources and patient populations could contribute to inconsistencies in study outcomes, thereby increasing heterogeneity.
Conclusion
In summary, our study demonstrated that prophylactic infusion of norepinephrine (0.10 μg/kg/min) is non-inferior to phenylephrine (0.60 μg/kg/min) for preventing spinal anesthesia-induced hypotension during cesarean delivery in terms of neonatal UA pH, and was associated with a lower risk of bradycardia. Our findings support the use of norepinephrine at an initial infusion rate of 0.10 μg/kg/min in obstetric anesthesia. However, further research is warranted to evaluate the long-term safety of norepinephrine in postpartum maternal hemodynamics and neonatal neurodevelopment.
Abbreviations
SAIH, spinal anesthesia-induced hypotension; ED90, the 90% effective dose; CI, confidence interval; NIBP, noninvasive blood pressure; SBP, systolic blood pressure; HR, heart rate; CO, cardiac output; UA, umbilical artery; ASA, American Society of Anesthesiologist; CONSORT, Consolidated Standards of Reporting Trials.
Data Sharing Statement
The data supporting the findings of this study can be obtained from the corresponding author (Zhimin Sheng) upon legitimate request.
Ethics Approval
This study received approval from the Ethics Committees of Wenling Maternity and Child Health Hospital (No. 2024-IRB-101) and the Affiliated Xiaoshan Hospital of Hangzhou Normal University (No. 2023-IRB-015). This study was conducted in strict accordance with the principles outlined in the Declaration of Helsinki.
Acknowledgments
We would like to acknowledge the staff in the Anesthesiology Departments of Wenling Maternity and Child Health Care Hospital and Affiliated Xiaoshan Hospital of Hangzhou Normal University for their assistance in this study.
Funding
This study was funded by the Zhejiang Medical and Health Science and Technology Plan Project (No. 2024XY101) and the Wenling Science and Technology Bureau (No. 2023S00164 and No. 2024S00158).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Lee A, Ngan Kee W. Effects of vasoactive medications and maternal positioning during cesarean delivery on maternal hemodynamics and neonatal acid-base status. Clin Perinatol. 2019;46(4):765–783. doi:10.1016/j.clp.2019.08.009
2. Consensus Statement Collaborators; Kinsella SM, Carvalho B, Dyer RA, et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia. 2018;73(1):71–92. doi:10.1111/anae.14080
3. Sklebar I, Bujas T, Habek D. Spinal anaesthesia-induced hypotension in obstetrics: prevention and therapy. Acta Clin Croat. 2019;58(1):90–95. doi:10.20471/acc.2019.58.s1.13
4. Lirk P, Haller I, Wong C. Management of spinal anaesthesia-induced hypotension for caesarean delivery: a European survey. Eur J Anaesthesiol. 2012;29(9):452–453. doi:10.1097/EJA.0b013e328352ab10
5. Wang X, Shen X, Liu S, Yang J, Xu S. The efficacy and safety of norepinephrine and its feasibility as a replacement for phenylephrine to manage maternal hypotension during elective cesarean delivery under spinal anesthesia. Biomed Res Int. 2018;2018:1869189. doi:10.1155/2018/1869189
6. Heesen M, Kölhr S, Rossaint R, Straube S. Prophylactic phenylephrine for caesarean section under spinal anaesthesia: systematic review and meta-analysis. Anaesthesia. 2014;69(2):143–165. doi:10.1111/anae.12445
7. Ngan Kee WD, Lee SWY, Ng FF, et al. Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology. 2015;122(4):736–745. doi:10.1097/ALN.0000000000000601
8. Mon W, Stewart A, Fernando R, et al. Cardiac output changes with phenylephrine and ephedrine infusions during spinal anesthesia for cesarean section: a randomized, double-blind trial. J Clin Anesth. 2017;37:43–48. doi:10.1016/j.jclinane.2016.11.001
9. Chen Y, Zou L, Li Z, et al. Prophylactic norepinephrine infusion for postspinal anaesthesia hypotension in patients undergoing caesarean section: a randomized, controlled, dose-finding trial. Pharmacotherapy. 2021;41(4):370–378. doi:10.1002/phar.2514
10. Hasanin A, Amin S, Refaat S, et al. Norepinephrine versus phenylephrine infusion for prophylaxis against post-spinal anaesthesia hypotension during elective caesarean delivery: a randomised controlled trial. Anaesth Crit Care Pain Med. 2019;38(6):601–607. doi:10.1016/j.accpm.2019.03.005
11. Kondo Y, Hirose N, Maeda T, et al. Relationship between changes in regional cerebral blood volume and oxygenation and changes in cardiac output and systemic vascular resistance during spinal anesthesia in women undergoing cesarean section. J Anesth. 2019;33(5):579–586. doi:10.1007/s00540-019-02670-0
12. Ngan Kee WD, Lee SWY, Ng FF, Lee A. Norepinephrine or phenylephrine during spinal anaesthesia for Caesarean delivery: a randomised double-blind pragmatic non-inferiority study of neonatal outcome. Br J Anaesth. 2020;125(4):588–595. doi:10.1016/j.bja.2020.05.057
13. Sun L, Tang Y, Guo F, et al. Norepinephrine or phenylephrine for the prevention of post-spinal hypotension after caesarean section: a double-blinded, randomized, controlled study of fetal heart rate and fetal cardiac output. J Clin Anesth. 2024;97:111533. doi:10.1016/j.jclinane.2024.111533
14. Liu T, Cheng Z, Zou S, et al. Effect of weight-adjusted phenylephrine, norepinephrine, and metaraminol for elective cesarean delivery on neonatal acid-base status: a randomized controlled trial. Drug Des Devel Ther. 2022;16:3215–3223. doi:10.2147/DDDT.S381048
15. Hasanin AM, Amin SM, Agiza NA, et al. Norepinephrine infusion for preventing postspinal anesthesia hypotension during cesarean delivery: a randomized dose-finding trial. Anesthesiology. 2019;130(1):55–62. doi:10.1097/ALN.0000000000002483
16. Singh PM, Singh NP, Reschke M, et al. Vasopressor drugs for the prevention and treatment of hypotension during neuraxial anaesthesia for caesarean delivery: a Bayesian network meta-analysis of fetal and maternal outcomes. Br J Anaesth. 2020;124(1):95–107. doi:10.1016/j.bja.2019.09.045
17. Jin WD, Mao JQ, Liu J, et al. Comparative dose-response study on the infusion of norepinephrine combined with crystalloid coload versus colloid coload for preventing hypotension during spinal anesthesia for cesarean delivery. Drug Des Devel Ther. 2022;16:2617–2626. doi:10.2147/DDDT.S378453
18. Xiao F, Shen B, Xu WP, et al. Dose-response study of 4 weight-based phenylephrine infusion regimens for preventing hypotension during cesarean delivery under combined spinal-epidural anesthesia. Anesth Analg. 2020;130(1):187–193. doi:10.1213/ANE.0000000000004092
19. Qian J, Zhao YP, Deng JL, et al. Determination of the relative potency of norepinephrine and phenylephrine given as infusions for preventing hypotension during combined spinal-epidural anesthesia for cesarean delivery: a randomized up-and-down sequential allocation study. Front Pharmacol. 2022;13:942005. doi:10.3389/fphar.2022.942005
20. Liu H, Huang Y, Diao M, et al. Determination of the 90% effective dose (ED90) of phenylephrine for hypotension during elective cesarean delivery using a continual reassessment method. Eur J Obstet Gynecol Reprod Biol. 2015;194:136–140. doi:10.1016/j.ejogrb.2015.07.001
21. Sharkey AM, Siddiqui N, Downey K, et al. Comparison of intermittent intravenous boluses of phenylephrine and norepinephrine to prevent and treat spinal-induced hypotension in cesarean deliveries: randomized controlled trial. Anesth Analg. 2019;129(5):1312–1318. doi:10.1213/ANE.0000000000003704
22. Theodoraki K, Hadzilia S, Valsamidis D, Stamatakis E. Prevention of hypotension during elective cesarean section with a fixed-rate norepinephrine infusion versus a fixed-rate phenylephrine infusion. Α double-blinded randomized controlled trial. Int J Surg. 2020;84:41–49. doi:10.1016/j.ijsu.2020.10.006
23. Yeh P, Emary K, Impey L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: analysis of 51,519 consecutive validated samples. BJOG. 2012;119(7):824–831. doi:10.1111/j.1471-0528.2012.03335.x
24. Knutzen L, Svirko E, Impey L. The significance of base deficit in acidemic term neonates. Am J Obstet Gynecol. 2015;213(3):
25. Theodoraki K, Hadzilia S, Valsamidis D, et al. Colloid preload versus crystalloid co-load in the setting of norepinephrine infusion during cesarean section: time and type of administered fluids do not matter. J Clin Med. 2023;12(4):1333. doi:10.3390/jcm12041333
26. Xu W, Drzymalski DM, Ai L, et al. The ED50 and ED95 of prophylactic norepinephrine for preventing post-spinal hypotension during cesarean delivery under combined spinal-epidural anesthesia: a prospective dose-finding study. Front Pharmacol. 2021;12:691809. doi:10.3389/fphar.2021.691809
27. Fu F, Xiao F, Chen W, et al. A randomised double-blind dose-response study of weight-adjusted infusions of norepinephrine for preventing hypotension during combined spinal-epidural anaesthesia for Caesarean delivery. Br J Anaesth. 2020;124(3):e108–e114. doi:10.1016/j.bja.2019.12.019
28. Ngan Kee WD, Lee A. Multivariate analysis of factors associated with umbilical arterial pH and standard base excess after Caesarean section under spinal anaesthesia. Anaesthesia. 2003;58(2):125–130. doi:10.1046/j.1365-2044.2003.02888.x
29. Murlewska J, Sylwestrzak O, Poszwa P, et al. The effect of nuchal umbilical cord on fetal cardiac and cerebral circulation: cross-sectional study. J Perinat Med. 2021;49(5):590–595. doi:10.1515/jpm-2020-0316
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.