Back to Journals » Journal of Pain Research » Volume 18
Effects of Transcutaneous Electrical Acustimulation on Patients with Fibromyalgia Syndrome: Study Protocol of a Randomized, Double-Blind, Sham-Controlled Trial
Authors Li Y , Fan B, Wang X , Hu N , Zhou X, Zhang Y, Mao P , Li Y
Received 4 March 2025
Accepted for publication 13 May 2025
Published 24 May 2025 Volume 2025:18 Pages 2663—2677
DOI https://doi.org/10.2147/JPR.S523538
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Yanpi Li,1 Bifa Fan,2 Xiyun Wang,1 Naichong Hu,1 Xinyi Zhou,1 Yi Zhang,2 Peng Mao,2 Yifan Li2
1Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 2Department of Pain Management, China-Japan Friendship Hospital, Beijing, People’s Republic of China
Correspondence: Yifan Li, Department of Pain Management, China-Japan Friendship Hospital, 2 Yinghuayuan East Street Chaoyang District, Beijing, 100029, People’s Republic of China, Email [email protected]
Background: Fibromyalgia Syndrome (FMS) is a chronic disorder characterized by widespread musculoskeletal pain, fatigue, and localized tenderness. Transcutaneous Electrical Acustimulation (TEA) is a non-invasive therapy that combines Traditional Chinese Medicine with electrical stimulation at specific acupoints. Previous studies have shown that TEA is effective in treating pain-related conditions. This study aims to evaluate the efficacy and mechanisms of TEA treatment for FMS.
Design: This is a prospective, randomized, double-blind, and placebo-controlled trial with two parallel groups, conducted at a single center. Forty participants will be randomly assigned to either the TEA group or the sham-TEA group in a 1:1 ratio. Participants will receive 2 weeks of treatment followed by 2 weeks of follow-up. The primary outcome is the change in VAS pain scores before and after treatment. Secondary outcomes include FMS and pain-related questionnaire scales, infrared thermography (IRT), vibration-controlled transient elastography (VCTE), blood neurobiological markers, cytokines, and metabolomics.
Trial Registration: http://itmctr.ccebtcm.org.cn/ identifier ITMCTR2024000638.
Keywords: FMS, traditional Chinese medicine, chronic pain, Hegu (LI4), Taichong (LR3)
Introduction
Fibromyalgia Syndrome (FMS) is a chronic and multidimensional condition predominantly characterized by chronic widespread pain (CWP), fatigue, sleep disturbances, and cognitive impairments. It is a relatively common, ranking as the third most prevalent musculoskeletal disease, with global prevalence ranging from 1.78% to 4% of the general population.1–3 FMS is most frequently observed in women aged 20 to 60, with a female-to-male ratio approximately between 2:1 and 7:1.1,4,5 The syndrome often presents with multiple comorbidities, which complicating the syndrome and influencing treatment outcomes.6 As a result, FMS is frequently misdiagnosed, with average diagnostic delays of around 2.3 years.7 Moreover, the economic burden of FMS is significant, impacting both healthcare systems and patients’ quality of life.8 Studies from Europe and North America indicate that FMS patients face a significant disease burden not only in terms of healthcare costs—which are estimated to be three times higher than for non-FMS individuals—but also in terms of lost productivity and reduced quality of life.9
The pathophysiological mechanism remains uncertain and multifactorial, involving factors like central sensitization, abnormal peripheral nociception, autoimmune, inflammatory, and hormonal dysregulation.10,11 Despite being recognized as a legitimate syndrome by numerous health organizations, there remains significant debate regarding its pathophysiology, diagnosis, and classification. Recent studies have identified small fiber neuropathy in patients with FMS,12 which, coupled with the observed efficacy of antiepileptic drugs typically used for neuropathic pain, has prompted a re-evaluation of FMS’s neuropathic pain characteristics and the exploration of new treatment strategies.13
A 2014 meta-analysis suggested that pharmacologic treatments for FMS, which primarily address pain, show limited effectiveness in managing the condition’s multidimensional nature. In contrast, non-pharmacologic approaches offer broader benefits across multiple symptom domains.14 A 2020 review highlights the necessity of multi-modal treatment approaches, given the complexity of the condition.1 Consequently, according to the latest EULAR recommendations for FMS, multicomponent therapies are supported (Evidence Ia, Grade A), and nonpharmacological interventions are prioritized as the initial treatment (Evidence IV, Grade D).15
Transcutaneous Electrical Acustimulation (TEA) is a promising non-invasive therapy that combines the principles of Traditional Chinese Medicine (TCM) with electrotherapy.16 Studies have shown that TEA can effectively manage pain, fertility issues, and immune system modulation.16–20 Basic studies have shown that TEA activates descending inhibitory pathways from the midbrain and brainstem, reducing the excitability of nociceptive neurons in the spinal cord.21,22 It exerts its effects through molecular pathways, including the release of endogenous opioids.23 TEA likely achieves analgesia by inhibiting pain-causing substances, promoting opioid peptide production, and disrupting mitogen-activated protein kinase signaling.20,24
However, research on TEA has been limited by methodological flaws, including insufficiently rigorous studies, poor replication, and inadequate control groups, making it difficult to conclusively establish its effectiveness for conditions like FMS.23,25,26 A 2017 Cochrane guideline27 noted that no strong evidence currently supports or refutes the use of TENS (transcutaneous electrical nerve stimulation, similar to TEA) for FMS pain. Therefore, we conducted this study further to investigate the effect of TEA treatment on FMS.
Our study is based on two hypotheses (Figure 1). First, we hypothesize that TEA alleviates central sensitization by reducing neuroinflammation-related factors.28 This reduction is proposed to mitigate systemic inflammation29 through improved microcirculation30 and metabolic regulation, thereby alleviating the core symptoms of FMS and improving quality of life. Second, we hypothesize that Body Mass Index (BMI), Waist-Hip Ratio (WHR), and TCM syndrome patterns are closely associated with pain severity and treatment response. Specifically, patients with higher BMI and WHR tend to have elevated inflammation levels,31 more severe pain,32 and poorer treatment outcomes. TCM syndrome patterns, particularly the Liver Qi Stagnation, may show a better response to TEA treatment.
 |
Figure 1 A hypothetical conceptual model of Transcutaneous Electrical Acustimulation (TEA), showing the expected outcomes and the mechanisms by which these outcomes will be assessed. |
The objectives of the study are threefold: First, to evaluate the effect of TEA on the core symptoms of FMS, with a comprehensively evaluate overall clinical efficacy, especially pain relief as measured by VAS scores. Second, to explore the mechanisms of TEA in FMS, focusing on neuroinflammatory factors, microcirculation, metabolomics and VCTE techniques. Third, as an exploratory component of this study, we plan to investigate potential FMS subtypes by examining the relationships between BMI, WHR, and TCM syndrome patterns with both the baseline severity of FMS and the therapeutic outcomes of TEA. This analysis aims to provide preliminary insights into how subtype characteristics may influence treatment efficacy.
Materials and Methods
Study Design
The study protocol adhered to the rigorous guidelines set forth by the CONSORT33 statements. This study is a prospective, randomized, double-blind (patients and researchers), single-center clinical, and placebo-controlled trial with two parallel groups. This trial is scheduled to start on July 1, 2024, and end on June 30, 2025, aiming to recruit 40 patients diagnosed with FMS. Forty participants will be randomly assigned to either the TEA group or the sham-TEA group in a 1:1 randomization ratio. Then they will undertake a 2-week treatment and a 2-week follow-up period. The flow chart is listed in Figure 2. The study schedule is listed in Table 1. The present protocol follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 guidelines34,35 and fulfills the SPIRIT checklist (see supplementary material). Any modifications to the protocol must be reported to and approved by the ethics committee.
 |
Table 1 Study Schedule of Enrollment, Intervention, and Assessments |
 |
Figure 2 Study flow diagram. |
Sample Size Calculation
The sample size for this study was calculated based on differences in VAS scores observed in pre-experimental results. The mean difference in VAS scores between the experimental and control groups was 1.0, with a standard deviation of 0.32. Using PASS 2023 software, we employed the sample size estimation method proposed by Chow et al, assuming a two-sided significance level (α) of 0.05 and a desired power (1-β) of 0.90.36 The minimum sample size per group was initially calculated to be 3 participants, with a total of 6 participants required for both groups. To account for potential dropouts, we anticipated a 10% loss, which increased the required sample size to 4 participants per group, resulting in a total of 8 participants. However, considering clinical feasibility and to enhance the robustness and generalizability of the findings, we ultimately recruited 40 participants, with 20 participants in each group. This sample size is deemed sufficient to detect significant differences in VAS scores while ensuring the reliability and external validity of the results.
Setting and Recruitment
The obtain informed consent, intervention and data collection will be independently administered by three research assistants from Beijing University of Chinese Medicine.37 Unless permission is obtained from the participant, any information that can identify their identity will not be disclosed to anyone outside the research team. Data will be reviewed periodically by a designated investigator. This single-center study is conducted at the China-Japan Friendship Hospital, which also serves as the China National Clinical Research Center for Pain. Participants will be recruited through online platforms, outpatient clinics, and inpatient wards.
Participant Eligibility
Participants must adhere to their ongoing treatment plans and are explicitly instructed not to alter their therapeutic interventions throughout the 4-week trial phase. The use of “as needed” rescue medications will be documented. We will also document factors that may influence treatment in the “treatment diary”, such as medication changes, exercise, and emotional status.
The inclusion criteria for participants are as follows: (1) Adults aged 18–80; (2) Diagnosed with FMS based on the 2016 Revisions to the 2010/2011 FMS Diagnostic Criteria. (3) VAS≥3 scores. (4) Volunteer for this clinical study and sign an informed consent form.
Exclusion criteria include: (1) The state of electronic devices such as pacemakers and brain nerve stimulators etc. (2) Serious damage to the heart liver or kidneys or mental disorders or failure to cooperate with examination and treatment. (3) Women in pregnancy. (4) Active malignant tumor. (5) Fragile skin prone to bleeding rupture allergies etc. (6) Used TEA in the last 3 months. (7) Participation in other clinical studies.
Participants will be withdrawn if they cannot complete the study protocol for any of the following reasons: (1) Withdrawal of informed consent at any stage of the trial. (2) Inability to comply with the study protocol, including missing scheduled visits or not following treatment instructions. (3) Serious adverse events.
This study will strictly adhere to the ethical standards as outlined in the revised Helsinki Declaration of 2013. Before the intervention, all participants will be provided with a detailed explanation of the research process and will voluntarily sign a written informed consent form. Participants will be compensated for their time and effort. Upon completion of the study, patients in the sham-TEA group will receive TEA treatment using the same parameters as the experimental group as a form of compensation. If a participant suffers any injury related to the study, they will be entitled to receive free treatment provided by the China-Japan Friendship Hospital and compensation in accordance with relevant Chinese laws.
Randomization, Allocation, and Blinding
Randomization was conducted using a table of random numbers. Random numbers from 1 to 100 was performed using SPSS 26.0 software, and the numbers were sorted in ascending order. The first 20 numbers were designated as the TEA group, while the subsequent 20 numbers were assigned to the sham-TEA group. Then the TEA devices from 1 to 40 according to the table of random numbers. The TEA devices and the sham-TEA devices will be visually indistinguishable. An independent statistician expert who is not involved in the trial will complete the aforementioned steps.
After confirming the participants’ eligibility based on the inclusion and exclusion criteria, researchers will allocate treatment devices in chronological order of enrollment based on the participants’ screening number. Allocation concealment will be maintained using the sealed-envelope method, where the randomization plan is kept in opaque envelopes. These envelopes will be distributed in sequence according to the enrollment order, and unblinding will occur by opening the envelopes to reveal group assignments when necessary.
Intervention
The TEA device is a watch-sized stimulator with two electrodes (SNM-FDC01; Ningbo Maida Medical Device, Ningbo, China), allowing simultaneous stimulation of a single acupoint bilaterally.38
The TEA group and sham-TEA group interventions will last for 14 days, with treatments administered twice daily. In the morning (0:00–12:00), participants will receive 30 minutes of stimulation at both sides and the He Gu (LI4) acupoint (depicted in Figure 3), followed by 30 minutes of stimulation at the Tai Chong (LR3) acupoint in the afternoon (12:00–24:00). Participants are instructed to adjust the stimulation intensity (current intensity) to the maximum tolerable level during the treatment period. Additionally, patients are advised to perform 10 minutes of relaxation and stretching exercises during each treatment session.
 |
Figure 3 Placement of TEA electrodes at LI4. |
The parameters will be set to 15 hz, with a pulse width of 0.5 ms. The only difference between the TEA and sham-TEA groups is that the TEA group will receive a stimulation mode of 30 minutes on, while the sham-TEA group will follow a stimulation pattern of 2 seconds on and 120 seconds off.
Outcome Assessment
Primary Outcome
We used Visual Analogue Scale (VAS) to quantify the immediate and long-term pain relief effects of TEA. The VAS is a quantitative tool for assessing pain intensity, represented by a 100-mm horizontal line ranging from “no pain” (0 points) to “worst imaginable pain” (100 points).39 We calculated the VAS reduction rate pre- and post-treatment for statistical analysis using the formula: 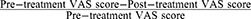 ×100%. Previous studies have confirmed the construct validity and reliability of this scale.40,41
×100%. Previous studies have confirmed the construct validity and reliability of this scale.40,41
Secondary Outcomes
TCM Syndrome Patterns
We use a symptom questionnaire to determine the Traditional Chinese Medicine (TCM) syndrome patterns of patients, followed by subgroup analysis to preliminarily evaluate which syndrome type responds best to TEA treatment. Each patient was independently evaluated by at least two licensed TCM practitioners using the four diagnostic methods—inspection, auscultation and olfaction, inquiry, and palpation. Diagnoses were made in accordance with the standardized criteria specified in Traditional Chinese Medicine Diagnostics (ISBN: 9787117366571). Only cases with consistent diagnoses between the two evaluators were included. Disagreements were resolved through discussion or by consultation with a senior TCM expert. Detailed diagnostic records, including symptom profiles, tongue and pulse characteristics, were documented in case report forms to ensure traceability and support quality assurance.
FMS are diagnosed differentially by Western and Chinese medicine.42 In TCM, FMS is considered a type of “jinbi” (muscle blockage) with a primary pathomechanism of Liver Qi Stagnation.43–45 When Liver Qi becomes stagnant, the body’s Qi circulation is disrupted, leading to muscle pain, cold intolerance in the limbs, and fatigue. TCM, as a holistic system, views this pathomechanism as potentially triggering a range of symptoms and comorbidities, similar to a “butterfly effect.” For example, it can affect the Spleen(In TCM, the spleen is not considered an anatomical organ but rather a functional system responsible for digestion and transformation of food and fluids, which is distinct from the anatomical spleen), leading to symptoms like irritable bowel syndrome (IBS), which is often comorbid with FMS.1 Additionally, since the Liver governs emotions in TCM, Liver Qi Stagnation can manifest as emotional disturbances, such as depression, which often improves with increased physical activity. This pattern is similar to the symptom characteristics commonly observed in FMS patients.
In summary, TCM believes that the pathogenesis of FMS is related to the liver. From the perspective of epidemiological studies, current research supports a close association between FMS and liver diseases.4,46 FMS patients exhibit a higher incidence of liver cirrhosis.47 Furthermore, a cross-sectional study identified significant associations between FMS and Hepatitis C Virus (HCV), Non-Alcoholic Steatohepatitis (NASH)-related cirrhosis, as well as psychiatric symptoms.48 Moreover, There is growing evidence that chronic inflammatory liver diseases are linked to changes in central neural transmission, contributing to symptoms like fatigue, cognitive dysfunction, mood disorders, and sleep disturbances.49
These findings indicate a strong relationship between FMS and liver health. To further explore this, we conducted initial measurements of liver fibrosis/fatty liver in FMS patients using VCTE, a technique capable of detecting early-stage liver abnormalities.
Physical Examinations
The physical examinations need to be done last, because exercise may induce pain to worsen to the extent that the relevant questionnaire is affected.
- Digit Span Test (DST): DST is a neuropsychological assessment that evaluates short-term attention and working memory through two tasks: digit span forward and digit span backward. In the forward task, participants repeat a sequence of digits, while in the backward task, they repeat the same digits in reverse order. Higher DST scores are indicative of better performance. For FMS patients, cognitive symptoms, often called “fibro fog”, can impact memory and concentration, making the DST useful for evaluating these cognitive limitations. Studies indicate that patients with FMS tend to show reduced performance on DST,50 highlighting attention and working memory deficits which are part of the cognitive challenges faced by individuals with FMS.
- Five Times Sit to Stand Test(FTSTS): The FTSTS is a validated functional assessment tool used to quantitatively evaluate lower body strength, endurance, and flexibility by measuring the time required for an individual to rise from a seated position to standing and return to sitting five times consecutively.51 In patients with FMS, who commonly experience compromised muscle endurance and chronic fatigue, FTSTS serves as an objective indicator of functional mobility and physical endurance, both of which are typically reduced in this population. Additionally, diminished performance on the FTSTS in FMS patients has been correlated with an elevated risk of falls, attributable to muscle weakness and balance impairments associated with the condition. Consequently, FTSTS results can provide valuable insights into the physical limitations and fall risks inherent in FMS, aiding in the assessment of therapeutic efficacy and guiding targeted interventions for mobility and stability improvement in these patients.
- Single-Leg Stand Test (SLS): The SLS is a straightforward clinical assessment tool that evaluates balance, lower limb strength, and proprioception. In this test, patients are instructed to maintain stability while standing on one leg for a designated period, allowing for the assessment of neuromuscular control. The SLS is widely utilized in orthopedic and neurological assessments as well as in fall-risk evaluations. This test is particularly relevant in the context of FMS, where impairments in static balance and postural control are common. A small randomized controlled trial (RCT) conducted in 2016 indicated that patients with FMS exhibit poorer bilateral coordination than control subjects,52 further underscoring the utility of the SLS in evaluating balance deficits and neuromuscular function in this population.
Body Mass Index(BMI) and Waist-Hip Ratio (WHR): Emerging evidence underscores the interrelationship between chronic pain and obesity.53 In patients with FMS, obesity and increased BMI are commonly associated with greater disease severity and poorer quality of life.54 Obesity, particularly central obesity, is characterized by the expansion of adipocytes, leading to localized hypoxia which triggers inflammatory responses and stimulates the production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6).31,55,56 Both BMI and WHR are widely used indicators for assessing obesity.57 The purpose of measuring BMI and WHR is twofold: First, to verify the association between obesity and the severity of FMS, and analyze the correlation between obesity and the course of FMS. Second, to compare baseline obesity between the TEA and sham-TEA groups, ensuring it does not affect the VCTE results. Waist circumference was measured midway between the lowest rib and the iliac crest during expiration, while hip circumference was assessed at the maximum hip width.57 The BMI calculation formula is:  . The WHR calculation formula is:
. The WHR calculation formula is: 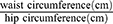 .
.
Multidimensional Questionnaires
- Brief Pain Inventory (BPI): The BPI can be used to evaluate pain severity, pain interference, average pain, worst pain and its impact on daily functioning. Studies have demonstrated strong reliability of BPI(Cronbach’s α = 0.85)58 and BPI-SF (Cronbach’s α = 0.84).59
- Fibromyalgia Survey Questionnaire (FSQ): The FSQ is a valuable tool for assessing the number and intensity of pain sites and tender points in individuals with FMS.60 The FSQ scale effectively measures the primary symptoms of FMS and provides a reliable assessment of their severity.61 The FSQ has shown good reliability(Cronbach’s α = 0.814), convergent and discriminant validity.61,62 The FSQ consists of two components: the Widespread Pain Index (WPI) and the Somatic Severity Score (SSS).
- Short Form 12 Item (version 2) Health Survey (SF-12 v2): The SF-12 v2 is widely used for assessing health status and is considered a generic health-related quality of life (QoL) instrument. It evaluates eight domains of functioning and well-being, including physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), general health perceptions (GH), energy and vitality (VT), social functioning (SF), role limitations due to emotional problems (RE), and mental health (MH).63 The eight domains can be summarized into two composite scores: the physical component summary (PCS) and the mental component summary (MCS).64 The scale ranges from 0 to 100, with lower scores indicating a lower quality of life. Research across multiple domains shown SF-12 had satisfactory reliability (Cronbach’s α = 0.7 to 0.910) and validity.65–74
- The Patient-Reported Outcomes Measurement Information System® (PROMIS)-Fatigue Short Form 7a (SF-7a) scale(PROMIS-Fatigue 7a) and PROMIS Sleep Disturbance 8b short form(PROMIS-Sleep Disturbance 8b): PROMIS could standardize patient-reported outcomes for chronic conditions through short-form (SF) instruments.75 The PROMIS-Fatigue 7a consists of seven items, and the PROMIS-Sleep Disturbance 8b comprises eight items. Both utilize a 5-point scale to assess experiences over the past seven days. For FMS, the PROMIS-SF showed moderate test-retest reliability (ICC 0.62 to 0.71) and strong internal consistency (Cronbach’s α= 0.89 to 0.92).76–78
- Patient Health Questionnaire-9 (PHQ-9): The PHQ-9 consists of nine items, including symptoms such as sleep disturbances, fatigue, and decreased appetite, which are common in FMS. It uses a 4-point rating scale, with the total score reflecting the severity of depressive symptoms. The score ranges from 0 to 27, with higher scores indicating greater depression severity. A systematic review has confirmed PHQ-9 is a validated instrument for identifying and monitoring depression, anxiety, and somatization.79
Laboratory Tests and Imaging
- Infrared thermography (IRT)
We use IRT to detect the highest, lowest, and average temperatures of a patient’s hands and face, and the facial image can also generate a TCM Pattern Identification Report. IRT can capture the body’s infrared emissions to generate “IR thermograms”, reflecting skin surface temperature variations that indicate underlying physiological conditions.80,81 IRT has demonstrated a sensitivity of 90% and a specificity of 86%.81 Previous studies have revealed the presence of microcirculatory abnormalities in patients with FMS, which may be associated with dysfunction of the autonomic nervous system (ANS)82,83 and pain modulation.84 FMS often exhibits exacerbation upon cold exposure and alleviation with heat, suggesting that improved blood circulation through TEA may be one of its therapeutic mechanisms.85 We will record IR thermograms before and after TEA treatment, including the facial region and the palmar and dorsal aspects of both hands. In our study using the D-LU384 model IRT camera produced by Xi’an Zhongke Lead IR-Tech Co., Ltd, China. This device employs a non-cooled focal plane infrared detector (polysilicon), with a working wavelength range of 8-14μm, a frame rate of ≥25Hz, thermal sensitivity of <60mK, temperature measurement range of 28-42°C, and effective pixels of ≥384×288. The imaging distance is 0.5 to 3m, with temperature measurement accuracy of ≤0.4°C, temperature consistency of ≤0.2°C, operating within an ambient temperature range of 10 °C to 30 °C. Results were analyzed using the Xi’an Taihao Infrared Management System V1.0 software, which generates reports on maximum, minimum, and average temperatures. Additionally, the software produces an extra report on TCM constitution identification. All IR thermograms were collected in compliance with the standards of the International Academy of Clinical Thermology. (IACT)
- Vibration controlled transient elastography (VCTE)
VCTE, provided by the FibroScan box device from Echosens in Paris, France, is a state-of-the-art, non-invasive method for assessing liver fibrosis/fatty liver.86 This device enables the real-time, non-invasive acquisition of both Liver Stiffness Measurement (LSM) and Controlled Attenuation Parameter (CAP), where LSM evaluates liver fibrosis and CAP assesses steatosis.
LSM, which is calculated from shear wave velocity measurements and converted via Hooke’s law, offers a quantitative assessment of hepatic fibrosis in kilopascals (kPa).87 Fibrosis stages are categorized as follows: F0 for 0–5.9 kPa, F1 for 6.0–6.9 kPa, F2 for 7.0–9.0 kPa, F3 for 9.1–10.3 kPa, and F4 for values ≥10.4 kPa. LSM values below 8 kPa effectively rule out advanced fibrosis, while values above 12–15 kPa are indicative of its presence, thereby facilitating the diagnosis and staging of liver fibrosis.88
The CAP, utilizing ultrasonic attenuation, is specifically designed to stage hepatic steatosis by quantifying liver fat content.87 Elevated CAP values suggest more severe steatosis, rendering it a valuable tool for investigating and monitoring liver diseases associated with fatty liver conditions. Patients were classified into three steatosis categories based on CAP values: low (<250 dB/m), intermediate (250–300 dB/m), and high (>300 dB/m). A CAP value >275 dB/m diagnoses significant steatosis.88
The FibroScan is performed by specially trained physicians. Before the examination, patients should fast for at least 3 hours and refrain from alcohol consumption for 24 hours.89 Measurements are taken with patients lying supine, their right arm placed above the head and the right leg crossed over the left, creating a “C” shape to enhance intercostal access for measuring the right liver lobe.90
- Biomarker Testing:
To better understand the mechanisms of tea treatment, we conducted blood biomarker assessments before and after the intervention. Venous blood samples were collected on days −7 to 0 and at 18 ± 4 days, with each collection yielding 4–6 mL (divided into two tubes) using EDTA anticoagulant tubes. One tube was sent immediately to China-Japan Friendship Hospital Laboratory for cytokine analysis(IL-1β, IL-2, IL-4, IL-6, IL-5, IL-10, IL-12p70, IL-17, TNF-α, IFN-α, IFN-γ, IL-8), while the other tube was processed for centrifugation within 30 minutes at 2–8°C, at 1000×g for 15 minutes. After centrifugation, the supernatant was aliquoted into four 200 µL samples and stored in a −80°C freezer within 5 minutes. One for measuring TSPO, S100B, BDNF, and CGRP, another for metabolomics analysis, and the remaining two as backups. The assays for TSPO, S100B, CGRP, and BDNF were conducted every four months using the same batch of ELISA plates. To minimize inter-group variability, metabolomics samples will be sent for analysis after the completion of the trial.Translocator Protein (TSPO),91 S100 calcium-binding protein B (S100B),92 Brain-Derived Neurotrophic Factor (BDNF),93 and Calcitonin Gene-Related Peptide (CGRP)94,95 were detected using commercial enzyme-linked immunosorbent assay (ELISA) kits from FineTest (Wuhan, China) with the following catalog numbers: EH4447 for TSPO, EH0543 for S100B, EH0043 for BDNF, and EH2808 for CGRP. We analyzed the metabolites extracted from a 200 µL plasma sample using liquid chromatography-mass spectrometry (LC-MS), followed by peak extraction and alignment of the resulting mass spectrometry data.96,97
Covariables
- Demographic and Medical history: The demographic information including date of birth, age, sex, ethnicity, marital status, occupation, and education level. Medical history including past medical history, concurrent medications, personal history (smoking, alcohol consumption), allergy history, menstrual history (for female participants), and family medical history.
- Rescue medications.
Quality Control
All researchers must undergo training and pass a training assessment before performing trial procedures. All distributed TEA devices can connect via Bluetooth, allowing researchers to monitor TEA operating time on their terminals. Researchers will check device performance daily at 10 AM and 8 PM to identify participants who have not completed their treatment and provide reminders. This feature facilitates the supervision of participants and enables the assessment of treatment adherence post-therapy. We have also designed a “treatment diary”, which must be completed before and after each day’s treatment. Sample collection will strictly follow the “Sample Handling Manual.” Our electronic “treatment diary” questionnaire will prompt participants daily at 9 AM and 6 PM to engage in their treatment.
Statistical Analyses
We will perform an Intention-To-Treat (ITT) analysis and will analyze the study data using SPSS 26.0. Missing data will be addressed using multiple imputation. Descriptive statistics summarize participants’ baseline and outcome characteristics. Normally distributed continuous data are expressed as mean ± standard error of the mean (SEM), while non-normally distributed data are presented as median (P25, P75). Categorical variables are reported as numbers and percentages (n, %). Parametric and non-parametric tests will be used to compare baseline differences between the two groups. To assess the magnitude of changes in primary and secondary outcomes across time and between the two groups for continuous variables, linear mixed models for repeated measures were employed, with baseline severity included as a covariate.98 The categorical variables were analyzed using McNemar’s test or Cochran’s Q test. Bonferroni correction was applied for the primary outcome analyses to account for multiple comparisons. Hypothesis testing was performed using two-tailed tests with a significance level of α = 0.05. The fixed effects include group, visit time and covariates (eg gender, sex).
Discussion
This study, grounded in TCM theory, explores the therapeutic efficacy of TEA in treating FMS. FMS’s multisystem involvement and complex symptomatology pose significant challenges for treatment. While pharmacological treatments offer some benefits, their single-target mechanisms often provide limited relief. Non-pharmacological approaches, by contrast, address a broader range of FMS symptoms, including pain, sleep disturbances, mood changes, and cognitive impairments, offering a more comprehensive management strategy.
Our approach seeks to overcome the drawbacks of traditional therapies, which are often prolonged and time-consuming. Evidence suggests that combining TEA with exercise significantly enhances therapeutic outcomes compared to TEA alone.27 In this study, we implemented a daily 1-hour treatment protocol, consisting of 10 minutes of simple stretching and relaxation exercises in the morning and afternoon. This regimen, known as “Dongqi Therapy” in TCM (mobilizing the affected area during acupuncture-related treatments), aims to amplify therapeutic effects without disrupting patients’ daily routines.
Emotional stress is closely linked to the severity of FMS,99 and TCM attributes its primary pathogenesis to Liver Qi Stagnation. Guided by the principle of soothing the liver and regulating Qi, we selected acupoints such as Hegu (LI4) and Taichong (LR3), integrating these with “Dongqi Therapy” to facilitate Qi flow to affected areas.100 Moreover, since previous research suggests that FMS patients experience microcirculatory insufficiency,101 we employed infrared thermography to measure surface temperature, exploring TEA’s mechanisms of action.
We also hypothesize that FMS-related fatigue may be linked to liver dysfunction. Patients with chronic liver diseases, such as inflammatory liver conditions or non-alcoholic fatty liver disease (NAFLD), often exhibit cognitive, emotional, and behavioral disturbances due to liver-brain axis alterations,102 which resemble FMS symptoms. Therefore, our study included VCTE assessments to preliminarily explore whether FMS-related fatigue has a liver-related pathological basis.
Despite certain limitations, including the absence of a healthy control group and only a single-centre, our findings provide preliminary evidence supporting TEA as a viable treatment for FMS. Future studies should expand sample sizes, include subgroup analyses, and investigate precision treatments tailored to patient-specific factors such as age, gender, body weight, TCM syndrome types, and non-depressive FMS.103 These analyses will deepen our understanding of FMS heterogeneity and enable the development of more personalized and effective treatment plans.
Abbreviations
FMS, Fibromyalgia Syndrome; TEA, Transcutaneous Electrical Acustimulation; IRT, infrared thermography; VCTE, vibration-controlled transient elastography; CWP, chronic widespread pain; TCM, Traditional Chinese Medicine; BMI, Body Mass Index; WHR, Waist-Hip Ratio; S100B, S100 calcium-binding protein B; TSPO, Translocator Protein; BDNF, Brain-Derived Neurotrophic Factor; CGRP, Calcitonin Gene-Related Peptide; QOL, Quality of Life; SF-12 v2, Short Form 12 Item (version 2) Health Survey; FSQ, Fibromyalgia Survey Questionnaire; VAS, Visual Analogue Scale; BPI, Brief Pain Inventory; PROMIS-Fatigue 7a, The Patient-Reported Outcomes Measurement Information System® (PROMIS)-Fatigue Short Form 7a (SF-7a) scale; PROMIS-Sleep Disturbance 8b, PROMIS Sleep Disturbance 8b short form; PHQ-9, Patient Health Questionnaire-9; DST, Digit Span Test; FTSTS, Five Times Sit to Stand Test; SLS, Single-Leg Stand Test; SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials; LI4, Hegu; LR3, Taichong; LSM, Liver Stiffness Measurement; CAP, Controlled Attenuation Parameter; kPa, Kilopascals; ELISA, Enzyme-Linked Immunosorbent Assay; LC-MS, Liquid Chromatography-Mass Spectrometry; ITT, Intention-To-Treat; SEM, Error of the Mean; NAFLD, Non-Alcoholic Fatty Liver Disease.
Ethics
The study has been approved by the China-Japan Friendship Hospital Ethics Committee (approval number: 2024-KY-278-1).
Acknowledgments
All investigators sincerely thank the two patients, Linlin Dong and Shanji Lin, for their contributions to our study.
Funding
This work was supported by the National High Level Hospital Clinical Research Funding (Number 2024-NHLHCRF-PYII-22 and 2022-NHLHCRF-YSPY-02).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sarzi-Puttini P, Giorgi V, Marotto D, et al. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16(11):645–660. doi:10.1038/s41584-020-00506-w
2. Alciati A, Nucera V, Masala IF, et al. One year in review 2021: fibromyalgia. Clinical and Experimental Rheumatology. 2021;39(3):3–12. doi:10.55563/clinexprheumatol/gz4i3i
3. Jones GT, Atzeni F, Beasley M, et al. The prevalence of fibromyalgia in the general population: a comparison of the American college of rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis & Rheumatology. 2015;67(2):568–575. doi:10.1002/art.38905
4. Queiroz LP. Worldwide Epidemiology of Fibromyalgia. Curr Pain Headache Rep. 2013;17(8):356. doi:10.1007/s11916-013-0356-5
5. White KP, Speechley M, Harth M, et al. The London Fibromyalgia Epidemiology Study: the prevalence of fibromyalgia syndrome in London, Ontario. J Rheumatol. 1999;26(7):1570–1576.
6. Zhao SS, Duffield SJ, Goodson NJ. The prevalence and impact of comorbid fibromyalgia in inflammatory arthritis. Best Practice & Research Clinical Rheumatology. 2019;33(3):101423. doi:10.1016/j.berh.2019.06.005
7. Varrassi G, Rekatsina M, Perrot S, et al. Is fibromyalgia a fashionable diagnosis or a medical mystery? cureus. doi:10.7759/cureus.44852
8. Amris K, Ibsen R, Duhn PH, et al. Health inequities and societal costs for patients with fibromyalgia and their spouses: a Danish cohort study. RMD Open. 2024;10(1):e003904. doi:10.1136/rmdopen-2023-003904
9. Branco JC, Bannwarth B, Failde I, et al. Prevalence of fibromyalgia: a survey in Five European countries. Seminars in Arthritis and Rheumatism. 2010;39(6):448–453. doi:10.1016/j.semarthrit.2008.12.003
10. Paroli M, Gioia C, Accapezzato D, et al. Inflammation, autoimmunity, and infection in fibromyalgia: a narrative review. IJMS. 2024;25(11):5922. doi:10.3390/ijms25115922
11. Jones EA, Asaad F, Patel N, et al. Management of fibromyalgia: an update. Biomedicines. 2024;12(6):1266. doi:10.3390/biomedicines12061266
12. Cheng CW, Wong CS, Hui GK, et al. Fibromyalgia: is it a neuropathic pain? Pain Manag. 2018;8(5):377–388. doi:10.2217/pmt-2018-0024
13. Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane pain, palliative and supportive care group. Cochrane Database of Systematic Reviews. 2013;2019(5). doi:10.1002/14651858.CD010567.pub2
14. Perrot S, Russell IJ. More ubiquitous effects from non‐pharmacologic than from pharmacologic treatments for fibromyalgia syndrome: a meta‐analysis examining six core symptoms. European Journal of Pain. 2014;18(8):1067–1080. doi:10.1002/ejp.564
15. Macfarlane GJ, Kronisch C, Dean LE, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017;76(2):318–328. doi:10.1136/annrheumdis-2016-209724
16. Zhou X, gen CS, Jie X, et al. TanEffects of Transcutaneous Electrical Acupoint Stimulation (TEAS) on Postoperative Recovery in Patients With Gastric Cancer: A Randomized Controlled Trial.doi:10.2147/CMAR.S292325
17. ying LL, Su Y, rong WR, et al. Transcutaneous electrical acupoint stimulation benefits postoperative pain relief of oocyte retrieval: a randomized controlled trial. Journal of Integrative Medicine. 2024;22(1):32–38. doi:10.1016/j.joim.2024.01.005
18. Chen J, Zhang Y, Li X, et al. Efficacy of transcutaneous electrical acupoint stimulation combined with general anesthesia for sedation and postoperative analgesia in minimally invasive lung cancer surgery: a randomized, double‐blind, placebo‐controlled trial. Thoracic Cancer. 2020;11(4):928–934. doi:10.1111/1759-7714.13343
19. Zhang H, Wang L, Zheng Z, et al. The use of transcutaneous electrical acupoint stimulation to reduce opioid consumption in patients undergoing off-pump CABG: a randomized controlled trial. Perioper Med. 2024;13(1):68. doi:10.1186/s13741-024-00427-2
20. Yan W, Kan Z, Yin J, et al. Efficacy and safety of Transcutaneous Electrical Acupoint Stimulation (TEAS) as an analgesic intervention for labor pain: a network meta-analysis of randomized controlled trials. Pain Ther. 2023;12(3):631–644. doi:10.1007/s40122-023-00496-z
21. Noehren B, Dailey DL, Rakel BA, et al. Effect of transcutaneous electrical nerve stimulation on pain, function, and quality of life in fibromyalgia: a double-blind randomized clinical trial. Physical Therapy. 2015;95(1):129–140. doi:10.2522/ptj.20140218
22. Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS). J Pharmacol Exp Ther. 2001;298(1):257–263. doi:10.1002/tox.22084
23. Szmit M, Krajewski R, Rudnicki J, et al. Application and efficacy of transcutaneous electrical acupoint stimulation (TEAS) in clinical practice: a systematic review. Adv Clin Exp Med. 2023;32(9):1063–1074. doi:10.17219/acem/159703
24. Wang L, An J, Song S, et al. Electroacupuncture preserves intestinal barrier integrity through modulating the gut microbiota in DSS-induced chronic colitis. Life Sciences. 2020;261:118473. doi:10.1016/j.lfs.2020.118473
25. Meng D, Fei MY, Mei SQ, et al. Efficacy and safety of Transcutaneous Electrical Acupoint Stimulation (TEAS) for postoperative pain in laparoscopy: a systematic review and meta‐analysis of randomized controlled trials. Medicine. 2021;100:e26348. doi:10.1155/2022/9922879
26. Yang H, hui HW, Xing XG, et al. Transcutaneous electrical acupoint stimulation for pregnancy outcomes in women undergoing in vitro fertilization-embryo transfer: a systematic review and meta-analysis. Front Public Health. 2022;10:892973. doi:10.3389/fpubh.2022.892973
27. Johnson MI, Claydon LS, Herbison GP, et al. Transcutaneous electrical nerve stimulation (TENS) for fibromyalgia in adults. Cochrane pain, palliative and supportive care group. Cochrane Database of Systematic Reviews. 2017;2017(10). doi:10.1002/14651858.CD012172.pub2
28. Tan Z, Dong F, Wu L, et al. Transcutaneous electrical acupoint stimulation attenuated neuroinflammation and oxidative stress by activating SIRT1-induced signaling pathway in MCAO/R rat models. Experimental Neurology. 2024;373:114658. doi:10.1016/j.expneurol.2023.114658
29. Li Q, Wang L, Wang Y, et al. Transcutaneous electrical acupoint stimulation for immunologic function after surgery in patients with gastrointestinal tumor: a meta-analysis. Biotechnol Genet Eng Rev. 2024;40(2):1001–1023. doi:10.1080/02648725.2023.2191090
30. Liu T, Yin C, Li Y, et al. Effects of transcutaneous electrical acupoint stimulation on postoperative cognitive decline in elderly patients: a pilot study. CIA. 2021;16:757–765. doi:10.2147/CIA.S309082
31. Kosovski IB, Bacârea V, Ghiga D, et al. Exploring the link between inflammatory biomarkers and adipometrics in healthy young adults aged 20–35 years. Nutrients. 2024;16(2):257. doi:10.3390/nu16020257
32. Zhong Y, Tian K, Zhu Y, et al. Chronic pain and obesity in community-dwelling adults: findings from the national health and nutrition examination survey. JPR. 2024;17:3115–3125. doi:10.2147/JPR.S470855
33. Juszczak E, Altman DG, Hopewell S, et al. Reporting of multi-arm parallel-group randomized trials: extension of the CONSORT 2010 statement. JAMA. 2019;321(16):1610. doi:10.1001/jama.2019.3087
34. Chan AW, Tetzlaff JM, Altman DG, et al. Declaración SPIRIT 2013: definición de los elementos estándares del protocolo de un ensayo clínico. Rev Panam Salud Publica. 2015;38(6):506–514.
35. Chan AW, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346(jan08 15):e7586–e7586. doi:10.1136/bmj.e7586
36. Chow SC, Shao J, Wang H, et al. Sample Size Calculations in Clinical Research.
37. Li Y, Yu Z, Li H, et al. The influence of different intensity of Tan Tui exercises on the posture control of students in the Tai Chi Elective Course: protocol for a randomized controlled trial. Trials. 2024;25(1):599. doi:10.1186/s13063-024-08447-5
38. Xiao Y, Xu F, Lin L, et al. Transcutaneous electrical acustimulation improves constipation by enhancing rectal sensation in patients with functional constipation and lack of rectal sensation. Clin Transl Gastroenterol. 2022;13(5):e00485. doi:10.14309/ctg.0000000000000485
39. Bjelkarøy MT, Benth JŠ, Simonsen TB, et al. Measuring pain intensity in older adults. Can the visual analogue scale and the numeric rating scale be used interchangeably? Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2024;130:110925. doi:10.1016/j.pnpbp.2023.110925
40. Vas J, Modesto M, Aguilar I, et al. Effects of acupuncture on patients with fibromyalgia: study protocol of a multicentre randomized controlled trial. Trials. 2011;12(1):59. doi:10.1186/1745-6215-12-59
41. Von Korff M, Jensen MP, Karoly P. Assessing Global Pain Severity by Self-Report in Clinical and Health Services Research. Spine. 2000;25(24):3140–3151. doi:10.1097/00007632-200012150-00009
42. Ng HP, Chong SY, Li YH, et al. Objective analysis of traditional Chinese medicine syndrome differentiation of patients with diabetes and prediabetes: protocol for a nonrandomized, exploratory, observational case-control study using digitalized traditional Chinese medicine diagnostic tools. JMIR Research Protocols. 2024;13(1):e56024. doi:10.2196/56024
43. Li Y, Sun Y, Fu J, et al. Distribution and characteristics of traditional Chinese medicine patterns in 165 patients with fibromyalgia syndrome. Journal of Beijing University of Traditional Chinese Medicine. 2022;45(6):630–636.
44. Guo Z, Chen F, Liu J, et al. Analysis of clinical characteristics and distribution rules of TCM patterns in 148 patients with fibromyalgia syndrome. Journal of Beijing University of Traditional Chinese Medicine. 2023;46(11):1481–1489.
45. Mist SD, Wright CL, Jones KD, et al. Traditional Chinese medicine diagnoses in a sample of women with fibromyalgia. Acupunct Med. 2011;29(4):266–269. doi:10.1136/acupmed-2011-010052
46. Wolfe F, Michaud K, Li T, et al. Chronic conditions and health problems in rheumatic diseases: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, systemic lupus erythematosus, and fibromyalgia. J Rheumatol. 2010;37(2):305–315. doi:10.3899/jrheum.090781
47. Rogal SS, Bielefeldt K, Wasan AD, et al. Fibromyalgia symptoms and cirrhosis. Dig Dis Sci. 2015;60(5):1482–1489. doi:10.1007/s10620-014-3453-3
48. Schatz RA, Moshiree B. Gastrointestinal and hepatic disease in fibromyalgia. Rheumatic Disease Clinics of North America. 2018;44(1):131–142. doi:10.1016/j.rdc.2017.09.009
49. D’Mello C, Swain MG. Liver-brain inflammation axis. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2011;301(5):G749–G761. doi:10.1152/ajpgi.00184.2011
50. Elgueta-Aguilera N, Guede-Rojas F, Mendoza C, et al. Self-perceived cognitive function and neuropsychological performance in women with fibromyalgia. Rev méd Chile. 2022;150(11):1450–1457. doi:10.4067/S0034-98872022001101450
51. De Melo TA, Silva Guimarães F, Silva LE. The five times sit-to-stand test: safety, validity and reliability with critical care survivors’s at ICU discharge. Arch Physiother. 2022;13(1):2. doi:10.1186/s40945-022-00156-z
52. Heredia-Jimenez J, Orantes-Gonzalez E, Soto-Hermoso VM. Variability of gait, bilateral coordination, and asymmetry in women with fibromyalgia. Gait & Posture. 2016;45:41–44. doi:10.1016/j.gaitpost.2016.01.008
53. Qian M, Shi Y, Yu M. The association between obesity and chronic pain among community-dwelling older adults: a systematic review and meta-analysis. Geriatric Nursing. 2021;42(1):8–15. doi:10.1016/j.gerinurse.2020.10.017
54. Rossi A, Lollo ACD, Guzzo MP, et al. Fibromyalgia and nutrition: what news? Clinical & Experimental Rheumatology. 2015;33(1 Suppl 88):S117.
55. Zhang Y, Jia R, Zhang Y, et al. Effect of non-surgical periodontal treatment on cytokines/adipocytokines levels among periodontitis patients with or without obesity: a systematic review and meta-analysis. BMC Oral Health. 2023;23(1):717. doi:10.1186/s12903-023-03383-3
56. Al Khathlan N. Association of inflammatory cytokines with obesity and pulmonary function testing. PLoS One. 2023;18(11):e0294592. doi:10.1371/journal.pone.0294592
57. Cancela-Carral JM, Bezerra P, Lopez-Rodriguez A, et al. Degree of association between the body mass index (BMI), waist-Hip ratio (WHR), waist-height ratio (WHtR), body adiposity index (BAI) and conicity index (CI) in physically active older adults. Clinical Nutrition ESPEN. 2023;58:335–341. doi:10.1016/j.clnesp.2023.10.007
58. Tan G, Jensen MP, Thornby JI, et al. Validation of the brief pain inventory for chronic nonmalignant pain. The Journal of Pain. 2004;5(2):133–137. doi:10.1016/j.jpain.2003.12.005
59. Im DD, Jambaulikar GD, Kikut A, et al. Brief pain inventory–short form: a new method for assessing pain in the emergency department. Pain Medicine. 2020;21(12):3263–3269. doi:10.1093/pm/pnaa269
60. Häuser W, Jung E, Erbslöh-Möller B, et al. Validation of the fibromyalgia survey questionnaire within a cross-sectional survey. PLoS One. 2012;7(5):e37504. doi:10.1371/journal.pone.0037504
61. Carrillo-de-la-Peña MT, Triñanes Y, González-Villar A, et al. Convergence between the 1990 and 2010 ACR diagnostic criteria and validation of the Spanish version of the Fibromyalgia Survey Questionnaire (FSQ). Rheumatol Int. 2015;35(1):141–151. doi:10.1007/s00296-014-3074-3
62. Bidari A, Ghavidel-Parsa B, Amir Maafi A, et al. Validation of fibromyalgia survey questionnaire and polysymptomatic distress scale in a Persian population. Rheumatol Int. 2015;35(12):2013–2019. doi:10.1007/s00296-015-3340-z
63. Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34(3):220–233. doi:10.1097/00005650-199603000-00003
64. Tawiah AK, Al Sayah F, Ohinmaa A, et al. Discriminative validity of the EQ-5D-5 L and SF-12 in older adults with arthritis. Health Qual Life Outcomes. 2019;17(1):68. doi:10.1186/s12955-019-1129-6
65. Yoshida M, Igawa Y, Higashimura S, et al. Translation and Reliability and Validity Testing of a Japanese Version of the Intermittent Self‐Catheterization Questionnaire Among Disposable and Reusable Catheter Users.doi:10.1002/nau.23111
66. Montazeri A, Vahdaninia M, Mousavi SJ, et al. The Iranian version of 12-item short form health survey (SF-12): factor structure, internal consistency and construct validity. BMC Public Health. 2009;9:341. doi:10.1186/1471-2458-9-341
67. Ibrahim AA, Akindele MO, Ganiyu SO, et al. The Hausa 12-item short-form health survey (SF-12): translation, cross-cultural adaptation and validation in mixed urban and rural Nigerian populations with chronic low back pain. PLoS One. 2020;15(5):e0232223. doi:10.1371/journal.pone.0232223
68. Gottlieb U, Yona T, Lumbroso DS, et al. Reliability and validity of patient-reported outcome measures for ankle instability in Hebrew. Med Sci Monit. 2022;28:e937831–1–e937831–10. doi:10.12659/MSM.937831
69. Shou J, Ren L, Wang H, et al. Reliability and validity of 12-item short-form health survey (SF-12) for the health status of Chinese community elderly population in Xujiahui district of Shanghai. Aging Clin Exp Res. 2016;28(2):339–346. doi:10.1007/s40520-015-0401-9
70. Amir M, Lewin-Epstein N, Becker G, et al. Psychometric properties of the SF-12 (Hebrew version) in a primary care population in Israel. Medical Care. 2022;40(10):918. doi:10.1097/00005650-200210000-00009
71. Lin Y, Yu Y, Zeng J, et al. Comparing the reliability and validity of the SF-36 and SF-12 in measuring quality of life among adolescents in China: a large sample cross-sectional study. Health Qual Life Outcomes. 2020;18:360. doi:10.1186/s12955-020-01605-8
72. Shah RM, Banahan BF, Holmes ER, et al. An evaluation of the psychometric properties of the sf-12v2 health survey among adults with hemophilia. Health Qual Life Outcomes. 2018;16:229. doi:10.1186/s12955-018-1059-8
73. White MK, Maher SM, Rizio AA, et al. A meta-analytic review of measurement equivalence study findings of the SF-36® and SF-12® Health Surveys across electronic modes compared to paper administration. Qual Life Res. 2018;27(7):1757–1767. doi:10.1007/s11136-018-1851-2
74. De Smedt D, Clays E, Doyle F, et al. Validity and reliability of three commonly used quality of life measures in a large European population of coronary heart disease patients. International Journal of Cardiology. 2013;167(5):2294–2299. doi:10.1016/j.ijcard.2012.06.025
75. Merriwether EN, Rakel BA, Zimmerman MB, et al. Reliability and construct validity of the Patient-Reported Outcomes Measurement Information System (PROMIS) instruments in women with fibromyalgia. Pain Med. doi:10.1093/pm/pnw187
76. Chimenti RL, Rakel BA, Dailey DL, et al. Test–retest reliability and responsiveness of PROMIS sleep short forms within an RCT in women with fibromyalgia. Front Pain Res. 2021;2:682072. doi:10.3389/fpain.2021.682072
77. Cook KF, Dunn W, Griffith JW, et al. Pain assessment using the NIH Toolbox. Neurology. 2013;80(11 Suppl 3):S49–S53. doi:10.1212/WNL.0b013e3182872e80
78. Boomershine CS. A comprehensive evaluation of standardized assessment tools in the diagnosis of fibromyalgia and in the assessment of fibromyalgia severity. Pain Research and Treatment. 2012;2012:1–11. doi:10.1155/2012/653714
79. Kroenke K, Spitzer RL, Williams JBW, et al. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. General Hospital Psychiatry. 2010;32(4):345–359. doi:10.1016/j.genhosppsych.2010.03.006
80. Kesztyüs D, Brucher S, Wilson C, et al. Use of infrared thermography in medical diagnosis, screening, and disease monitoring: a scoping review. Medicina. 2023;59(12):2139. doi:10.3390/medicina59122139
81. Aguilar-Ferrándiz ME, Casas-Barragán A, Tapia-Haro RM, et al. Evaluation of sympathetic adrenergic branch of cutaneous neural control throughout thermography and its relationship to nitric oxide levels in patients with fibromyalgia. Journal of Thermal Biology. 2021;95:102813. doi:10.1016/j.jtherbio.2020.102813
82. Casas-Barragán A, García-Ríos MC, Rus A, et al. Associations among serum VEGF and CGRP levels with the peripheral vascular blood flow of the skin of the hands in women with Fibromyalgia. Journal of Thermal Biology. 2023;112:103469. doi:10.1016/j.jtherbio.2023.103469
83. Elmas O, Yildiz S, Bilgin S, et al. Physiological parameters as a tool in the diagnosis of fibromyalgia syndrome in females: a preliminary study. Life Sciences. 2016;145:51–56. doi:10.1016/j.lfs.2015.12.029
84. Sempere-Rubio N, Aguilar-Rodríguez M, Inglés M, et al. Thermal imaging ruled out as a supplementary assessment in patients with fibromyalgia: a cross-sectional study. PLoS One. 2021;16(6):e0253281. doi:10.1371/journal.pone.0253281
85. Kamali F, Mirkhani H, Nematollahi A, et al. The effect of transcutaneous electrical nerve stimulation of sympathetic ganglions and acupuncture points on distal blood flow. Journal of Acupuncture and Meridian Studies. 2017;10(2):120–124. doi:10.1016/j.jams.2017.01.003
86. He T, Li J, Ouyang Y, et al. FibroScan detection of fatty liver/liver fibrosis in 2266 cases of chronic hepatitis B. Journal of Clinical and Translational Hepatology. 2020;8(2):113–119. doi:10.14218/JCTH.2019.00053
87. Tapper EB, Castera L, Afdhal NH. FibroScan (Vibration-Controlled Transient Elastography): where does it stand in the United States practice. Clinical Gastroenterology and Hepatology. 2015;13(1):27–36. doi:10.1016/j.cgh.2014.04.039
88. Martínez‐Arenas L, Vinaixa C, Conde I, et al. FibroScan compared to liver biopsy for accurately staging recurrent hepatic steatosis and fibrosis after transplantation for MASH. Liver International. 2024;44:3174–3182. doi:10.1111/liv.16085
89. Eddowes PJ, Sasso M, Allison M, et al. Accuracy of fibroscan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717–1730. doi:10.1053/j.gastro.2019.01.042
90. Mikolasevic I, Orlic L, Franjic N, et al. Transient elastography (FibroScan ®) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease - Where do we stand? WJG. 2016;22(32):7236. doi:10.3748/wjg.v22.i32.7236
91. Mueller C, Fang YHD, Jones C, et al. Evidence of neuroinflammation in fibromyalgia syndrome: a [18F]DPA-714 positron emission tomography study. Pain. 2023;164(10):2285–2295. doi:10.1097/j.pain.0000000000002927
92. Vega-Ramírez MT, Becerril-Villanueva E, Maldonado-García JL, et al. S100 proteins: a new frontier in fibromyalgia research. Mol Brain. 2024;17(1):29. doi:10.1186/s13041-024-01102-9
93. Zanette SA, Dussan-Sania JA, Souza A, et al. Higher Serum S100B and BDNF levels are correlated with a lower pressure-pain threshold in fibromyalgia. Mol Pain. 2014;10:1744–8069–10–46. doi:10.1186/1744-8069-10-46
94. Tapia-Haro RM, Molina F, Rus A, et al. Serum VEGF and CGRP biomarkers: relationships with pain intensity, electric pain, pressure pain threshold, and clinical symptoms in fibromyalgia—an observational study. IJMS. 2023;24(21):15533. doi:10.3390/ijms242115533
95. Korucu RU, Karadağ A, Taş A, et al. Serum calcitonin gene-related peptide and receptor protein levels in patients with fibromyalgia syndrome: a cross-sectional study. Arch Rheumatol. 2020;35(4):463–467. doi:10.46497/ArchRheumatol.2020.7783
96. Lesnak J, Merriwether E, Taylor E, et al. (328) select metabolomics reveal potential biomarkers of fibromyalgia that correlate with pain and fatigue. The Journal of Pain. 2019;20(4):S57. doi:10.1016/j.jpain.2019.02.024
97. Clos-Garcia M, Andrés-Marin N, Fernández-Eulate G, et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. eBioMedicine. 2019;46:499–511. doi:10.1016/j.ebiom.2019.07.031
98. Mg D, M MDB, Cr M, et al. Combined physiotherapy and cognitive behavioral therapy for functional movement disorders: a randomized clinical trial. JAMA neurology. 2024;81(9). doi:10.1001/jamaneurol.2024.2393
99. Ueda H. Systems pathology of neuropathic pain and fibromyalgia. Biological & Pharmaceutical Bulletin. 2019;42(11):1773–1782. doi:10.1248/bpb.b19-00535
100. Shi G, Bai H. Clinical study on balance acupuncture combined with moving qi therapy treatment of acute Lumbar Sprain. China Journal of Chinese Medicine. 2015;30(5):759–760.
101. Choi DH, Kim HS. Quantitative analysis of nailfold capillary morphology in patients with fibromyalgia. Korean J Intern Med. 2015;30(4):531. doi:10.3904/kjim.2015.30.4.531
102. Zou J, Li J, Wang X, et al. Neuroimmune modulation in liver pathophysiology. J Neuroinflammation. 2024;21(1):188. doi:10.1186/s12974-024-03181-w
103. Cheng DK, Lai KSP, Pico-Espinosa OJ, et al. Interventions for depressive symptoms in people living with chronic pain: a systematic review of meta-analyses. Pain Medicine. 2022;23(5):934–954. doi:10.1093/pm/pnab248
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

