Back to Journals » Journal of Pain Research » Volume 18
Efficacy and Safety of a Novel Low-Dose Water-Dispersible Turmeric Extract in the Management of Knee Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Authors Thanawala S , Shah R , Alluri KV , Bhupathiraju K, Prasad N, Agarwal Y
Received 17 October 2024
Accepted for publication 11 January 2025
Published 25 January 2025 Volume 2025:18 Pages 411—427
DOI https://doi.org/10.2147/JPR.S501505
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Robert B. Raffa
Shefali Thanawala,1 Rajat Shah,2 Krishnaraju Venkata Alluri,3 Kiran Bhupathiraju,4 Nandlal Prasad,5 Yash Agarwal6
1Medical Science and Research Department, Nutriventia Limited, Mumbai, Maharashtra, 400069, India; 2Executive Management, Nutriventia Limited, Mumbai, Maharashtra, 400069, India; 3Pharmacology and Clinical Research, Laila Nutraceuticals, Vijayawada, Andhra Pradesh, 520010, India; 4Research & Development, Laila Nutraceuticals, Vijayawada, Andhra Pradesh, 520010, India; 5Department of Orthopedics, Bajarang Memorial Fracture Accidental and Surgical Center, Varanasi, Uttar Pradesh, 221003, India; 6Department of Orthopedics, Sri Ram Ortho and Physio Rehab Centre, Varanasi, Uttar Pradesh, 221007, India
Correspondence: Shefali Thanawala, Medical Science and Research Department, Unit 703 & 704, 7 th Floor Solaris One Premises Co-Operative Society Limited, N.S. Phadke Marg, Andheri (East), Nutriventia Limited, Mumbai, 400069, Maharashtra, India, Tel +912269113721, Email [email protected]
Purpose: Turmeric extract is a well-known nutraceutical ingredient recognized for its benefits in managing musculoskeletal health. This study evaluated the efficacy and safety of a novel low-dose water-dispersible turmeric extract containing 60% natural curcuminoids (WDTE60N) in participants with mild-to-moderate knee osteoarthritis.
Patients and Methods: This double-blind, randomized, placebo-controlled trial was conducted at two orthopedic centers in Uttar Pradesh, India (July 2023–November 2023). Participants aged 45– 75 years with unilateral or bilateral OA of the knee for > 3 months were randomized in 1:1 ratio to receive WDTE60N (250 mg) or placebo capsules once daily for three months. Study endpoints included assessment of changes from baseline to day 90 in pain intensity (visual analog scale [VAS], knee injury and osteoarthritis outcome score [KOOS]), inflammatory biomarkers, and safety profile. Data were analyzed using independent t-test, chi-square test, and analysis of co-variance test.
Results: In total, 139 participants (WDTE60N, n = 70; placebo, n = 69) with mean age and BMI of 56.35 years and 23.89 kg/m2, respectively, were included. The mean reduction (95% CI) in VAS score from baseline to day 90 was significantly higher in the WDTE60N group than in the placebo group (14.41 [13.08, 15.75] vs 6.02 [5.00, 7.05]; p < 0.0001). In the WDTE60N group, the mean change in VAS scores was significantly reduced from as early as day 07 (p = 0.0076), which continued until day 90 (p < 0.0001), compared to the placebo group. Improvement in the mean KOOS scores (baseline-Day 90) was evident, with significantly higher mean scores for each domain (pain, symptoms, activities of daily living, function in sport and recreation, and knee-related quality of life [QoL]) on Day 90 in the WDTE60N group than in the placebo group (p < 0.05). Inflammatory biomarkers (hsCRP, TNF-α, IL-6, and IL-1β) were significantly reduced from baseline to day 90 in the WDTE60N group compared to the placebo group (p < 0.0001). Four mild adverse events were reported during the study period.
Conclusion: Supplementation with the low-dose water-dispersible turmeric extract containing 60% natural curcuminoids for three months was safe and effective in alleviating pain, improving functional status and quality of life and reducing inflammation in participants with mild-to-moderate knee osteoarthritis.
CTRI Registration Number: CTRI/2023/07/055411
Keywords: curcuminoids, inflammatory biomarkers, KOOS, pain, QoL, visual analog scale
Introduction
Knee osteoarthritis (OA), the most prevalent form of OA, is a degenerative joint disease of the knee that typically results from wear and tear and progressive loss of articular cartilage. According to the International Classification of Diseases (ICD) classification, knee OA is classified as unilateral or bilateral primary knee OA, unilateral or bilateral post-traumatic knee OA, and other unilateral or bilateral secondary knee OA.1 Clinically, it is characterized by progressive worsening of symptoms such as pain, inflammation, stiffness-related discomfort, and reduced mobility over the course of the disease. If left untreated, the disease can advance, potentially resulting in disability and impaired quality of life (QoL).2,3 According to the latest Global Burden of Disease Study (GBD) report, approximately 7.6% of the global population was diagnosed with OA in 2020. Knee OA was a major contributor to this global burden, affecting 4307 individuals per 100,000 population. Furthermore, it is anticipated that the prevalence of knee OA will increase by 74.9% from 2020 to 2050, indicating a substantial increase in the disease burden that will pose a critical challenge for healthcare systems worldwide.3
Given that knee pain is the central cause of disability in patients with OA and adversely affects their functional abilities and QoL, the primary goal of treatment is to alleviate the intensity of knee pain.4–6 Among the currently available management strategies including pharmacological, non-pharmacological, and surgical interventions, pharmacological treatments (such as non-steroidal anti-inflammatory drugs [NSAIDs], acetaminophen, naproxen, selective cyclooxygenase-2 [COX-2] inhibitor, glucosamine, capsaicin, steroids, etc) are the most commonly used.2,7,8 Despite various advancements in the available management strategies over the past few decades, no cure exists for knee OA; treatments primarily aim to delay disease progression.2 In particular, due to the safety concerns associated with the long-term use of these pharmacological treatments, there is growing interest in exploring safer natural therapies. In the past decade, supplementation with nutraceuticals for the management of OA has gained attention owing to their promising role in alleviating pain, inflammation, oxidative stress, and joint discomfort while also promoting cartilage formation.9
The potential of turmeric to offer a myriad of health benefits has been well recognized since ancient times in Asian countries. Curcuminoids are the major phytochemicals found in the rhizome of the turmeric plant (Curcuma longa L.) of which curcumin is the major phytoactive and the other two are demethoxycurcumin and bisdemethoxycurcumin. Curcuminoids, also commonly called curcumin, have several pharmacological effects, such as anti-inflammatory, antitumor, antioxidant, lipid-regulating, and anticoagulant effects.10,11 Within the wide range of dietary supplements evaluated, curcumin has been reported as one of the most potent and effective nutraceutical ingredients in reducing the severity of pain in patients with OA.12,13 A strong wall of evidence supports the efficacy and safety of curcumin in alleviating pain and improving functional abilities in individuals with OA.14–17 However, its poor absorption and low bioavailability, which necessitate the administration of multiple daily doses, might affect treatment adherence, especially during long-term use. In addition, high doses of turmeric extract and curcumin (>4000 mg/day) are associated with an increased risk of adverse events such as gastrointestinal symptoms, liver enzyme abnormalities, and urticaria.18–20 Currently, most bioavailable turmeric extracts in the market are formulated with synthetic excipients and contain up to 20% active ingredients, still requiring higher doses to achieve clinical efficacy.21 Thus, there exists a gap in developing a turmeric formulation that can deliver therapeutic benefits at lower doses. To address these limitations, a novel low-dose, water-dispersible turmeric extract containing 60% natural curcuminoids (WDTE60N of Nutriventia Limited and Laila Nutraceuticals, India) was developed. This novel formulation was designed to deliver optimal benefits with a single daily low dose of 250 mg, containing 150 mg of curcuminoids.
Robust evidence from previous pharmacokinetic studies demonstrated a higher bioavailability of WDTE60N compared to the commercially available standard turmeric extract formulations, both with and without the bioavailability enhancer – piperine.21,22 In particular, once a day, administration of WDTE60N can provide the clinical benefits of curcumin at a ten-fold lower dose of 150 mg of curcuminoids compared to multiple daily doses of the standard 95% turmeric extract, which contains 1500 mg of curcuminoids.21 This was demonstrated in two clinical trials. A randomized, double-blind, placebo-controlled trial conducted among healthy adults with chronic knee pain demonstrated the efficacy of WDTE60N, administered for 3 months, in terms of significant improvements in joint function and mobility and alleviation of chronic knee pain.23 Another similarly designed clinical trial was conducted on the delayed onset muscle soreness in recreationally active healthy adults after a bout of acute exercise. The WDTE60N was well tolerated in these studies.24
These promising outcomes, along with multiple clinical studies evaluating other curcumin supplements in patients with knee OA,14,16,25–28 highlight the growing body of evidence supporting the use of curcumin supplements in the management of knee OA. However, to date, none of these studies have assessed the efficacy of curcumin at shorter time intervals after the initiation of product administration. In view of this lacuna, the present study aimed to assess the efficacy and safety of a WDTE60N in participants with mild-to-moderate knee OA, with a focus on understanding its effects at earlier timepoints, starting as early as day 03 following administration.
Materials and Methods
Study Design
This prospective, double-blind, randomized, multicenter, parallel-group, two-arm, placebo-controlled trial included participants with mild-to-moderate knee OA. This study was conducted at two sites in Uttar Pradesh, India (Bajarang Memorial, Fracture, Accidental and Surgical Center, Varanasi and Sri Ram Ortho, and Physio Rehab Center, Varanasi) between July 27, 2023, and November 20, 2023. The study protocol was approved by the Vatsalya Ethics Committee, Vatsalya Hospital Multispecialty Center, Sikraul, Varanasi, Uttar Pradesh – 221002 (vide registration no: ECR/1611/Inst/UP/2021). This trial was registered in the Clinical Trials Registry (CTRI) on 29/07/2023 (CTRI registration no. CTRI/2023/07/055411). This study was conducted in compliance with the International Conference on Harmonization-Good Clinical Practices (ICH-GCP) guidelines E6 (R2), 2016; ICMR guidelines – National Ethical Guidelines for Biomedical and Health Research Involving Human Participants, 2017; Declaration of Helsinki, 2013; applicable local government regulations, institutional research policies, and standard operating procedures. Written informed consent was obtained from each participant before initiation of the clinical study.
Study Population
Men and women aged 45–75 years with a body mass index (BMI) of 18–29 kg/m2, who had unilateral or bilateral OA of the knee for >3 months (National Institute for Health and Care Excellence [NICE] criteria29) with Kellgren-Lawrence grade II or III by radiographic classification and with a visual analog scale (VAS) score between 40 and 70 mm during the most painful knee movement, and who had mild-to-moderate pain (diagnosed based on VAS score between 5 and 44 mm as mild pain and 45 and 75 mm as moderate pain30) not adequately or completely controlled with anti-inflammatory drugs were enrolled in the study. Additional inclusion criteria were as follows: otherwise, healthy participants determined by medical history, physical examination, and clinical judgment of the principal investigator (PI) and participants willing to provide informed consent and comply with the study procedure. Participants with the following criteria were excluded from the study: those who underwent treatment for Coronavirus disease 2019 (COVID-19) within the last three months or tested positive during the study; known allergy to NSAIDs (including aspirin); suspected hypersensitivity, allergy, or other contraindications to any compound present in the study products; history or presence of injury in the area affected by OA of the knee, underlying inflammatory arthropathy, rheumatoid arthritis, severe OA, gout, systemic lupus erythematosus or any other systemic disorder affecting joints, congestive heart failure; evidence or history of clinically significant hematological, renal, endocrine, pulmonary, gastrointestinal, cardiovascular, hepatic, neurologic diseases, or malignancies (as per the discretion of the PI); or who received any corticosteroid, indomethacin, glucosamine or chondroitin within three months prior to the treatment period, or intra-articular treatment/injections with corticosteroid, or hyaluronic acid, or Omega-3 Fatty acids dietary supplements within six months preceding the study.
Study Procedure and Treatment Randomization
After providing written informed consent, the participants underwent a detailed screening assessment on visit 1 (seven days before treatment administration) by trained study personnel. The evaluations included physical examination, assessment of vital signs, demographic and anthropometric measurements, medical history (including substance abuse and/or addiction to alcohol), treatment and surgical history, OA screening according to NICE criteria,29 X-ray examination of the knee, measurement of pain score using VAS, clinical laboratory examinations for hematology, liver function, renal function, serological determination of human immunodeficiency virus (HIV), Hepatitis B, and Hepatitis C, COVID-19 screening with rapid antigen test, urine routine analysis, and urine pregnancy test for women of childbearing potential. The diagnosis of knee OA was confirmed by the PIs, who are qualified orthopedic surgeons with over ten years of clinical experience. For the clinical laboratory evaluations, 5–8 mL of blood was collected via venipuncture from each participant at each visit.
An independent statistician generated the randomization codes (employing a permuted block design with a block size of 4) using the Jamovi (2022, Version 2.3) and R programming (2021, Version 4.1) software. To ensure impartiality in the randomization process, the block size was disclosed only to the trial statistician until the statistical analysis was completed. The randomization code list was centrally maintained by the sponsor designee and was distributed to the site coordinator in a concealed envelope, with instructions to unblind only at the discretion of PI and sponsor. The blinding and labelling were carried out by the unblinded personnel. The study products were packaged in identical containers, and each labelled a randomization code. The sponsor, clinical research organization, PIs, study personnels, site staff and participants were all blinded in the study. At visit 2 (Day 01), the PI randomly assigned eligible participants in a 1:1 ratio to WDTE60N (TurmXTRA® 60N: 250 mg natural, water-dispersible turmeric extract capsules containing 60% natural curcuminoids, manufactured by Nutriventia Limited, Mumbai, India, and Laila Nutraceuticals, India) or placebo (identical capsules that matched the color, size, and shape of the WDTE60N, but without active, manufactured by Nutriventia Limited, Mumbai, India). The participants were provided 33 capsules of either WDTE60N or placebo for 30 ± 3 days on days 1, 30, and 60. They were instructed to take one capsule orally once daily in the morning after breakfast, with adequate water. All participants were followed up at different interim time points, starting from visit 3 (Day 3 ± 1) until the end of the study (visit 9 [Day 90 ± 3]) (Figure 1). Additionally, all participants were provided with a diary to maintain a daily log of study product administration and record any adverse events (AE) experienced during the study period. Treatment compliance and monitoring of treatment-emergent adverse events (TEAEs) for each participant were assessed by reviewing their respective diaries at each follow-up visit until the end of the study. All participants were prohibited from using ibuprofen, aspirin, or any other NSAIDs, as well as any other pain relievers, including over-the-counter or prescribed topical treatments, throughout the entire trial, except for acetaminophen/paracetamol as a rescue medication. For participants for whom the study treatment was deemed ineffective, paracetamol 650 mg (with a maximum daily dose of three tablets) was permitted as a rescue medication at the discretion of the PI.
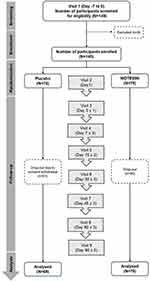 |
Figure 1 Study flowchart and participants’ disposition. Abbreviation: WDTE60N, water-dispersible turmeric extract containing 60% natural curcuminoids. |
Study Endpoints
The primary efficacy endpoint was to evaluate and compare the change in VAS pain score (100 mm) from baseline (Day 01) to the end of treatment (Day 90) between the WDTE60N and placebo groups. The secondary efficacy endpoints were to determine changes in the VAS pain score (100 mm) at all follow-up visits (days 03, 07, 15, 30, 45, and 60) from baseline, and changes in the pain intensity based on knee injury and osteoarthritis outcome score (KOOS) domain scores at all interim study visits until the end of the study from baseline (days 03, 07, 15, 30, 45, 60, and 90). Furthermore, we evaluated changes in serum inflammatory markers (tumor necrosis factor-α [TNF-α], high-sensitivity C-reactive protein [hsCRP], interleukin-6 [IL-6], and interleukin-1β [IL-1β]) from baseline to days 45 and 90. The subject’s global assessment (SGA) and physicians’ global assessment (PGA) of therapy at the end of treatment (Day 90) were also evaluated. During the safety assessment, we monitored TEAEs at all interim visits until the end of the study. The collected data included the nature of the AE, date and time of onset, intensity, duration, causality, action taken, and the outcome of the event.
Assessment Scales
In this study, pain intensity was measured using VAS and KOOS assessment scales. The VAS tool involves a horizontal line marked from 0 to 100 (100 mm scale), anchored by word descriptors at each end (“0” indicates no pain and “100” indicates severe pain).31 The KOOS is a self-administered validated instrument that evaluates five patient-relevant dimensions through 42 items: pain (nine items), symptoms (seven items), activities of daily living (ADL) function (17 items), sport and recreation function (five items), and quality of life (four items). It helps assess short- and long-term patient-relevant outcomes following knee injury and OA.32,33 Levels of inflammatory biomarkers were determined by enzyme-linked immunosorbent assay (ELISA) method in the NABL-accredited and ISO (9001:2015)-certified laboratory. In addition, the SGA and PGA were evaluated using an internally developed scale ranging from 1 to 7 (1 indicates improved knee pain and overall health, and 7 indicates worsened knee pain and overall health). A lower score typically indicates a better improvement in overall health and treatment satisfaction, whereas a higher score suggests poorer knee health and treatment dissatisfaction.
Statistical Analysis
The sample size was calculated using the G*Power (version 3.1.9.3) software.34 The primary outcome of the VAS score after the 80 m fast-paced walk test in the previous clinical trial of WDTE60N in chronic knee pain was used to estimate the sample size and power using the effect size.23 With an effect size of at least 0.535, a sample size of 56 participants in each group was required to achieve a power of 80% at a significance level (alpha) of 0.05. Considering the 20% dropout rate, a sample size of approximately 70 participants (56+14=70) per group was considered adequate for randomization in this study.
All subjects who consumed at least one dose of the study product were included in the intention-to-treat (ITT) population. The missing observations were inputted using the last observation carried forward (LOCF) approach. Participants who had no major protocol violations affecting the efficacy variables and had treatment compliance of ≥80% were included in the per-protocol (PP) population. All efficacy evaluations were conducted in the PP population. Participants who received at least one dose of study treatment were included in the safety analysis population.
Statistical analysis was performed using the SAS® software (SAS Institute Inc., Cary, NC, USA), version 9.4. Descriptive statistics was used to summarize categorical data as frequencies and percentages, and continuous data as mean and standard deviation (SD). An independent t-test was used for intergroup comparisons of continuous data, and the chi-square test was used for intergroup comparisons of categorical data. The change from the baseline was calculated by subtracting the baseline value from the post-dose value at that evaluation time point. Comparison of the mean changes from baseline to other time points between the groups was performed using analysis of co-variance (ANCOVA) test. Statistical significance was set at p <0.05.
Results
Demographic Details
Of the 149 participants screened, 140 eligible participants were randomized to either the WDTE60N or placebo group. One participant (dropout rate = 0.71%) from the placebo group dropped out, and 139 participants (WDTE60N, n = 70; placebo, n = 69) were included in the PP analysis (adherence rate = 99.29%) (Figure 1). The mean (SD) age and BMI of participants was 56.35 (6.86) years and 23.89 (1.63) kg/m2, respectively. The demographic and anthropometric characteristics of the study population at the baseline are summarized in Table 1.
 |
Table 1 Demographic Parameters |
Assessment of Pain Intensity Using VAS
Figure 2 presents the changes in the mean VAS scores of both study groups over 90 days of the study duration. The mean reduction in VAS scores from baseline to day 90 was significantly higher in the WDTE60N group (14.41) than that in the placebo group (6.02) (p < 0.0001) with Cohen’s d effect size of 1.07. Improvements in pain intensity on Day 90 were 25.05% and 10.57% in the WDTE60N and placebo groups, respectively. The mean VAS score in the WDTE60N group was significantly lower from Day 30 onwards (p = 0.0064) and continued to be significantly lower on days 45 (p < 0.0001), 60 (p < 0.0001), and 90 (p < 0.0001) than that in the placebo group. The comparative assessment of the mean change in VAS scores showed a significantly higher mean change in VAS scores (from baseline) as early as Day 07 (2.73 [95% confidence interval {CI}: 2.29, 3.17] vs 1.15 [95% CI: 0.65, 1.67]; p = 0.0076), with the trend continuing on all subsequent evaluation visits on days 15 (4.76 [95% CI: 4.10, 5.43] vs 2.59 [95% CI: 2.03, 3.16]; p = 0.0008), 30 (7.11 [95% CI: 6.11, 8.12] vs 3.78 [95% CI: 3.09, 4.46]; p < 0.0001), 45 (9.96 [95% CI: 8.76, 11.16] vs 4.83 [95% CI: 4.00, 5.66]; p < 0.0001), 60 (13.50 [95% CI: 12.30, 14.69] vs 5.31 [95% CI: 4.27, 6.31]; p < 0.0001), and 90 (14.41 [95% CI: 13.08, 15.75] vs 6.02 [95% CI: 5.00, 7.05]; p < 0.0001) in the WDTE60N group compared to the placebo group (Figure 2).
Assessment of Pain Intensity Using KOOS
The changes in KOOS subscale scores from baseline to the end of treatment (Day 90) are summarized in Table 2. Over the 90-day treatment period, the mean KOOS scores for all five subscales showed an upward trend towards improvement from baseline in both groups; however, notably, at Day 90, the mean KOOS scores for each of the domains in the WDTE60N group were significantly higher than those in the placebo group (p < 0.0001 for Pain [Cohen’s d effect size = 1.53], Symptom [Cohen’s d effect size = 0.90], and ADL [Cohen’s d effect size = 1.33]; p = 0.0323 for Function in sports and recreation [Cohen’s d effect size = 0.37]; p = 0.0087 for Knee-related QoL[Cohen’s d effect size = 0.45]). This improvement in KOOS scores on Day 90 in the WDTE60N and placebo groups was 42.98% and 10.99% for the KOOS-pain scale, 33.79% and 19.45% for the KOOS-symptom scale, 39.68% and 18.36% for KOOS-ADL, 25.60% and 16.15% for KOOS-function in sports and recreation, and 19.68% and 8.38% for KOOS-knee-related QoL, respectively.
 |
Table 2 Comparative Analysis of Change in KOOS Domain Scores Between WDTE60N and Placebo From Baseline to Day 90 |
KOOS – Pain
Compared to placebo, a significant improvement was reported in the mean KOOS-pain score in the WDTE60N group on Day 07 (p = 0.0148), and then from day 45 onwards (p = 0.0422), which continued on days 60 (p = 0.0005) and 90 (p < 0.0001). Compared to placebo, the mean change from baseline was significantly improved in the WDTE60N group as early as Day 03 (p < 0.0001) and Day 07 (p = 0.0035) and then was consistently observed on days 45 (p = 0.0309), 60 (p = 0.0002), and 90 (p < 0.0001) (Table 2).
KOOS – Symptom
The mean KOOS-symptom score in the WDTE60N group was significantly higher on days 15 (p = 0.0060), 45 (p = 0.0065), and 90 (p < 0.0001) than that in the placebo group. The mean change from baseline was significantly higher in the WDTE60N group on days 07 (p = 0.0311), 15 (p = 0.0043), 45 (p = 0.0065), and 90 (p < 0.0001) than that observed in the placebo group (Table 2).
KOOS – Activities of Daily Living
Compared to placebo, a significant improvement was reported in the mean KOOS score for ADL in the WDTE60N group on Day 07 (p = 0.0024), and then on Day 60 (p < 0.0001), and Day 90 (p < 0.0001). Furthermore, the mean change from baseline was significantly higher in the WDTE60N group on days 07 (p = 0.0008), 60 (p < 0.0001), and 90 (p < 0.0001) than that in the placebo group (Table 2).
KOOS – Function in Sports and Recreation
The mean KOOS score for Function in sports and recreation in the WDTE60N group was significantly higher on Day 15 (p = 0.004) and Day 90 (p = 0.0323) than that in the placebo group. Similarly, a significant improvement in the mean change from baseline was observed on days 15 (p = 0.0025) and 90 (p = 0.0267) in the WDTE60N group compared to the placebo group (Table 2).
KOOS – Knee-Related QoL
Compared to the placebo group, the WDTE60N group showed a significant improvement in the mean KOOS – knee-related QoL score from day 30 onwards (p = 0.004) (Day 45, p = 0.0006; Day 60, p = 0.0425) until day 90 (p = 0.0087). Similarly, the WDTE60N group demonstrated a significant improvement in the mean change from baseline on days 30 (p = 0.0038), 45 (p = 0.0006), 60 (p = 0.0403), and 90 (p = 0.0086) (Table 2).
Assessment of Inflammatory Biomarkers
The mean change in the levels of inflammatory biomarkers (including hsCRP, TNF-α, IL-6, and IL-1β) from baseline to Day 45 and Day 90 showed significant reductions in the WDTE60N group compared to placebo group (hsCRP [ng/mL]: Day 45, −16.57 [95% CI: −18.85, −14.30] vs −3.91 [95% CI: −4.73, −3.10] [p < 0.0001] and Day 90, −27.33 [95% CI: −29.78, −24.88] vs −6.27 [95% CI: −7.47, −5.07] [p < 0.0001; Cohen’s d = 1.04]; TNF-α [pg/mL]: Day 45, −0.81 [95% CI: −0.92, −0.71] vs −0.09 [95% CI: −0.16, −0.03] [p < 0.0001] and Day 90, −1.55 [95% CI: −1.68, −1.42] vs −0.17 [95% CI: −0.25, −0.08] [p < 0.0001; Cohen’s d = 1.49]; IL-6 [pg/mL]: Day 45, −0.15 [95% CI: −0.18, −0.13] vs −0.04 [95% CI: −0.06, −0.01] [p < 0.0001] and Day 90, −0.30 [95% CI: −0.35, −0.27] vs 0.04 [95% CI: 0.01, 0.07] [p < 0.0001; Cohen’s d = 0.66]); and IL-1β [pg/mL]: Day 45, −0.24 [95% CI: −0.32, −0.16] vs −0.06 [95% CI: −0.10, −0.03] [p = 0.0128] and Day 90, −0.61 [95% CI: −0.69, −0.53] vs −0.02 [95% CI: −0.08, 0.05] [p < 0.0001; Cohen’s d = 0.2]) (Figures 3 and 4). In particular, in the WDTE60N group, the percentage change in all inflammatory biomarkers on Day 90 from baseline was higher than that observed in the placebo group (hsCRP, −21.11 and −4.95; TNF-α, −22.86 and −2.59; IL-6, −14.08 and 1.89; IL-1β, −8.74 and −0.29, respectively).
Subject’s and Physician’s Global Assessment of Therapy
The mean (SD) scores for SGA and PGA of therapy were significantly lower in the WDTE60N group than in the placebo group (SGA: 2.17 [0.66] vs 3.29 [0.91], p < 0.0001; PGA: 2.04 [0.62] vs 3.25 [0.79], p < 0.0001).
Safety Assessment
Four AEs were reported during the study, all of which were of mild intensity and resolved without any sequalae. In the WDTE60N group, one participant experienced abdominal pain, and in the placebo group, three participants reported vomiting (n = 1), nausea (n = 1), and headache (n = 1). AEs were analyzed and concluded to be unrelated to the treatment received during the study. None of the participants required any concomitant medications for the AEs.
Discussion
Overall, the present study demonstrated that WDTE60N (250 mg) at a dose of a single capsule daily for three months substantially alleviated pain and improved the functional status and QoL of participants with mild-to-moderate knee OA compared to placebo. The salient findings of this study were as follows: i) significant improvement in pain intensity, as assessed by VAS, was observed as early as day 07 and continued to improve over the course of 90 days of treatment with WDTE60N compared to that of placebo; ii) significantly higher KOOS scores for all the five KOOS subscales, which included improvement in pain, functional status, and QoL, were observed at Day 90 in the WDTE60N group compared to the placebo group, indicating a significantly superior improvement; iii) the WDTE60N group also showed a significant reduction in the inflammatory biomarkers (hsCRP, TNF-α, IL-6, and IL-1β) at days 45 and 90 from baseline compared to the placebo group, highlighting the potential of WDTE60N in reducing inflammation; iv) significantly lower SGA and PGA scores in the WDTE60N group compared to the placebo group indicated a better improvement in their overall health or condition; and v) Incidence of one mild AE in WDTE60N group with unrelatedness to the treatment suggested acceptable safety profile of WDTE60N.
Pain is a critical factor that significantly impairs physical function, daily activities, and overall QoL in knee OA. Despite the administration of multiple treatments, persistent pain remains the most common challenge in managing knee OA and tends to worsen as the disease progresses.2,4,35 Typically, the intensity of pain is assessed using the VAS tool as it offers advantages such as quick administration, strong test–retest reliability, and sensitivity to changes in chronic inflammatory and degenerative joint pain.30,36 In the present study, pain intensity measured using the VAS showed a significant reduction in the VAS scores starting from day 7 in the WDTE60N group, which continued through day 90, compared with placebo. This progressive trend indicated that WDTE60N was effective in achieving continued and substantial relief from knee pain throughout the study. Furthermore, the improvement in pain intensity on day 90 was approximately 2.4 times greater with WDTE60N than with the placebo (25.05% vs 10.57%) and had a large effect size of 1.07 compared to the placebo. This suggests that supplementation with low-dose WDTE60N capsules for three months had a positive impact on pain alleviation in participants with mild-to-moderate knee OA. These observations are in line with those of previous studies evaluating the efficacy and safety of turmeric extracts for their analgesic and anti-inflammatory properties in individuals with knee OA.25,37 A randomized placebo-controlled trial in patients with primary knee OA reported a significant reduction in pain (assessed using VAS) on Day 21 (38.83 vs 53.83) and Day 42 (19.48 vs 46.03) from baseline in patients treated with polysaccharide-rich Curcuma longa extract compared to placebo (p < 0.05).37 Similarly, another randomized placebo-controlled study observed that 8 weeks of supplementation with a surface-controlled water-dispersible curcumin significantly improved VAS scores (p = 0.023), demonstrating notable pain relief.25 Furthermore, a recently published network meta-analysis involving 23 studies with 2175 patients provided a comprehensive evidentiary basis that highlights the efficacy and safety of curcumin, whether used alone or in combination with other treatments, in the management of knee OA. Curcumin may offer the dual benefits of enhancing therapeutic effectiveness and minimizing adverse reactions of NSAIDs and chondroprotective drugs, when used in combination. Particularly, curcumin was effective in significant reduction of VAS pain score than placebo (mean difference: −1.63, 95% CI: −2.91 to −0.45).38 These trials have used multiple daily doses of turmeric extracts. In our study, a better improvement was noted in the VAS score (mean reduction from baseline to day 90: 14.41) with a single daily dose of a comparatively lower dose of WDTE60N. In patients with knee OA, clinically important difference for VAS scores was reported as a decrease by a minimum of 8.4 points.39 In our study, WDTE60N demonstrated clinically important improvement in VAS scores (≥8.4 points decrease) starting from day 45 which continued till day 90. In contrast, the placebo group showed no clinically important improvement in VAS scores (<8.4 points) throughout the study period. This further confirms that the reduction in pain intensity with WDTE60N was clinically relevant.
The KOOS, an expanded version of the Western Ontario and McMaster Universities Arthritis Index (WOMAC) osteoarthritis index, is a pivotal advancement for assessing short- and long-term symptoms and functions in participants with knee injury and OA.36 Limited placebo-controlled trials have used KOOS to evaluate the effects of turmeric extracts or curcumin on the functional status and QoL in patients with knee OA.26,28,40 As a validated patient-reported tool with proven effectiveness across various orthopedic procedures, KOOS evaluation holds significant importance in the management of knee OA. An improvement in the KOOS score by a minimum of 8 to 10 points is generally considered clinically important.32,33,41 Our study demonstrated a clinically important improvement (>8 points increase) in all KOOS subscales by day 90. In the WDTE60N group, Pain, Symptom, and Function in sports and recreation subscales showed clinically significant improvement from day 45, whereas ADL and QoL domains showed improvement by Day 60 and Day 90, respectively. In contrast, the placebo group showed no clinically important improvement in KOOS scores (≤8 points), except for the Symptom subscale on days 60 and 90. These findings, along with the trend of improvement in the KOOS subscales with WDTE60N and medium to large effect sizes (ranging from 0.37 to 1.53), compared to placebo, emphasize its potential, with a once-daily low dose, to substantially improve pain, functional abilities, and QoL in individuals with knee OA. These findings are corroborated by a Japanese study wherein a significantly improved KOOS knee-pain score (11.98 vs 5.52; p = 0.009) was observed in participants receiving 500 mg of a standardized curcuminoid extract containing 50% curcuminoids (vs placebo) twice daily for two months. However, no improvement was noted in other four KOOS subscales.28 Interestingly, in our study, with WDTE60N supplementation, the clinically significant improvement in KOOS-pain score (11.55) was achieved earlier, ie, from Day 45, in comparison to this study, and we also observed clinical improvement in all KOOS domains. These noteworthy findings depict the potential of WDTE60N supplementation with a single daily low-dose of 150 mg curcuminoids to clinically improve functional status and knee-related QoL in individuals with mild-to-moderate knee OA. These observations are in concordance with the findings of Shep et al study in patients with knee OA, which reported that 4-weeks supplementation with curcumin 500 mg thrice daily was efficacious in consistent improvement across all five KOOS subscales on days 14 and 28 from baseline, with the difference on day 28 reaching statistical significance (p < 0.01).26 However, a Phase II placebo-controlled trial in patients with symptomatic knee OA evaluating the effect of bio-optimized curcumin extract on KOOS scores reported comparable improvement to placebo after three months of treatment period.40
Inflammation is a vital aspect of the pathophysiology of OA, and alterations in inflammatory marker levels are considered as both diagnostic and prognostic tools in the management of OA.42 A defining feature of OA is the disruption of the balance between inflammatory and anti-inflammatory signals in chondrocytes and synovial cells. This imbalance leads to abnormal activation of cytokine pathways and excessive production of inflammatory mediators.43 Evidence suggests an association between altered serum levels of certain inflammatory markers (including TNF-α, IL-1β, IL-6, and CRP) in the early stages of OA and knee pain.44,45 Therefore, changes in inflammatory markers over 90 days of study treatment were also evaluated to understand the prognostic impact of WDTE60N supplementation on inflammation in participants with knee OA. A significant reduction in inflammatory biomarkers from day 45 that was sustained until day 90 in participants receiving WDTE60N emphasized the potential of this novel low-dose turmeric extract in controlling inflammation, thereby possibly delaying the progression of knee OA. The medium to large effect sizes observed for the improvement in most of the inflammatory biomarkers (hsCRP, TNF-α, and IL-6) further substantiates clinical significance of effectiveness of WDTE60N. These findings corroborate the evidence from a previous trial of WDTE60N conducted among healthy adults with chronic knee pain, wherein the CRP and TNF-α levels showed numerically higher reduction after 90 days of treatment in the WDTE60N group compared to the placebo group.23 In contrast to our findings, a 90-day treatment with a low-dose curcumin (160 mg daily, using Optimized Curcumin with solid lipid curcumin particles) compared to ibuprofen (400 mg/day) did not result in significant reduction of inflammation (TNF-α, IL-6, IL-1β) in patients with knee OA, even though a significant improvement in symptoms was observed.46 However, some studies of other turmeric extracts have shown their efficacy in reducing inflammation in patients with OA, supporting the findings of the present study.47,48 A study by Singhal et al reported a significant reduction in CRP levels (37.21%) and TNF-α levels (74.81%) at 6 weeks from baseline in patients with knee OA who were administered a bioavailable turmeric extract (500 mg capsule twice daily) compared to those administered with paracetamol 650 mg tablet (thrice daily).47 Furthermore, observations of a double-blind, randomized, placebo-controlled study of a standardized turmeric extract in patients with primary knee OA are in line with our study, where the authors reported significant reduction of IL-1β levels on days 60 and 120 from baseline.48
This study also assessed the perspectives of both participants and physicians regarding treatment efficacy using SGA and PGA scales. Both rated WDTE60N as significantly effective in improving the overall health and patient satisfaction. The safety assessment in this study found that supplementation with WDTE60N for three months was safe, with one mild AE, compared to three AEs in the placebo group. These findings are consistent with the safety profile of WDTE60N reported in previous studies, further confirming its acceptable safety profile in patients with knee OA.23,24
The results of WDTE60N in the current study are in line with those of previous studies that have established the efficacy and tolerability of WDTE60N in the musculoskeletal health. In healthy adults with chronic knee pain, the administration of a single daily dose of WDTE60N 250 mg capsules with 150 mg curcuminoids for three months was beneficial in relieving pain, improving joint function and mobility, and was well tolerated. Similarly, when administered before and after eccentric exercise in recreationally active participants, the formulation in the same low single daily dose was effective in alleviating muscle soreness and enhancing psychological well-being.23,24 Taken together, these data, along with the findings of the present study, underscore the promising role of WDTE60N in enhancing musculoskeletal health.
The larger effect sizes observed for improvements in the majority of pain intensity and functional assessment scales and inflammatory biomarkers, along with their statistically significant improvements over the three months strongly support the potential of WDTE60N supplementation for the management of mild-to-moderate knee OA. The alleviation of pain intensity using this novel low-dose water-dispersible turmeric extract can be attributed to the anti-inflammatory, antioxidant, and cartilage-protective effects of curcumin.10,11 Curcumin can aid to preserve the structural integrity of the joints and alleviate pain associated with the degenerative process by scavenging free radicals and reducing oxidative damage in the cartilage tissues.49 Additionally, it helps reduce inflammation and damage to joint structure by promoting the synthesis of collagen and protecting chondrocytes from apoptosis and degeneration and modulates pain signaling pathways through interaction with transient receptor potential vanilloid 1 receptor involved in pain sensation.50–52 These multiple mechanisms make curcumin a natural and effective nutraceutical ingredient in the management of knee OA.
The potential drug interactions of curcumin with other standard of care drugs for osteoarthritis are an independent subject of research; however, preliminary evidence suggests a possible synergistic effect of curcumin with NSAIDs.53 However, further herb–drug interaction studies are required before any strong recommendations can be made regarding the long-term coadministration of curcumin with these medications.
To the best of our knowledge, this study is the first to use extensive time-points covering a wide range of improvement assessments at short time intervals. This approach could help to precisely understand the time point when patients experience the benefits from the supplementation. We found a profound effect of curcuminoids at a single daily dose of 150 mg on pain reduction and functional status improvement from as early as day 07 on the VAS and KOOS subscales (pain, symptoms, and ADL) compared with placebo. Furthermore, WDTE60N, which is substantially effective in reducing inflammation starting from day 45, is another unique efficacy outcome that highlights its crucial role in the effective management of knee OA. Despite these strengths, this study has some limitations that impact the broader applicability of its findings. This study was conducted in a population with knee OA, which is the most common form of OA. Additionally, participants with severe knee OA were excluded. Future studies exploring the potential of WDTE60N in severe knee OA and OA of other joints would help better understand its broader role in managing joint health. Furthermore, future research with a longer study duration would be valuable in assessing the long-term effects of WDTE60N in the knee OA management.
Conclusion
The salient findings of this study indicate that a three-month supplementation with WDTE60N 250 mg capsules once daily was safe and effective in alleviating pain, improving functional status, and enhancing QoL in participants with mild-to-moderate knee OA, with beneficial effects observed in some of the KOOS domains from day 7 onwards. Additionally, WDTE60N demonstrated substantial efficacy in reducing levels of inflammatory biomarkers. Collectively, the assessment of these subjective and objective parameters depicts the potential of WDTE60N as an effective adjuvant for the management of knee OA, with a favorable safety profile.
Institutional Review Board Statement
The study protocol was approved by the Vatsalya Ethics Committee, Vatsalya Hospital Multispecialty Center, Sikraul, Varanasi, Uttar Pradesh – 221002 (vide registration no: ECR/1611/Inst/UP/2021). This study was conducted in compliance with the International Conference on Harmonization-Good Clinical Practices (ICH-GCP) guidelines E6 (R2), 2016; ICMR guidelines – National Ethical Guidelines for Biomedical and Health Research Involving Human Participants, 2017; Declaration of Helsinki, 2013; applicable local government regulations, and institutional research policies, and standard operating procedures.
Abbreviations
ADL, activities of daily living; AE, adverse event; ANCOVA, analysis of co-variance; BMI, body mass index; 95% CI, 95% confidence interval; COVID-19, coronavirus disease 2019; GBD, global burden of disease study; HIV, human immunodeficiency virus; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; IL-1β, interleukin 1β; KOOS, knee injury and osteoarthritis outcome score; NICE, National Institute for Health and Care Excellence; NSAIDs, non-steroidal anti-inflammatory drugs; OA, osteoarthritis; PGA, physician’s global assessment; PI, principal investigator; PP, per-protocol; QoL, quality of life; SD, standard deviation; SGA, subject’s global assessment; TEAEs, treatment-emergent adverse events; TNF-α, tumor necrosis factor-α; VAS, visual analog scale; WDTE60N, water-dispersible turmeric extract containing 60% natural curcuminoids; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
Data Sharing Statement
The datasets used in this study will be available from the corresponding author on a reasonable request.
Informed Consent Statement
Written informed consent was obtained from each participant before initiation of the clinical study.
Consent for Publication
All authors confirm that the details of images, videos, recordings, etc., can be published and that all authors provide their consent for the same.
Acknowledgments
The authors would like to thank all the study participants who were enrolled in this study. Medical writing and editorial support were provided by Tejal Dhotre and Alphy Lopes of Nutriventia Limited.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas, took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Nutriventia Limited, and Laila Nutraceuticals, India.
Disclosure
Ms. Rajat Shah and Dr. Shefali Thanawala are employees of Nutriventia Limited. Ms. Rajat Shah also has ownership interests. Dr. Krishnaraju Venkata Alluri and Mr. Kiran Bhupathiraju are employees of Laila Nutraceuticals, India. Dr. Nandlal Prasad and Dr. Yash Agarwal were the principal investigators in this study. Ms Rajat Shah has patents US11666535, JP7547324 and GB2591679 issued to Nutriventia Limited. Dr Shefali Thanawala, Dr Nandlal Prasad and Dr Yash Agarwal report patents US11666535, JP7547324 and GB2591679 issued to Nutriventia Limited. The authors do not have any other conflicts of interest to declare.
References
1. ICD-10 version: 2019. Available from: https://icd.who.int/browse10/2019/en#M17.
2. Hsu H, Siwiec RM. Knee Osteoarthritis. [Updated 2023 Jun 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507884/.
3. Steinmetz JD, Culbreth GT, Haile LM; GBD; Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the global burden of disease study 2021. Lancet Rheumatol. 2023;5(9):e508–e522. doi:10.1016/S2665-9913(23)00163-7
4. Wojcieszek A, Kurowska A, Majda A, Liszka H, Gądek A. The impact of chronic pain, stiffness and difficulties in performing daily activities on the quality of life of older patients with knee osteoarthritis. Int J Environ Res Public Health. 2022;19(24):16815. doi:10.3390/ijerph192416815
5. Kim IJ, Kim HA, Seo YI, et al. Prevalence of knee pain and its influence on quality of life and physical function in the Korean elderly population: a community based cross-sectional study. J Korean Med Sci. 2011;26(9):1140–1146. doi:10.3346/jkms.2011.26.9.1140
6. Prado LD, Ramos MEK, Camargo JDC, Bertoncelo GL, Reginatto CC, Siqueira L de O. Relationship between pain, functional limitations, dependence, depression and osteoarthritis in older adults. Fisioter mov. 2023;36:e36202. doi:10.1590/fm.2023.36202
7. Zeng L, Yang T, Yang K, et al. Efficacy and safety of curcumin and curcuma longa extract in the treatment of arthritis: a systematic review and meta-analysis of randomized controlled trial. Front Immunol. 2022;13:891822. doi:10.3389/fimmu.2022.891822
8. Uivaraseanu B, Vesa CM, Tit DM, et al. Therapeutic approaches in the management of knee osteoarthritis (Review). Exp Ther Med. 2022;23(5):328. doi:10.3892/etm.2022.11257
9. Colletti A, Cicero AFG. Nutraceutical approach to chronic osteoarthritis: from molecular research to clinical evidence. Int J mol Sci. 2021;22(23):12920. doi:10.3390/ijms222312920
10. Peng Y, Ao M, Dong B, et al. Anti-inflammatory effects of curcumin in the inflammatory diseases: status, limitations and countermeasures. Drug Des Devel Ther. 2021;15:4503–4525. doi:10.2147/DDDT.S327378
11. Sharifi-Rad J, Rayess YE, Rizk AA, et al. Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front Pharmacol. 2020;11:01021. doi:10.3389/fphar.2020.01021
12. Liu X, Machado GC, Eyles JP, Ravi V, Hunter DJ. Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis. Br J Sports Med. 2018;52(3):167–175. doi:10.1136/bjsports-2016-097333
13. Bannuru RR, Osani MC, Al-Eid F, Wang C. Efficacy of curcumin and Boswellia for knee osteoarthritis: systematic review and meta-analysis. Semin Arthritis Rheum. 2018;48(3):416–429. doi:10.1016/j.semarthrit.2018.03.001
14. Paultre K, Cade W, Hernandez D, Reynolds J, Greif D, Best TM. Therapeutic effects of turmeric or curcumin extract on pain and function for individuals with knee osteoarthritis: a systematic review. BMJ Open Sport Exerc Med. 2021;7(1):e000935. doi:10.1136/bmjsem-2020-000935
15. Hsiao AF, Lien YC, Tzeng IS, Liu CT, Chou SH, Horng YS. The efficacy of high- and low-dose curcumin in knee osteoarthritis: a systematic review and meta-analysis. Complement Ther Med. 2021;63:102775. doi:10.1016/j.ctim.2021.102775
16. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014;9:451–458. doi:10.2147/CIA.S58535
17. Kuptniratsaikul V, Thanakhumtorn S, Chinswangwatanakul P, Wattanamongkonsil L, Thamlikitkul V. Efficacy and safety of Curcuma domestica extracts in patients with knee osteoarthritis. J Altern Complement Med. 2009;15(8):891–897. doi:10.1089/acm.2008.0186
18. Fadus MC, Lau C, Bikhchandani J, Lynch HT. Curcumin: an age-old anti-inflammatory and anti-neoplastic agent. J Tradit Complement Med. 2016;7(3):339–346. doi:10.1016/j.jtcme.2016.08.002
19. Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10(20):6847–6854. doi:10.1158/1078-0432.CCR-04-0744
20. Irving GR, Karmokar A, Berry DP, Brown K, Steward WP. Curcumin: the potential for efficacy in gastrointestinal diseases. Best Pract Res Clin Gastroenterol. 2011;25(4–5):519–534. doi:10.1016/j.bpg.2011.09.005
21. Thanawala S, Shah R, Alluri KV, Somepalli V, Vaze S, Upadhyay V. Comparative bioavailability of curcuminoids from a water-dispersible high curcuminoid turmeric extract against a generic turmeric extract: a randomized, cross-over, comparative, pharmacokinetic study. J Pharm Pharmacol. 2021;73(6):816–823. doi:10.1093/jpp/rgab028
22. Thanawala S, Shah R, Doyle L, Upadhyay V. Comparative pharmacokinetics of curcuminoids from water-dispersible turmeric extract against a curcuminoids-piperine combination: an open-label, randomized, balanced, 2-treatment, 2-sequence, 2-period crossover study. Altern Ther Health Med. 2024;30(4):18–23.
23. Thanawala S, Shah R, Somepalli V, et al. Placebo-controlled trial assessing efficacy and safety of a novel low-dose turmeric extract formulation in healthy adults with chronic knee pain [published correction appears in Clin Pharmacol. Clin Pharmacol. 2023;15:63–65. doi:10.2147/CPAA.S427333]
24. Thanawala S, Shah R, Karlapudi V, Desomayanandam P, Bhuvanendran A. Efficacy and safety of TurmXTRA® 60N in delayed onset muscle soreness in healthy, recreationally active subjects: a randomized, double-blind, placebo-controlled trial. Evid Based Complement Alternat Med. 2022;2022:9110414. doi:10.1155/2022/9110414
25. Nakagawa Y, Mukai S, Yamada S, et al. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: a randomized, double-blind, placebo-controlled prospective study. J Orthop Sci. 2014;19(6):933–939. doi:10.1007/s00776-014-0633-0
26. Shep D, Khanwelkar C, Gade P, Karad S. Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: a randomized open-label parallel-arm study. Trials. 2019;20(1):214. doi:10.1186/s13063-019-3327-2
27. Rahimnia AR, Panahi Y, Alishiri G, Sharafi M, Sahebkar A. Impact of supplementation with curcuminoids on systemic inflammation in patients with knee osteoarthritis: findings from a randomized double-blind placebo-controlled trial. Drug Res. 2015;65(10):521–525. doi:10.1055/s-0034-1384536
28. Lopresti AL, Smith SJ, Jackson-Michel S, Fairchild T. An Investigation into the effects of a curcumin extract (Curcugen®) on osteoarthritis pain of the knee: a randomised, double-blind, placebo-controlled study. Nutrients. 2021;14(1):41. doi:10.3390/nu14010041
29. Osteoarthritis in over 16s: diagnosis and management. London: National Institute for Health and Care Excellence (NICE);
30. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS Pain), numeric rating scale for pain (NRS Pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res. 2011;63 Suppl 11:S240–S252. doi:10.1002/acr.20543
31. Alghadir AH, Anwer S, Iqbal A, Iqbal ZA. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J Pain Res. 2018;11:851–856. doi:10.2147/JPR.S158847
32. Roos EM, Lohmander LS. The knee injury and osteoarthritis outcome score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi:10.1186/1477-7525-1-64
33. Collins NJ, Prinsen CA, Christensen R, Bartels EM, Terwee CB, Roos EM. Knee injury and osteoarthritis outcome score (KOOS): systematic review and meta-analysis of measurement properties. Osteoarthritis Cartilage. 2016;24(8):1317–1329. doi:10.1016/j.joca.2016.03.010
34. Kang H. Sample size determination and power analysis using the G*Power software. J Educ Eval Health Prof. 2021;18:17. doi:10.3352/jeehp.2021.18.17
35. Abujaber S, Altubasi I, Hamdan M, Al-Zaben R. Impact of end-stage knee osteoarthritis on perceived physical function and quality of life: a descriptive study from Jordan. PLoS One. 2023;18(6):e0286962. doi:10.1371/journal.pone.0286962
36. Thirumaran AJ, Deveza LA, Atukorala I, Hunter DJ. Assessment of pain in osteoarthritis of the knee. J Pers Med. 2023;13(7):1139. doi:10.3390/jpm13071139
37. Madhu K, Chanda K, Saji MJ. Safety and efficacy of Curcuma longa extract in the treatment of painful knee osteoarthritis: a randomized placebo-controlled trial. Inflammopharmacology. 2013;21(2):129–136. doi:10.1007/s10787-012-0163-3
38. Zhao J, Liang G, Zhou G, et al. Efficacy and safety of curcumin therapy for knee osteoarthritis: a Bayesian network meta-analysis. J Ethnopharmacol. 2024;321:117493. doi:10.1016/j.jep.2023.117493
39. Eberle E, Ottillinger B. Clinically relevant change and clinically relevant difference in knee osteoarthritis. Osteoarthritis Cartilage. 1999;7(5):502–503. doi:10.1053/joca.1999.0246
40. Henrotin Y, Malaise M, Wittoek R, et al. Bio-optimized Curcuma longa extract is efficient on knee osteoarthritis pain: a double-blind multicenter randomized placebo controlled three-arm study. Arthritis Res Ther. 2019;21(1):179. doi:10.1186/s13075-019-1960-5
41. Roos EM. 30 years with the knee injury and osteoarthritis outcome score (KOOS). Osteoarthritis Cartilage. 2024;32(4):421–429. doi:10.1016/j.joca.2023.10.002
42. Mabey T, Honsawek S. Cytokines as biochemical markers for knee osteoarthritis. World J Orthop. 2015;6(1):95–105. doi:10.5312/wjo.v6.i1.95
43. Yunus MHM, Nordin A, Kamal H. Pathophysiological perspective of osteoarthritis. Medicina. 2020;56(11):614. doi:10.3390/medicina56110614
44. Jin X, Beguerie JR, Zhang W, et al. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(4):703–710. doi:10.1136/annrheumdis-2013-204494
45. Stannus O, Jones G, Cicuttini F, et al. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18(11):1441–1447. doi:10.1016/j.joca.2010.08.016
46. Gupte PA, Giramkar SA, Harke SM, et al. Evaluation of the efficacy and safety of Capsule Longvida® Optimized Curcumin (solid lipid curcumin particles) in knee osteoarthritis: a pilot clinical study. J Inflamm Res. 2019;12:145–152. doi:10.2147/JIR.S205390
47. Singhal S, Hasan N, Nirmal K, et al. Bioavailable turmeric extract for knee osteoarthritis: a randomized, non-inferiority trial versus paracetamol. Trials. 2021;22(1):105. doi:10.1186/s13063-021-05053-7
48. Srivastava S, Saksena AK, Khattri S, Kumar S, Dagur RS. Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: a four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology. 2016;24(6):377–388. doi:10.1007/s10787-016-0289-9
49. Park HJ, Lee CK, Song SH, Yun JH, Lee A, Park HJ. Highly bioavailable curcumin powder suppresses articular cartilage damage in rats with mono-iodoacetate (MIA)-induced osteoarthritis. Food Sci Biotechnol. 2019;29(2):251–263. doi:10.1007/s10068-019-00679-5
50. Jin Z, Chang B, Wei Y, et al. Curcumin exerts chondroprotective effects against osteoarthritis by promoting AMPK/PINK1/Parkin-mediated mitophagy. Biomed Pharmacother. 2022;151:113092. doi:10.1016/j.biopha.2022.113092 Epub 2022 May 10. PMID: 35550528.
51. Li X, Feng K, Li J, et al. Curcumin inhibits apoptosis of chondrocytes through activation ERK1/2 signaling pathways induced autophagy. Nutrients. 2017;9(4):414. doi:10.3390/nu9040414 PMID: 28430129; PMCID: PMC5409753.
52. Yeon KY, Kim SA, Kim YH, et al. Curcumin produces an antihyperalgesic effect via antagonism of TRPV1. J Dent Res. 2010;89(2):170–174. doi:10.1177/0022034509356169
53. De Paz-Campos MA, Ortiz MI, Chávez Piña AE, Zazueta-Beltrán L, Castañeda-Hernández G. Synergistic effect of the interaction between curcumin and diclofenac on the formalin test in rats. Phytomedicine. 2014;21(12):1543–1548. doi:10.1016/j.phymed.2014.06.015
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




