Back to Journals » Nature and Science of Sleep » Volume 16
Effects of Tobacco Use on the Macrostructure and Microstructure of Sleep in Patients with OSA
Authors Ji W , Shi L , Ji Z , Zhao Z , Lin L, Wang X , Cheng J, Chen X
Received 27 May 2024
Accepted for publication 23 November 2024
Published 29 November 2024 Volume 2024:16 Pages 1849—1868
DOI https://doi.org/10.2147/NSS.S480116
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Valentina Alfonsi
Wei Ji,1,* Liyong Shi,1,2,* Zhiqiang Ji,3,* Zhihuang Zhao,1 Lianshun Lin,1,2 Xiali Wang,4 Jing Cheng,4 Xiaoyang Chen1,2
1The Second Clinical College of Fujian Medical University, Quanzhou, Fujian Province, 362000, People’s Republic of China; 2Department of Pulmonary and Critical Care Medicine, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, Fujian Province, 362000, People’s Republic of China; 3Zunyi Medical University, Zunyi, Guizhou Province, 563006, People’s Republic of China; 4Quanzhou Medical College, Quanzhou, Fujian Province, 362000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoyang Chen, Email [email protected]
Objective: Both tobacco use and obstructive apnea-hypopnea syndrome (OSA) can affect sleep, and it is speculated that tobacco use may further affect the sleep of those with OSA. Our primary objective is to clarify the associations between tobacco use and the macrostructure and microstructure of sleep in patients with OSA.
Methods: This retrospective study encompasses a cohort of 1017 patients who were hospitalized between January 2020 and January 2023 for the investigation of sleep disorders. Rigorous inclusion criteria were applied, and all patients underwent a comprehensive polysomnography (PSG) assessment and completed a Pittsburgh Sleep Quality Index (PSQI) questionnaire.
Results: Patients with OSA who concurrently used tobacco exhibited markedly inferior sleep quality than those who did not. Notably, there was no association between the degree of tobacco dependence and sleep quality. Those with OSA who used tobacco demonstrated a significant prolongation of stage 1 light sleep and a reduction of deep sleep duration (N3). In this group, those who demonstrated poor sleep quality had more pronounced alterations in light sleep stages with prolonged N1 and shortened N2 stages.
Conclusion: Our findings reveal a substantial reduction in sleep quality amongst OSA patients who also use tobacco, compared to those with OSA who do not use tobacco. The rate of poor sleep quality was not linearly associated with the level of tobacco dependence. Tobacco use was associated with alterations in both light and slow wave sleep in those with OSA. Importantly, the effects of tobacco dependence on sleep structure were more pronounced in those with more severe OSA.
Keywords: tobacco, sleep structure, sleep quality, OSA
Introduction
Obstructive sleep apnea-hypopnea syndrome (OSA) represents a significant global public health concern, affecting a substantial number of individuals worldwide. The estimated global prevalence of OSA is approximately 1 billion individuals, with approximately 425 million individuals experiencing moderate to severe OSA.1 Currently, OSA is the most common type of sleep-breathing disorder.2 OSA is characterized by the repeated occurrence of partial or complete obstruction of the upper airway during sleep, resulting in recurrent episodes of apnea or hypopnea, and typical manifestations of OSA include chronic snoring, as well as nocturnal hypoxemia and excessive daytime sleepiness.3 The repetitive occurrence of apnea in individuals with OSA disrupts the normal sleep architecture, leading to notable alterations in sleep stages. One distinctive feature of OSA-related sleep architecture disorders is an increase in stages of light sleep (N1 and N2) and a decrease in slow wave sleep (SWS).4 Multiple studies have identified OSA as a significant contributor to the reduction in SWS observed in humans.5
With more than 1.3 billion people around the world using tobacco products, it’s important to be wary of the fact that tobacco use has been shown to alter the essential physiological function of sleep.6 Tobacco use is associated with various symptoms of insomnia, including an increase in light sleep stages, a reduction in slow-wave sleep, an elevation in rapid eye movement sleep, prolonged sleep latency, diminished sleep efficiency, and a decrease in total sleep duration.6–9 The so-called Tobacco-induced sleep disturbances (TISDs) is also known by this term.10 However, some studies have not found significant differences in REM, total sleep time or other sleep structures in those who use tobacco.6 Tobacco is widely recognized for its impact on the central nervous system. Tobacco directly stimulates cholinergic nerves at the widespread acetylcholine receptor, and indirectly alters the glutaminergic, dopaminergic and 5-hydroxytryptaminergic systems in the brain.9,11 Cessation of tobacco use is associated with symptoms of depression.6
Several studies have underscored tobacco use as a notable risk factor for the development of OSA.12 According to findings from the University of Wisconsin Sleep Cohort Study, which is one of the largest cohort studies in the United States investigating OSA, current smokers exhibited a higher likelihood of presenting with moderate to severe OSA in comparison to non-smokers.13 Nevertheless, it is crucial to acknowledge that divergent viewpoints exist within scholarly discourse. Some researchers have put forth the notion that tobacco use may potentially decrease the frequency of sleep apneas, while others have posited that nicotine itself may not exert a significant effect on OSA.14 Insomnia and OSA are often coincident. Those with insomnia and OSA have more severely impaired sleep than those with insomnia or OSA alone.15 In a study of 231 OSA patients, up to 50% suffered from insomnia.16
The impact of tobacco use on OSA remains a subject of debate within the scientific community. Existing studies investigating the effects of tobacco use on sleep structure have predominantly focused on unspecified populations, while comprehensive investigations specifically targeting individuals with OSA are lacking. Particularly, the examination of sleep quality in this specific population remains notably limited.
As tobacco use and OSA both independently affect sleep, we hypothesize that the use of tobacco may further impact sleep in those with OSA. To advance our understanding and facilitate the diagnosis and treatment of OSA, it is crucial to refine our knowledge in this area. Therefore, the objective of this study is to investigate the effects of tobacco use on sleep structure and sleep quality in patients with OSA. This will be achieved through the integration of objective measurements obtained from polysomnography (PSG) and subjective perceptions assessed using the Pittsburgh Sleep Quality Index (PSQI). By doing so, we aim to elucidate the relationship between tobacco use, sleep structure, and sleep quality. Furthermore, we endeavor to explore the factors influencing OSA in both tobacco and non-tobacco using populations.
Materials and Methods
Participants, Inclusion and Exclusion Criteria
This retrospective study involved a total of 1017 participants who were selected from the Sleep Medicine Center of the Second Affiliated Hospital of Fujian Medical University (Fujian Provincial Sleep Medicine Center) (Figure 1).
 |
Figure 1 Inclusion and exclusion process in this study. |
The primary inclusion criteria for this study were as follows: participants were individuals who were admitted to the hospital between January 2020 and January 2023 with suspected sleep disorders. To ensure consistent sleep structure parameters, the age range of participants was restricted to 15–64 years, excluding individuals in childhood and older adults due to potential variations in sleep patterns. Before enrollment, all participants provided voluntary informed consent, which was an essential component of the study protocol.
The primary exclusion criteria for this study encompassed the following: individuals undergoing treatments that could impact muscle function and sleep structure, those diagnosed with neurological disorders or experiencing neuropathic pain, individuals with active inflammation or confirmed active malignancy, individuals with severe respiratory or cardiac insufficiency (Including hypertension, chronic obstructive pulmonary disease, and asthma, etc)., those with severe psychiatric disorders, cognitive impairment, and individuals displaying non-compliance during the study duration.17 And individuals with comorbidities of sleep-related disorders which are related to sleep architecture such as bruxism or periodic limb movement during sleep.
To minimize potential interference with sleep quality and sleep structure, participants were instructed to abstain from consuming substances known to affect the nervous system and sleep, such as tea, coffee, alcohol, and eszopiclone, for 3 days preceding the polysomnography test. Moreover, participants with a history of prolonged usage of the aforementioned substances, or other addictive substances, were excluded from the study to mitigate potential withdrawal reactions that could impact the examination results. On the day of the polysomnography test, participants were advised to refrain from engaging in strenuous physical exercise. The polysomnography test was conducted after 2 hours of tranquil rest in a suitable environment, ensuring optimal conditions for data acquisition.
Sample Size
The sample size was determined according to the formula (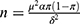 ).18 π is the detection rate of tobacco use. In order to minimize the error, we set π as 0.3 (Based on the smoking prevalence rate of 26.6% among people ≥15 years old in China).19 δ is the allowable error, it is the maximum error of the sample rate and the overall rate that should be controlled range, we set the allowable error is 0.03π. We set α as 0.05, a confidence interval is taken as 95%, and then the corresponding μ is 1.96. Using this method, we determine that n is 498 (patients with OSA), and considering the 20% lost interview rate, 623 patients with OSA need to be surveyed. To minimize error, there were 1017 patients with OSA in this study.
).18 π is the detection rate of tobacco use. In order to minimize the error, we set π as 0.3 (Based on the smoking prevalence rate of 26.6% among people ≥15 years old in China).19 δ is the allowable error, it is the maximum error of the sample rate and the overall rate that should be controlled range, we set the allowable error is 0.03π. We set α as 0.05, a confidence interval is taken as 95%, and then the corresponding μ is 1.96. Using this method, we determine that n is 498 (patients with OSA), and considering the 20% lost interview rate, 623 patients with OSA need to be surveyed. To minimize error, there were 1017 patients with OSA in this study.
Polysomnography
PSG is internationally acknowledged as the gold standard diagnostic tool for assessing OSA. In this study, the sleep structure of the participants was meticulously evaluated using PSG. PSG is a comprehensive diagnostic tool that employs the monitoring of various bioelectrical signals, including electroencephalogram (EEG), electrooculogram (EOG), electromyogram (EMG), electrocardiogram (ECG), and airflow detectors. In addition to these bioelectrical measurements, PSG incorporates visual recordings and audio recordings throughout the nocturnal period to provide a comprehensive assessment of sleep patterns and associated physiological parameters.17,20,21
To ensure the precision of the examination outcomes, the fitting of PSG equipment was performed on each patient by a skilled sleep technician affiliated with the Sleep Medicine Center. The PSG fitting procedures adhered to the established guidelines outlined in the American Academy of Sleep Medicine (AASM) recommendations, specifically referring to “The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications, Version 2.3”.22 The PSG results were mutually subjected to simultaneous analysis and evaluation by two experienced physicians specializing in pulmonary and critical care. In events of discordance in result analysis, a third pulmonary and critical care physician, equally qualified and experienced, will undertake a reassessment. Following this reassessment, the three physicians will discuss their findings. Consensus among them will be a prerequisite for including the participant’s findings in the study. The analysis and evaluation process followed the guidelines recommended by the AASM. The PSG recordings were conducted from 22:00 on the day of the study until 06:00 the following day, encompassing a total duration of eight hours. To accommodate individual circumstances, fluctuations in the PSG duration were limited to ±1 hours.
OSA Definitions
The ASSM provides the following classification for OSA severity.22 OSA severity was categorized into three levels: mild, moderate, and severe. Mild OSA was defined as an apnea-hypopnea index (AHI, AHI is number of apneas plus hypopneas per hour of sleep22) ranging from 5 to 15, accompanied by one or more of the following sleep symptoms: (1) Nocturnal breathing disturbances, such as snoring or apnea episodes (occurring during snoring, gasping, or sleep); (2) Daytime sleepiness, fatigue, or non-refreshing sleep despite adequate opportunities for sleep, not attributable to another mental disorder (including sleep disorders) or a somatic condition. Moderate OSA was defined by an AHI between 15 and 30. Severe OSA was defined as an AHI exceeding 30.
Sleep Structure Staging Definition
The sleep structure encompasses two main stages: non-rapid eye movement sleep (NREM) and rapid eye movement sleep. NREM sleep is further divided into three stages: N1, N2, and N3. Stages N1 and N2 collectively represent the light sleep stage, while stage N3 is referred to as the deep sleep stage, also known as SWS.
It is important to note that there are variations in sleep structure across different age groups, leading to ongoing debates regarding the staging of sleep structure worldwide. In accordance with the recommendations outlined in the Principles and Practice of Sleep Medicine (4th Edition), we established specific percentage ranges to define the normal distribution of each sleep stage for the participants in this study. These ranges are as follows: 2–5% for stage N1, 45–55% for stage N2, 10–20% for stage N3, and 20–25% for REM sleep (stage R).23 In this study, the participants’ sleep stages (PSG results) were manually analyzed and assessed by two qualified respiratory and critical care physicians, following the recommendations of The AASM Manual for The Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications, Version 2.3.
Pittsburgh Sleep Quality Index
We used the PSQI scale to assess participants’ sleep quality in the last 1 month. The Chinese version of the PSQI has good reliability and validity, with a retest reliability of 0.81, a specificity of 90.2%, and a sensitivity of 98.3%.24 The PSQI scale comprises 7 components, encompassing a total of 18 items. These components assess various aspects of sleep quality and related factors. The components of the PSQI scale include sleep quality, sleep latency (time taken to fall asleep), sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction.18 Each component is scored on a scale ranging from 0–3. The total PSQI score is the sum of the cumulative scores for each of the components. The total PSQI score ranges from 0–21, with higher scores indicating poorer sleep quality. 0–5 is classified as great sleep quality, 6–10 is common sleep quality, 11–15 is bad sleep quality, and 16–21 is terrible sleep quality. A total PSQI score>7 is the criterion for poor sleep quality.18
Tobacco Use and the Heaviness of Smoking Index Definition
Participants who had smoked at least one traditional cigarette in the past 1 month, and had smoked more than 100 traditional cigarettes in their lifetime, were defined as having a valid history of tobacco use.25 Participants who matched a valid history of tobacco use and had no previous history of quitting as well as no current quitting status were included in the tobacco use population. Never-smokers (non-tobacco users) were subjects who had never smoked for 1 year or more, and had a total cigarette exposure of less than 0.5 pack-years.26
The Heaviness of Smoking Index (HSI) was used to measure participants’ tobacco addiction, which consisted of 2 items, including the number of cigarettes currently smoked per day and the time they woke up in the morning to smoke for the first time.27 The number of cigarettes currently smoked per day: ≤10 cigarettes is a 0 score, 11–20 cigarettes is a 1 score, 21–30 cigarettes is a 2 score, >30 cigarettes is a 3 score. The time they woke up in the morning to smoke for the first time: more than 60 minutes is a 0 score, 31–60 minutes is 1 score, 6–30 minutes is a 2 score, ≤5 minutes is a 3 score. Each item score ranges from 0–3, and the total HSI score is the sum of the two item scores. The total HSI score ranges from 0–6, and those with an HSI ≥4 are defined as severely tobacco dependent.28 In addition, body mass index (BMI) was defined as follows: <18.5 as thin, 18.5–24 as normal weight, 24–28 as overweight, and ≥28 as obese.29
Data Analysis
Data analysis for this study was conducted using SPSS 27.0 software, which is protected by copyright license. To determine the statistical significance of differences between variables, we initially employed a one-way analysis of variance (ANOVA) test using SPSS 27.0. Significance was determined at a threshold of p<0.05, indicating statistically significant differences. The model was optimized through iterative attempts, considering main effects and full factorial interactions. Subsequently, a multi-logistic regression analysis was performed using the optimized model. All statistical tests were two-tailed, with a significance level (α) set at 0.05.
When exploring sleep quality and sleep structure, participants were divided into two categories: those who used tobacco and those who did not. In the analysis conducted on the tobacco-using and non-tobacco-using population, a one-way ANOVA was performed with “sleep structure (Stage N1/N2/N3/R)” and “total PSQI score” as dependent variables. The independent variables included “OSA grading, gender, BMI, and sleep quality”. There were no individuals with a BMI less than 18.5, so the underweight category was not included in the analysis.
When exploring the severity of tobacco addiction, the tobacco-using population was further divided into two groups: severe tobacco addiction and non-severe tobacco addiction. A one-way ANOVA was conducted with “sleep structure (Stage N1/N2/N3/R) and total PSQI score” as the dependent variable, and “OSA grading, gender, BMI, and sleep quality” as independent variables.
Sleep structure (Stage N1/N2/N3/R) shows the percentage of total sleep, PSQI is presented as a total score, with higher scores representing poorer sleep quality. Scores greater or equal to 7 were defined as “poor sleep quality”. Sleep quality shows the boundary between the good and bad of sleep. Variables with correlation were then tested for Pearson correlation (labelled r).
Results
Demographic Characteristics of Participants
A total of 1017 participants diagnosed with OSA were included in this study. The study population consisted of 288 (28.3%) tobacco users and 729 (71.7%) non-tobacco users, with a mean age of 42.6±11.8 years. Specific details are shown in Table 1.
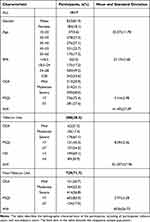 |
Table 1 Demographic Characteristics of Participants |
Sleep Quality and Sleep Structure in Tobacco/Non-Tobacco Using Populations
The results indicated a significant correlation between OSA grading and sleep stages (Stage N1/N3) (Pa<0.001, Pc<0.001) (ra= 0.207, rc= 0.083) in the non-tobacco-using population. However, no significant correlation was observed between OSA grading and sleep stages (Stage N2/R) or the total PSQI score. Gender showed a significant correlation with sleep stages (Stage N1/N3) (Pa<0.001, Pc<0.001) (ra= 0.132, rc= −0.205) but not with sleep stages (Stage N2/R) or the total PSQI score. BMI demonstrated significant correlations with sleep stages (Stage N2/N3) (Pb=0.026, Pc=0.007) (rb= 0.044, rc=- 0.116) and the total PSQI score (Pe= 0.038) (rc= 0.071), while no significant correlation was found with sleep stages (Stage N1/R). Sleep quality did not exhibit a significant correlation with sleep stages (Stage N1/N2/ N3/R). Specific details are shown in Table 2 and Figures 2–4.
 |
Table 2 One-Way ANOVA for Sleep Structure and Sleep Quality in Tobacco/Non-Tobacco Using Populations |
 |
Figure 3 Comparison of sleep structure among tobacco/non-tobacco using populations (OSA Grade/Gender). |
 |
Figure 4 Comparison of sleep structure among tobacco/non-tobacco using populations (BMI/Sleep Quality). |
The results indicated a significant correlation between OSA grading and sleep stages (Stage N1/N3/R) (Pa<0.001, Pc<0.001, Pd=0.025) (ra= 0.228, rc= −0.287, rd= −0.125) in the tobacco-using population. However, no significant correlation was observed between OSA grading and sleep stages (Stage N2) or the total PSQI score. Gender showed a significant correlation with sleep stages (Stage R) (Pd=0.004) (rd= −0.170) and the total PSQI score (Pd=0.027) (rd= 0.131), but not with sleep stages (Stage N1/N2/N3). BMI did not exhibit a significant correlation with sleep stages (Stage N1/N2/N3/R) or the total PSQI score. Sleep quality demonstrated a significant correlation with sleep stages (Stage N1/N2) (Pa<0.001, Pb<0.001) (ra= 0.189, rb= −0.210), but not with sleep stages (Stage N3/R).
Comparison of Sleep Quality and Sleep Structure Between Severely Addicted and Non-Severely Addicted Tobacco Users
In the non-severe tobacco addiction population, the analysis revealed a significant correlation between OSA grading and sleep stages (Stage N1/N3/R) (Pa=0.002, Pc<0.001, Pd=0.016) (ra= 0.246, rc= −0.355, rd= −0.195), while no significant correlation was found with sleep stages (Stage N2) or the total PSQI score. Gender exhibited a significant correlation with sleep stages (Stage R) (Pd=0.002) (rd= −0.213) and the total PSQI score, but not with sleep stages (Stage N1/N2/N3). BMI demonstrated a significant correlation with sleep stages (Stage R) (Pd=0.017) (rd= −0.161), but no significant correlation was observed with sleep stages (Stage N1/N2/N3) or the total PSQI score. Sleep quality showed a significant correlation with sleep stages (Stage N1/N2) (Pa=0.002, Pb<0.001) (ra= 0.207, rb= −0.238), but not with sleep stages (Stage N3/R). Specific details are provided in Table 3 and Figures 5–7.
 |
Table 3 One-Way ANOVA for Sleep Structure and Sleep Quality in Severe Addiction/Non-Severe Addiction Populations |
 |
Figure 5 Comparison of sleep structure in severe addiction/non-severe addiction populations (OSA Grade/BMI). |
 |
Figure 6 Comparison of sleep structure in severe addiction/non-severe addiction populations (Gender/Sleep Quality). |
 |
Figure 7 Comparison of PSQI total scores in severe addiction/non-severe addiction populations. |
In the severe tobacco addiction population, the analysis indicated a significant correlation between OSA grading and sleep stages (Stage N1/N3) (Pa=0.031, Pc=0.025) (ra= 0.153, rc= −0.116), while no significant correlation was found with sleep stages (Stage N2/R) or the total PSQI score. There was no significant correlation between gender and sleep stages (Stage N1/N2/N3/R) or the total PSQI score. Likewise, there was no significant correlation between BMI and sleep stages (Stage N1/N2/N3/R) or the total PSQI score. Additionally, there was no significant correlation between sleep quality and sleep stages (Stage N1/N2/N3/R).
Multi-Logistics Regression Analysis of Factors Influencing OSA in Tobacco/Non-Tobacco Using Populations
In the non-tobacco-using population, we employed “age, gender, BMI, and sleep quality” as independent variables, while “OSA grading” served as the dependent variable. Multi-logistic regression analysis was conducted with mild OSA as the control (P<0.05 for inclusion and P>0.10 for exclusion). Our results demonstrate that relative to mild OSA, moderate OSA was significantly correlated with gender (OR=0.56[0.340–0.921], P=0.022), but was not significantly correlated with Age, BMI or sleep quality. Severe OSA showed a significant correlation with gender (OR=0.259[0.164–0.410], P<0.001) and BMI (OR=0.26[0.146–0.462], P<0.001 // OR=0.544[0.338–0.878], P=0.013), but not with age or sleep quality. Detailed information can be found in Table 4 and Figure 8. The rows with β of 0 and OR of 1 in Table 4 were utilized as controls for this variable.
 |
Table 4 Multi-Logistics Regression Analysis of Factors Influencing OSA Grading in Tobacco and Non-Tobacco Using Populations |
 |
Figure 8 Protection and risk factors for different grades of OSA in tobacco/non-tobacco using populations. |
In the tobacco-using population, we utilized “age, gender, BMI, sleep quality, and HIS” as independent variables, and “OSA grading” as the dependent variable. Multi-logistic regression analysis was performed with mild OSA as the control (P<0.05 for inclusion and P>0.10 for exclusion). Compared with mild OSA, moderate OSA was significantly correlated with gender (OR=0.184[0.037–0.919], P=0.039), but not age, BMI, sleep quality or HSI. Severe OSA was significantly correlated with Age ([35–45), OR=2.713[1.072–6.863], P=0.035), Gender (OR=0.165[0.051–0.534], P=0.003) and BMI (normal weight/overweight) (ORnormal-weight=0.218[0.075–0.637], Pnormal-weight=0.005//ORoverweight=0.451[0.224–0.908], Poverweight=0.026), but not with sleep quality or HSI.
Multi-Logistics Regression Analysis of Factors Influencing Sleep Stages
The sleep structure was categorized into normal and abnormal values, with the normal values of sleep stages (Stage N1/N3/R) serving as the baseline control. A multi-logistic regression analysis was conducted with “tobacco use, sleep quality, OSA grading, age, gender, and BMI” as independent variables and abnormal sleep stages (Stage N1/N3/R) as the dependent variable (P<0.05 for inclusion and P>0.10 for exclusion). N1 sleep was significantly associated with tobacco use (OR=0.585[0.308–0.732], P=0.02) and sleep quality (OR=0.328[0.158–0.683], P=0.003), but no significant associations were observed with OSA grade, age, gender or BMI. N3 sleep was significantly associated with OSA grading (ORMild =0.701[0.496–0.991], PMild =0.044 // ORModerate =0.506[0.362–0.706], PModerate-OSA<0.001), but did not show a significant association with tobacco use, sleep quality, age, gender or BMI. There was a significant association between R stage and BMI (ORnormal-weight =0.350[0.181–0.677], Pnormal-weight =0.002 // ORoverweight =0.506[0.292–0.879], Poverweight =0.016), but not with tobacco use, sleep quality, OSA grade, age or gender. Specific details are shown in Table 5 and Figure 9. The rows with β of 0 and OR of 1 in Table 5 were used as controls for this variable.
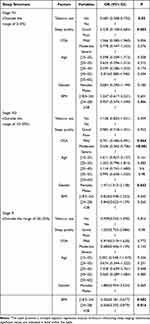 |
Table 5 Multi-Logistics Regression Analysis of Factors Influencing Sleep Stages |
 |
Figure 9 Protection and risk factors for sleep stage. |
Discussion
Obstructive sleep apnea-hypopnea syndrome represents the predominant form of sleep apnea, characterized by recurrent episodes of sleep fragmentation caused by repeated awakenings. These disruptions contribute to daytime sleepiness, memory impairment, and cognitive dysfunction in affected individuals, thereby increasing the risk of inadvertent accidents.30 OSA is associated with cardiovascular and cerebrovascular diseases, diabetes, neurocognitive dysfunction, and other diseases, it is a huge burden on society, and only the United States spends at least 10 billion dollars a year on it above the health care costs.31 However, what is even more worrying is that there are still significant shortcomings in the diagnosis and treatment of OSA in clinical practice.3,32,33 The association between OSA and tobacco use remains a subject of ongoing debate, and a definitive conclusion regarding their relationship has yet to be established with certainty.34
Tobacco use has been shown to approximately double the risk of developing sleep disorders when comparing tobacco-using groups to non-users.30 In our study, we observed a significant elevation in the prevalence of poor sleep quality among individuals with OSA who were tobacco users, as opposed to non-tobacco users within the OSA population. Tobacco withdrawal may increase sleep instability due to the inability of tobacco users to smoking after falling asleep.35 It is worth mentioning that tobacco promotes the release of neurotransmitters by stimulating cholinergic receptors in the central nervous system, and the decrease in the release of relevant neurotransmitters at night may be related to the decrease in the concentration of tobacco in the bloodstream after falling asleep, which is a property that may be involved in the regulation of the sleep-wake cycle, thereby leading to fluctuations in sleep.36 Therefore, we propose that the association between tobacco use and compromised sleep quality can be attributed to the interplay of tobacco’s dual effects, encompassing both the euphoric and withdrawal-related outcomes, along with the potential presence of psychological disorders prevalent among individuals with OSA, such as depression and anxiety. These overlapping factors likely contribute to the complex nature of the relationship between tobacco use and impaired sleep quality in OSA patients.37 Additionally, in exploring sleep quality across different degrees of tobacco dependence, we also found that rates of poor sleep quality did not continue to increase with tobacco dependence.
OSA patients frequently manifest clinical symptoms such as lethargy, depression, and cognitive dysfunction. Intriguingly, certain studies have suggested that tobacco use might serve as a form of self-medication among individuals with OSA.37,38 People with OSA may be ostracized by their peers due to factors such as loud nighttime snoring, daytime sleepiness, and decreased work productivity.35 While tobacco use has been shown to be potentially harmful,31 and smoking induces upper airway inflammation or increases airway resistance leading to OSA symptoms,30 it is worth noting that tobacco has been found to increase ventilation and decrease upper airway resistance, thereby counteracting the symptoms of OSA.35 The findings regarding the relationship between tobacco use and OSA appear contradictory. The apparent contradiction in these findings warrants further investigation and a more comprehensive understanding of the complex interactions between tobacco use and OSA. Importantly, there is insufficient evidence from long-term prospective studies to establish a definitive correlation between smoking cessation and the improvement of OSA.14
Several studies have demonstrated that long-term tobacco use in adults can disrupt sleep structure, specifically characterized by a notable increase in lighter sleep stages (Stage N1 and Stage N2) and a reduction in slow wave sleep (Stage N3), within the continuous sleep structure. However, no significant differences were observed between the sleep structures of total sleep time and REM sleep in individuals who use tobacco compared to healthy adults who do not use tobacco.6 Our investigation revealed significant alterations in sleep structure among individuals with OSA who engaged in tobacco use. Specifically, we observed a notable elongation of the N1 stage (light sleep) and a concurrent reduction in the N3 stage (deep sleep). Notably, the impact of tobacco on sleep structure appeared to be more prominent as the severity of OSA worsened. We hypothesize that these changes may be attributed to the influence of tobacco on the cholinergic system, which in turn disrupts the delicate balance between wakefulness and sleep states.39,40 Furthermore, tobacco use has been shown to elevate nasal resistance and cause inflammation of the nasal mucosa. And exposure to tobacco smoke irritates the bronchial mucosa and triggers acute reflex constriction of the airways, subsequently increasing airway resistance. These effects of tobacco use further exacerbate the symptoms of OSA.41 We postulate that the observed heightened impact of tobacco on sleep structure, particularly as the severity of OSA worsens, may be associated with this characteristic. More interestingly, we found that a shortening of the R stage occurred in severe OSA compared to mild-moderate OSA in the tobacco-using population. It is imperative to note that REM sleep predominantly occurs in the latter half of the night, while the concentration of tobacco in the blood gradually diminishes after the initiation of sleep. Consequently, the impact of nicotine on the central nervous system also diminishes gradually.42 We hypothesize that the reason behind this observation is likely due to a combination of the direct euphoric effect of tobacco being compromised by the presence of airway obstruction and the concurrent withdrawal effect of tobacco.43
Subsequently, we conducted further investigations to examine the influence of the degree of tobacco dependence on sleep structure. Our findings revealed that the lengthening of the N1 stage and the reduction of the N3 stage were more pronounced in the population with severe tobacco dependence as compared to those with non-severe tobacco dependence. On one hand, the effect of tobacco on sleep structure depends not only on the duration of use but also the dose used.31 In the slow wave sleep stage, a reduction of tobacco dosage is accompanied by an augmentation in duration.44 We speculate that the occurrence of a shortened N3 stage in this population may be associated with prolonged and intensive tobacco use over an extended period. On the other hand, as stated previously, we speculate that the prolonging of the N1 stage is partially attributable to the alteration of arousal and sleep by tobacco use.
Moreover, we examined the factors that influenced abnormal values in sleep structure. Intriguingly, tobacco use, sleep quality, and age emerged as influential factors in relation to the N1 stage. Recurrent sleep fragmentation and sleep arousal significantly impact sleep quality, particularly affecting the light sleep stage. Additionally, tobacco has been found to enhance mood, thereby exerting some influence on sleep quality.45,46 In addition, we observed a correlation between the severity of OSA and the N3 sleep stage. As the OSA grading worsens, there is an increase in the frequency and duration of apnea events. Patients with OSA experience hypoxia and upper airway occlusion during sleep, leading to the need for increased ventilation. However, the heightened activity of the vagus nerve at night makes it challenging to prevent sleep fragmentation and arousal caused by hypopnea or apnea.47 This feature also exacerbates the reduction of SWS and REM.48 While our multivariate regression analyses did not reveal a significant impact of tobacco on slow wave sleep, our previous univariate analyses did suggest an effect. We postulate that the discrepancy between these findings may be attributed to ongoing uncertainties in establishing the normal range of sleep structure values.23 Additionally, we observed that the REM stage was influenced by BMI. It is worth noting that although it is conventionally believed that patients with OSA typically experience difficulty in directly entering REM sleep after awakening and instead transition gradually from NREM sleep, we speculate that patients with abnormal body weight may encounter prolonged deficiencies in the REM stage due to recurrent sleep apnea, which perpetuates their sleep in the NREM stage. In compensatory situations, patients may rapidly enter the deficient REM stage shortly after falling asleep. It is worth noting that individuals of lower socio-economic status often face the need to engage in multiple concurrent jobs or reside in environments characterized by high levels of noise. These circumstances contribute to increased levels of stress experienced by such individuals, subsequently resulting in sleep deprivation.49 Our study did not control the potential confounding effect of participants’ socioeconomic status, which could partially account for this finding.
It is widely acknowledged that the primary risk factors for OSA include gender, age, weight, and craniofacial abnormalities.35 To investigate whether tobacco use constitutes an independent risk factor for OSA, Kashyap et al conducted a study involving over 100 individuals with OSA and corresponding healthy controls, ultimately determining that tobacco use is indeed an independent risk factor for OSA.50 Additionally, Moreno et al discovered that tobacco use was an independent risk factor for OSA through a survey of over 10,000 drivers using the Berlin Questionnaire (OR 1.16, P=0.014).51 In our current study, we were unable to conduct a specific investigation to ascertain whether tobacco is an independent risk factor for OSA, as we included all patients with OSA in the study. However, interestingly, we observed that the severity of tobacco dependence did not impact the progression of OSA in the tobacco-using OSA population. We speculate that this observation may be attributed to a balanced interplay between the mixed effects of tobacco (a combination of euphoric and withdrawal effects) in individuals who have used tobacco for an extended duration.35
Furthermore, we found that the progression of OSA was influenced by gender and BMI in the non-tobacco-using population, while it was influenced by age, gender, and BMI in the tobacco-using population. The two populations exhibited differences primarily in terms of age. Long-term tobacco users experience prolonged exposure to hypoxia, which can exacerbate OSA.52 We postulate that the effect of tobacco may not have reached equilibrium for those in the 35–45 age group, and that the effects of hypoxia are yet to be seen in the younger age group, whereas the older age group may have developed equilibrium adaption. Among middle-aged patients aged between 30 to 49, males are three times more likely to develop moderate-to-severe OSA compared to females.53 Consistent with our study’s findings, gender was identified as an influential factor in moderate-to-severe OSA regardless of tobacco use, with females exhibiting a protective effect against OSA in comparison to males. Additionally, multiple studies have indicated that up to 70% of patients undergoing weight loss treatment are diagnosed with OSA.32 This aligns with our results, which demonstrate a significant association between severe OSA and BMI.
Strengths and Limitations
To the best of our knowledge, this study represents the first extensive examination of the effects of tobacco on both macroscopic and microscopic aspects of sleep structure in individuals with OSA. By integrating subjective and objective measures, we have addressed the limitations of previous studies that solely relied on either subjective self-reports or objective assessments. Consequently, we have bridged important gaps in our understanding of the impact of tobacco on sleep structure and quality.
It is important to acknowledge certain limitations of our study. Firstly, this investigation was retrospective in nature, lacking the ability to capture longitudinal changes in sleep quality and structure due to varying durations of tobacco use. Additionally, the utilization of the initial night’s polysomnography introduces potential confounding factors associated with first-night effects. Despite these limitations, our findings shed crucial light on the significant influence of tobacco use on sleep structure and quality in individuals with OSA.
Conclusion
This study elucidated the impact of tobacco use on sleep quality and sleep structure in individuals with obstructive sleep apnea (OSA). Our findings revealed a substantial deterioration in sleep quality among the OSA population who engaged in tobacco use, as evidenced by a significantly higher prevalence of poor sleep quality compared to their non-tobacco-using counterparts. Notably, the rate of poor sleep quality did not exhibit a linear increase with escalating levels of tobacco dependence. Furthermore, tobacco use was associated with alterations in light sleep stages and slow wave sleep in the OSA population. Importantly, the effects of tobacco dependence on these sleep structures were more pronounced in individuals with more severe OSA.
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
During the preparation of this work the author(s) used [ChatGPT 3.5] in order to [improve language and readability]. After using this tool, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Ethics Approval
The study was approved by the Ethics Committee of the Second Affiliated Hospital of Fujian Medical University. Prior to completing the survey, participants were informed that participation was voluntary and that their data were anonymous. Participants gave informed consent to their participation in the survey being published. The informed consent was obtained from all subjects and/or their legal guardian(s). The study complies with the Declaration of Helsinki.
Acknowledgments
The authors thank all participants for contributing to this work.
Funding
Joint funds for the innovation of science and technology, Fujian province (2023Y9262); The Natural Science Foundation of Fujian Province (2023J01741, 2023J01102); Talent Training Project of Fujian Respiratory Medical Center (HXZX202202); The Fujian Medical University Sail Fund Project (2021QH1111).
Disclosure
The authors have no conflicts of interest to declare related to the current study.
References
1. Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi:10.1016/S2213-2600(19)30198-5
2. Chung F, Abdullah HR, Liao P. STOP-bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631–638. doi:10.1378/chest.15-0903
3. Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70.
4. Zhang B, Lu S, Guo H, Xu J, Xiao Z, Tang J. Relationship between ODI and sleep structure of obstructive sleep apnea and cardiac remodeling. Sleep Breath. 2023;28(1):173–181. doi:10.1007/s11325-023-02872-7
5. Dijk DJ. Regulation and functional correlates of slow wave sleep. J Clin Sleep Med. 2009;5(2 Suppl):S6–15. doi:10.5664/jcsm.5.2S.S6
6. Catoire S, Nourredine M, Lefebvre S, et al. Tobacco-induced sleep disturbances: a systematic review and meta-analysis. Sleep Med Rev. 2021;60:101544. doi:10.1016/j.smrv.2021.101544
7. Gordon HW. Differential Effects of Addictive Drugs on Sleep and Sleep Stages. J Addict Res. 2019;3(2):1.
8. Conroy DA, Arnedt JT. Sleep and substance use disorders: an update. Curr Psychiatry Rep. 2014;16(10):487. doi:10.1007/s11920-014-0487-3
9. Jaehne A, Loessl B, Bárkai Z, Riemann D, Hornyak M. Effects of nicotine on sleep during consumption, withdrawal and replacement therapy. Sleep Med Rev. 2009;13(5):363–377. doi:10.1016/j.smrv.2008.12.003
10. De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci. 2011;34:105–130.
11. Bertrand D. The possible contribution of neuronal nicotinic acetylcholine receptors in depression. Dialogues Clin Neurosci. 2005;7(3):207–216. doi:10.31887/DCNS.2005.7.3/dbertrand
12. Boussoffara L, Boudawara N, Sakka M, Knani J. Smoking habits and severity of obstructive sleep apnea hypopnea syndrome. Rev Mal Respir. 2013;30(1):38–43. doi:10.1016/j.rmr.2012.08.009
13. Krishnan V, Dixon-Williams S, Thornton JD. Where there is smoke…there is sleep apnea: exploring the relationship between smoking and sleep apnea. Chest. 2014;146(6):1673–1680. doi:10.1378/chest.14-0772
14. Zeng X, Ren Y, Wu K, et al. Association between smoking behavior and obstructive sleep apnea: a systematic review and meta-analysis. Nicotine Tob Res. 2023;25(3):364–371. doi:10.1093/ntr/ntac126
15. Sweetman A, Lack L, McEvoy RD, et al. Bi-directional relationships between co-morbid insomnia and sleep apnea (COMISA). Sleep Med Rev. 2021;60(101519):101519. doi:10.1016/j.smrv.2021.101519
16. Krakow B, Melendrez D, Ferreira E, et al. Prevalence of insomnia symptoms in patients with sleep-disordered breathing. Chest. 2001;120(6):1923–1929. doi:10.1378/chest.120.6.1923
17. Frosztega W, Wieckiewicz M, Nowacki D, et al. Polysomnographic assessment of effects of tobacco smoking and alcohol consumption on sleep bruxism intensity. J Clin Med. 2022;11(24):7453. doi:10.3390/jcm11247453
18. Ji W, Shi L, Lin X, et al. The relationship between sleep quality and daytime dysfunction among college students in China during COVID-19: a cross-sectional study. Front Public Health. 2023;11:1253834. doi:10.3389/fpubh.2023.1253834
19. China CW. TWCoRoHHoSi: 2020 report on health hazards of smoking in china: an updated summary. Chin Circ J. 2021;36:937–952.
20. Ommerborn MA, Walentek N, Bergmann N, Franken M, Gotter A, Schäfer R. Validation of a new diagnostic method for quantification of sleep bruxism activity. Clin Oral Investig. 2022;26(6):4351–4359. doi:10.1007/s00784-022-04398-w
21. Wang J, Zhao S, Zhou Y, et al. Narcolepsy diagnosis with sleep stage features using PSG recordings. IEEE Trans Neural Syst Rehabil Eng. 2023;31:3619–3629. doi:10.1109/TNSRE.2023.3312396
22. Berry RB, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV; for theAmerican Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and AssociatedEvents: rules, Terminology and Technical Specifications, Version 2.3. American Academy of Sleep Medicine: Darien, lllinois; 2016.
23. Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi:10.1093/sleep/27.7.1255
24. Guo S, Sun W, Liu C, Wu S. Structural validity of the Pittsburgh sleep quality index in Chinese undergraduate students. Front Psychol. 2016;7(1126). doi:10.3389/fpsyg.2016.01126
25. Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: international tobacco control four-country survey. Am J Prev Med. 2013;44(3):207–215. doi:10.1016/j.amepre.2012.10.018
26. Shi L, Dai X, Yan F, et al. Novel lipidomes profile and clinical phenotype identified in pneumoconiosis patients. J Health Popul Nutr. 2023;42(1):55. doi:10.1186/s41043-023-00400-7
27. Brown J, Beard E, Kotz D, Michie S, West R. Real-world effectiveness of e-cigarettes when used to aid smoking cessation: a cross-sectional population study. Addiction. 2014;109(9):1531–1540. doi:10.1111/add.12623
28. Schnoll RA, Goren A, Annunziata K, Suaya JA. The prevalence, predictors and associated health outcomes of high nicotine dependence using three measures among US smokers. Addiction. 2013;108(11):1989–2000. doi:10.1111/add.12285
29. Ji L, Jiang H, Cheng Z, et al. A Phase 2 randomised controlled trial of mazdutide in Chinese overweight adults or adults with obesity. Nat Commun. 2023;14(1):8289. doi:10.1038/s41467-023-44067-4
30. Pataka A, Kotoulas S, Kalamaras G, et al. Does smoking affect OSA? What about smoking cessation? J Clin Med. 2022;11(17):5164. doi:10.3390/jcm11175164
31. Htoo A, Talwar A, Feinsilver SH, Greenberg H. Smoking and sleep disorders. Med Clin North Am. 2004;88(6):1575–1591. doi:10.1016/j.mcna.2004.07.003
32. Ravesloot MJ, van Maanen JP, Hilgevoord AA, van Wagensveld BA, de Vries N. Obstructive sleep apnea is underrecognized and underdiagnosed in patients undergoing bariatric surgery. Eur Arch Otorhinolaryngol. 2012;269(7):1865–1871. doi:10.1007/s00405-012-1948-0
33. Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6(2):49–54. doi:10.1055/s-2002-32318
34. Deleanu OC, Pocora D, Mihălcuţă S, Ulmeanu R, Zaharie AM, Mihălţan FD. Influence of smoking on sleep and obstructive sleep apnea syndrome. Pneumologia. 2016;65(1):28–35.
35. Lin YN, Li QY, Zhang XJ. Interaction between smoking and obstructive sleep apnea: not just participants. Chin Med J. 2012;125(17):3150–3156.
36. Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70(4):531–549. doi:10.1016/S0091-3057(01)00651-7
37. Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994;154(19):2219–2224. doi:10.1001/archinte.1994.00420190121014
38. Schrand JR. Is sleep apnea a predisposing factor for tobacco use? Med Hypotheses. 1996;47(6):443–448. doi:10.1016/S0306-9877(96)90155-3
39. Khateb A, Fort P, Pegna A, Jones BE, Mühlethaler M. Cholinergic nucleus basalis neurons are excited by histamine in vitro. Neuroscience. 1995;69(2):495–506. doi:10.1016/0306-4522(95)00264-J
40. Saint-Mleux B, Eggermann E, Bisetti A, et al. Nicotinic enhancement of the noradrenergic inhibition of sleep-promoting neurons in the ventrolateral preoptic area. J Neurosci. 2004;24(1):63–67. doi:10.1523/JNEUROSCI.0232-03.2004
41. Kjaergaard T, Cvancarova M, Steinsvaag SK. Smoker’s nose: structural and functional characteristics. Laryngoscope. 2010;120(7):1475–1480. doi:10.1002/lary.20967
42. Zhang L, Samet J, Caffo B, Punjabi NM. Cigarette smoking and nocturnal sleep architecture. Am J Epidemiol. 2006;164(6):529–537. doi:10.1093/aje/kwj231
43. Colrain IM, Trinder J, Swan GE. The impact of smoking cessation on objective and subjective markers of sleep: review, synthesis, and recommendations. Nicotine Tob Res. 2004;6(6):913–925. doi:10.1080/14622200412331324938
44. Davila DG, Hurt RD, Offord KP, Harris CD, Shepard JW Jr. Acute effects of transdermal nicotine on sleep architecture, snoring, and sleep-disordered breathing in nonsmokers. Am J Respir Crit Care Med. 1994;150(2):469–474. doi:10.1164/ajrccm.150.2.8049831
45. Mihailescu S, Guzmán-Marín R, Domínguez Mdel C, Drucker-Colín R. Mechanisms of nicotine actions on dorsal raphe serotoninergic neurons. Eur J Pharmacol. 2002;452(1):77–82. doi:10.1016/S0014-2999(02)02244-6
46. Guzmán-Marín R, Alam MN, Mihailescu S, Szymusiak R, McGinty D, Drucker-Colín R. Subcutaneous administration of nicotine changes dorsal raphe serotonergic neurons discharge rate during REM sleep. Brain Res. 2001;888(2):321–325. doi:10.1016/S0006-8993(00)03104-8
47. Ryan CM, Wilton K, Bradley TD, Alshaer H. In-hospital diagnosis of sleep apnea in stroke patients using a portable acoustic device. Sleep Breath. 2017;21(2):453–460. doi:10.1007/s11325-016-1438-5
48. Macarthur KE, Bradley TD, Ryan CM, Alshaer H. Dissociation between objectively quantified snoring and sleep quality. Am J Otolaryngol. 2020;41(1):102283. doi:10.1016/j.amjoto.2019.102283
49. Petrovic D, Haba-Rubio J, de Mestral Vargas C, et al. The contribution of sleep to social inequalities in cardiovascular disorders: a multi-cohort study. Cardiovasc Res. 2020;116(8):1514–1524. doi:10.1093/cvr/cvz267
50. Kashyap R, Hock LM, Bowman TJ. Higher prevalence of smoking in patients diagnosed as having obstructive sleep apnea. Sleep Breath. 2001;5(4):167–172. doi:10.1055/s-2001-18805
51. Moreno CR, Carvalho FA, Lorenzi C, et al. High risk for obstructive sleep apnea in truck drivers estimated by the Berlin questionnaire: prevalence and associated factors. Chronobiol Int. 2004;21(6):871–879. doi:10.1081/CBI-200036880
52. Conway SG, Roizenblatt SS, Palombini L, et al. Effect of smoking habits on sleep. Braz J Med Biol Res. 2008;41(8):722–727. doi:10.1590/S0100-879X2008000800014
53. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi:10.1093/aje/kws342
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


