Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 20
Efficacy and Safety of Biologics Targeting Type 2 Inflammation in COPD: A Systematic Review and Network Meta-Analysis
Authors Li S, Yi B, Wang H, Xu X, Yu L
Received 6 November 2024
Accepted for publication 5 June 2025
Published 3 July 2025 Volume 2025:20 Pages 2143—2159
DOI https://doi.org/10.2147/COPD.S504774
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Shujie Li,1,* Baiyi Yi,1,* Huan Wang,2 Xianghuai Xu,1 Li Yu1
1Department of Pulmonary and Critical Care Medicine, Tongji Hospital, School of Medicine, Tongji University, Shanghai, 200065, People’s Republic of China; 2Clinical Research Center, Tongji Hospital, School of Medicine, Tongji University, Shanghai, 200065, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xianghuai Xu, Department of Pulmonary and Critical Care Medicine, Tongji Hospital, School of Medicine, Tongji University, No. 389 Xincun Road, Shanghai, 200065, People’s Republic of China, Email [email protected] Li Yu, Department of Pulmonary and Critical Care Medicine, Tongji Hospital, School of Medicine, Tongji University, No. 389 Xincun Road, Shanghai, 200065, People’s Republic of China, Email [email protected]
Purpose: This study aims to comparatively evaluate the efficacy and safety profiles of biologic agents targeting type 2 inflammation in COPD.
Methods: As of September 1, 2024, we identified and screened eight clinical studies evaluating biologic agents targeting type 2 inflammation for COPD treatment from multiple databases. Following data extraction, we conducted a network meta-analysis using R software to indirectly compare the efficacy and safety profiles of the five included biologic agents, incorporating visualization of the analytical results.
Results: In COPD patients with elevated eosinophil levels (peripheral blood eosinophil count ≥ 200 cells/μL), dupilumab demonstrated significant therapeutic efficacy by: (1) reducing the annualized rate of acute exacerbations (versus placebo: − 0.44; 95% CI − 0.77 to − 0.10), (2) decreasing SGRQ total scores (versus placebo: − 3.41; 95% CI − 6.00 to − 0.82), and (3) increasing pre-bronchodilator FEV1 (versus placebo: 0.06 L; 95% CI 0.00 to 0.12). Benralizumab also showed clinical benefits in reducing acute exacerbation rates (10 mg versus placebo: − 0.21; 95% CI − 0.39 to − 0.04) and improving SGRQ scores (100 mg versus placebo: − 1.70; 95% CI − 3.35 to − 0.04). Furthermore, all five biologic agents evaluated in this network meta-analysis exhibited favorable safety profiles.
Conclusion: This NMA demonstrates that both dupilumab and benralizumab show statistically significant efficacy in COPD management, particularly among patients with eosinophilic inflammation. And these biological agents maintain favorable safety profiles. Future research should focus on large-scale multicenter clinical trials, biomarker-based patient stratification, optimization of drug delivery regimens, and development of multi-target combination therapies.
Plain Language Summary: Biological therapy represents an emerging treatment approach for COPD, with several biological agents under development, including benralizumab, mepolizumab, itepekimab, astegolimab, and dupilumab. Which of these biological agents demonstrates optimal efficacy? Which exhibits the most favorable safety profile? These critical questions form the focus of the present investigation. In the absence of direct comparative clinical trials evaluating these biological agents, we employed network meta-analysis to estimate their relative efficacy and safety. Our analysis ultimately demonstrated that among the five biological agents targeting type 2 inflammation in COPD, only dupilumab and benralizumab exhibited significant therapeutic efficacy, particularly in patient populations with elevated eosinophil levels. Furthermore, all investigated biological agents demonstrated favorable safety profiles. This finding not only addresses the current lack of direct comparative evidence, but also indicates that patients with elevated eosinophil levels may represent the optimal target population for biological therapy.
Keywords: COPD, biologics, type 2 inflammation, network meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD) is recognized by the World Health Organization (WHO) as the third leading cause of global mortality, imposing substantial health and socioeconomic burdens worldwide. Currently, it is believed that COPD is a preventable, treatable disease marked by persistent airflow obstruction and chronic inflammatory airways disease, exhibiting pathogenic similarities to asthma.1,2 Inhaled corticosteroids (ICS) and bronchodilators constitute the cornerstone pharmacotherapy for COPD. While LABA/LAMA/ICS triple therapy effectively controls symptoms, its capacity to enhance lung function or prevent acute exacerbations is limited. Moreover, due to the inability of some patients to accurately use inhalation devices, the effectiveness of triple therapy cannot meet expectations. Therefore, it is of great significance to develop drugs with novel routes of administration that provide more comprehensive benefits to COPD patients. Furthermore, suboptimal inhaler technique among a subset of patients compromises the therapeutic efficacy of triple therapy. Consequently, developing novel drug formulations that offer broader clinical benefits for COPD patients represents a critical unmet need in respiratory medicine.
Recent years have witnessed significant advances in immune-targeted therapies, with monoclonal antibodies emerging as particularly promising biologic agents for COPD treatment.3,4 The type 1 inflammatory phenotype in COPD manifests as neutrophilic airway inflammation, mediated by a cytokine cascade involving TNF-α, IL-6, IL-8, IL-17, and IL-23. Early-stage therapeutic development therefore prioritized cytokine-targeting biologics for type 1 inflammation, but clinical evaluation of IL-8 inhibitor ABX-IL8, TNF-α blocker infliximab, and IL-1 antagonist MEDI8968 yielded unsatisfactory clinical outcomes.5–8 Subsequent investigations have revealed that approximately 20–40% of COPD patients exhibit type 2 inflammatory responses, primarily characterized by eosinophilic infiltration. And in patients with prior exacerbation history, type 2 inflammation demonstrates significant association with increased future exacerbation risk.9 The mechanistic pathway of type 2 inflammation in COPD begins with smoke- or virus-induced epithelial release of alarmins (TSLP and IL-33). These mediators activate Th2 and ILC2s to secrete IL-4, IL-5, and IL-13, culminating in the triad of: (1) acute bronchoconstriction and mucous hypersecretion, (2) chronic epithelial remodeling with goblet cell hyperplasia, and (3) progressive airway wall fibrosis with MMP-mediated alveolar destruction.3
Emerging consensus recognizes COPD as a pathobiologically heterogeneous disorder, exhibiting diverse morphological and biological manifestations.11–13 Mirroring asthma classification systems, COPD heterogeneity is described through either clinical phenotypes (observable traits) or endotypes (molecular mechanisms).14,15 The type 2 inflammation-associated “eosinophilic COPD” endotype is defined by type 2 inflammation and elevated eosinophil counts.16 This phenotype demonstrates significant pathophysiological overlap with asthma, a typical airway inflammatory disease mediated by type 2 immune response. Clinical evidence has established that biologics targeting severe eosinophilic asthma exhibit robust therapeutic efficacy, leading to their widespread adoption in clinical practice.17–20 Consequently, investigation of type 2 inflammation mechanisms in COPD and development of targeted biologics against related cytokine pathways have emerged as a prominent research focus in recent years.
Significant research advances have been made in developing biologic therapies targeting type 2 inflammatory pathways in COPD. Among biologics targeting the IL-5/IL-5R pathway, the most clinically advanced candidates are mepolizumab and benralizumab. The IL-5/IL-5R pathway plays a pivotal role in type 2 inflammation by regulating eosinophil differentiation, recruitment, maturation, activation, and degranulation. Both mepolizumab and benralizumab have received regulatory approval for severe asthma treatment.21 Current clinical evidence demonstrates that IL-5 pathway inhibitors significantly reduce the annualized exacerbation rate in COPD patients with eosinophilic phenotypes.22 IL-4 and IL-13 serve as pivotal mediators of type 2 inflammation, orchestrating the activation, recruitment, and trafficking of type 2 inflammatory cells through chemokine induction. Dupilumab can specifically bind to the IL-4Rα subunit, block IL-4 and IL-13 signaling, and alleviate type 2 inflammatory response. In COPD patients with blood eosinophil counts ≥300 cells/μL, dupilumab treatment significantly reduces the annualized exacerbation rate and improves lung function parameters.23 Dupilumab received regulatory approval in the European Union (July 2024), followed by China and the United States (September 2024), as an add-on maintenance treatment for uncontrolled COPD patients with eosinophilic phenotypes. In addition, therapeutic targeting of upstream alarmins in the type 2 inflammatory cascade has emerged as a promising research frontier in COPD management. The therapeutic method based on this mechanism is exemplified by the IL-33-targeting monoclonal antibody itepekimab and the ST2-blocking monoclonal antibody astegolimab, though their clinical efficacy in COPD remains to be fully established. Preliminary results from the itepekimab Phase IIa study indicated efficacy in lowering exacerbation frequency in a specific COPD cohort (former smokers with smoking cessation history). Further research is underway.
While multiple type 2 inflammation-targeting biologics have shown therapeutic benefits in COPD, the field currently lacks direct comparative evidence to establish their relative efficacy and safety advantages.3 To address this evidence gap, we conducted a network meta-analysis (NMA) to systematically compare the relative efficacy and safety profiles of these biologic therapies. NMA methodology synthesizes both direct and indirect comparisons through evidence network construction, with Bayesian or frequentist approaches generating posterior probability distributions for comparative effectiveness estimates and treatment hierarchy determination. Through this analytical approach, we aim to identify biologics with optimal efficacy and safety profiles, thereby informing evidence-based clinical decision-making for COPD management.
Methods
This network meta-analysis has been registered in the PROSPERO international prospective register of systematic reviews (Registration ID: CRD42023460937). The study adheres to the PRISMA-NMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Network Meta-Analyses) reporting guidelines.
Retrieval Strategy
As of September 1, 2024, we have searched relevant literature in multiple databases, including PubMed, Web of Science, Cochrane Controlled Trial Registry (Central), Wanfang Medical Database, China National Knowledge Infrastructure. For English-language databases like PubMed, we employed a combined search strategy using Medical Subject Headings (MeSH) terms and text words: ((chronic obstructive pulmonary disease[MeSH]) OR (COPD[Title/Abstract])) AND ((biological products[MeSH]) OR (biologics[Title/Abstract]) OR (biological therapy[MeSH])). For Chinese databases, the search incorporated the following free-text terms: “COPD”, “Man xing zu se xing fei bing”, “ Man zu fei”, “Sheng wu zhi ji” and “Sheng wu liao fa”. The included studies were restricted to randomized controlled trials (RCTs) published within the past decade. Two investigators independently conducted the literature screening process, performing initial assessments based on titles, abstracts, and keywords, followed by full-text evaluations to determine final study inclusion according to the predefined eligibility criteria for this network meta-analysis.
Inclusion Criteria
Studies eligible for inclusion in this meta-analysis were required to meet the following criteria:
- Randomized controlled trial (RCT) design;
- Enrollment of patients with moderate-to-severe COPD, with exclusion of comorbid pulmonary conditions including asthma, bronchiectasis, cystic fibrosis, sarcoidosis, interstitial lung disease, moderate-to-severe obstructive sleep apnea, or pulmonary hypertension;
- Background therapy with ICS/LABA/LAMA triple therapy in all participants;
- Intervention group receiving any biologic therapy.
Exclusion Criteria
Studies were excluded based on the following criteria:
- Unpublished or undisclosed trial data or non-peer-reviewed results;
- Lack of prespecified outcome measures, including: annualized rate of moderate-to-severe acute exacerbations (AECOPD), change in St. George’s Respiratory Questionnaire (SGRQ) total score, pre-bronchodilator forced expiratory volume in 1 second (FEV1) values, and incidence of serious adverse events (SAEs).
Data Extraction and Risk of Bias Assessment
Following PRISMA guidelines, two blinded reviewers conducted duplicate data abstraction using piloted forms capturing: (1) study identifiers, (2) intervention parameters, (3) baseline characteristics, and (4) efficacy/safety outcomes. Registry data (ClinicalTrials.gov) superseded journal publications when discrepancies occurred.
Two independent researchers assessed the methodological quality of included RCTs using the Cochrane Risk of Bias Tool (RoB 2.0). Discrepancies in quality assessments were resolved through consensus discussion with a third senior reviewer.
Primary efficacy endpoints comprised: (1) moderate-to-severe AECOPD rate (events/year), (2) SGRQ score improvement, and (3) pre-BD FEV1 increase (mL). Safety analysis evaluated SAE occurrence.
Statistical Analysis
Methodological execution involved two distinct analytical platforms: RevMan 5.4 for literature quality assessment and R (version 4.2.1) for network meta-analysis. R is a statistical programming language that can process various types of data, including count data, continuous data, or binary data, by loading the BUGSnet package. The R statistical environment, utilizing the BUGSnet package, enabled comprehensive data processing across variable types.24 The analysis process is as follows:
- Data entry and preparation: For continuous outcome variables - including the annualized rate of moderate-to-severe AECOPD, change in SGRQ total score, and pre-FEV1 value - we extracted the following parameters: mean values, standard deviations (SDs), and corresponding sample sizes. Where required, non-parametric data presentations were converted to standardized mean ± standard deviation (SD) format using established transformation methods. For dichotomous safety outcomes (number of SAE), the required extracted parameters included event frequency counts and total sample sizes.
- Draw the network geometry: Edge represents direct comparison. The width of the edge is proportional to the number of experiments conducted. The size of the node is directly proportional to the number of participants randomly assigned to take the drug.
- The network meta-analysis assumptions - homogeneity, transitivity, and consistency - were methodologically validated through comprehensive heterogeneity assessment and inconsistency testing. If the heterogeneity of the NMA is strong, sensitivity analysis should be used to screen for heterogeneity sources.
- Evaluate the effect model: Calculate the I2 value, P value, and 95% CI, and draw the forest chart and funnel plot, and use fitting code to generate lever plots and fitting statistical data for fixed effects and random effects models, and select the best fitting model.
- Treatment hierarchies were visualized through multiple graphical representations: rank probability heatmaps, cumulative ranking curves (SUCRA values), forest plots, and other statistical chart.
- Subgroup analysis: Depending on the analysis results, further exploration can be conducted on the efficacy and safety of biologics in patient populations with certain characteristics.
Results
Retrieval Process and Data Extraction
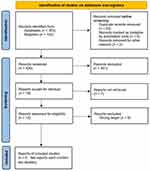
We ultimately included six published articles (Brightling et al;25 Pavord et al;22 Criner et al;26 Rabe et al;27 Yousuf et al;28 Bhatt et al23). Among these, two articles (Pavord et al, 2017; Criner et al, 2019) each comprised two distinct studies, resulting in a total of eight studies being incorporated into this NMA. The detailed retrieval process is illustrated in Figure 1.
Among the eight included studies, five were single-arm trials (Brightling et al, 2014; Pavord et al, 2017 [METREX]; Rabe et al, 2021; Yousuf et al, 2022; Bhatt et al, 2024), two were dual-arm studies (Pavord et al, 2017 [METREO]; Criner et al, 2019 [GALATHEA]), and one was a three-arm trial (Criner et al, 2019 [TERRANOVA]). This NMA evaluated a total of five biologics—benralizumab, mepolizumab, itepekimab, astegolimab, and dupilumab—with an aggregate sample size of 6880 participants. Detailed study characteristics are presented in Table 1.
 |
Table 1 Basic Characteristics of the Included Studies |
Literature Quality Evaluation
The potential biases identified in the literature encompass selection bias, performance bias, detection bias, attrition bias, reporting bias, and other sources of bias. The detailed quality assessment results are presented in Figure 2. Although the studies included in this NMA were predominantly randomized double-blind trials, certain studies failed to explicitly describe their randomization procedures or allocation concealment methods.
 |
Figure 2 Quality assessment of literature. |
Network Meta-Analysis
Figure 3 presents the network plot of biological agents targeting type 2 inflammation in COPD. The network diagram visually demonstrates the comparative relationships between different therapeutic regimens, including various drug types and dosage forms. This NMA incorporates both direct comparisons from multi-arm studies and indirect comparisons through common placebo controls. Notably, all closed-loop structures in the network diagram originate exclusively from multi-arm trials themselves, ensuring theoretical consistency. Consequently, no inconsistency test was required for this analysis. Heterogeneity assessment was performed for the studies included in the network meta-analysis (Figure 4). The results demonstrated low heterogeneity (I² = 15.6%; p = 0.291), with the 95% confidence interval not crossing zero. This NMA incorporates robust clinical data from high-quality RCTs, satisfying the three key assumptions of NMA methodology (homogeneity, similarity, and consistency). The validated transitivity and coherence of the network confirm the analytical feasibility and provide a reliable foundation for indirect treatment comparisons.
 |
Figure 4 Heterogeneity test. (A) The funnel plot of heterogeneity analysis. (B) Forest plot and I2 of heterogeneity analysis. |
For model selection, we compared the deviance information criterion (DIC) values between fixed-effect and random-effects models. The model with the lower DIC value was selected, as this indicates better model fit. For analysis of the annualized rate of moderate-to-severe AECOPD, we selected a fixed-effects model. For SAEs, we employed a random-effects model (Figure 5).
Figure 3 presents the forest plots comparing the efficacy and safety profiles of biological agents targeting type 2 inflammation in COPD. As shown in Figure 6A, among the five biologics evaluated in this network meta-analysis, only dupilumab demonstrated a statistically significant reduction in the annualized rate of AECOPD compared with placebo (95% CI excluding zero). The heatmap (Figure 7) displays the mean differences with 95% CIs, where dupilumab versus placebo shows a value of −0.44 (95% CI: −0.77 to −0.10), marked with double asterisks “**” to indicate statistical significance. Consequently, these findings preclude definitive efficacy ranking among the evaluated biological agents. The forest plot in Figure 6B reveals no statistically significant differences in adverse event incidence between the biological agents and placebo controls, indicating favorable safety characteristics of these therapeutic agents.
Subgroup Analysis
Based on the inclusion criteria, methodology, and results of the studies included in this meta-analysis, we identified that peripheral blood eosinophil levels may contribute to both the heterogeneity in our network meta-analysis and the phenotypic variability of COPD itself. Notably, in the clinical trial conducted by Bhatt et al, investigating dupilumab, enrolled patients had peripheral blood eosinophil counts ≥300 cells/μL. After comprehensive consideration of the aforementioned factors and to maximize the analytical sample size, we defined the subgroup with peripheral blood eosinophil counts ≥200 cells/μL as the high EOS group for subsequent subgroup analyses.
In the high eosinophil subgroup (≥200 cells/μL), we analyzed three key outcomes: (1) The annualized rate of moderate-to-severe AECOPD included five studies evaluating four biologics (benralizumab, dupilumab, itepekimab, and astegolimab); (2) Changes in SGRQ total scores included four studies assessing two biologics (benralizumab and dupilumab); (3) Changes in pre-FEV₁ values included three studies examining two biologics (benralizumab and dupilumab). The corresponding network diagrams are presented in Figure 8.
Figures 9–11 demonstrate that dupilumab and benralizumab show superior therapeutic effects compared with other biological agents in the high eosinophil subgroup (≥200 cells/μL). For the annualized rate of moderate-to-severe AECOPD, both dupilumab (MD: −0.44; 95% CI: −0.78 to −0.12) and benralizumab 10 mg (MD: −0.21; 95% CI: −0.39 to −0.04) demonstrated superior efficacy compared with placebo. However, no statistically significant difference was observed between dupilumab and benralizumab 10 mg. For changes in SGRQ total scores, both dupilumab (MD: −3.41; 95% CI: −6.00 to −0.82) and benralizumab 100 mg (MD: −1.70; 95% CI: −3.35 to −0.04) showed statistically significant improvements compared with placebo. But the difference between dupilumab and benralizumab 100 mg did not reach statistical significance. Although the network meta-analysis could not directly compare efficacy between dupilumab and benralizumab, the ranking plots suggest that dupilumab is more likely to be the highest-ranked biologic for both reducing the annualized rate of AECOPD and improving SGRQ total scores. Regarding pre-FEV₁ values changes, only dupilumab demonstrated statistically significant improvement compared with placebo (MD: 0.06 L; 95% CI: 0.00 to 0.12).
Discussion
The findings of this NMA further substantiate the feasibility of biologics targeting type 2 inflammation in COPD, while revealing efficacy variations among different biological agents. Dupilumab may demonstrate optimal therapeutic efficacy in three key clinical outcomes for COPD patients: (1) reducing the annualized rate of moderate-to-severe AECOPD, (2) improving SGRQ total scores, and (3) increasing pre-FEV₁ values. In COPD patients with elevated eosinophil levels (peripheral blood eosinophil count ≥200 cells/μL), benralizumab also demonstrated significant therapeutic effects, showing improvements in both SGRQ total scores and pre-FEV₁ values. However, the remaining biological agents evaluated in this network meta-analysis (excluding dupilumab and benralizumab) failed to demonstrate statistically significant efficacy. Importantly, all five investigated biologics showed comparable safety profiles, with no significant increase in serious adverse event incidence.
Dupilumab is a fully human monoclonal antibody targeting the IL-4Rα. By competitively inhibiting the binding of both IL-4 and IL-13 to their receptors, it suppresses the downstream janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathway, thereby inhibiting the activation of Th2 cells and ILC2, reducing eosinophil infiltration and activation, reducing IgE synthesis and reducing the release of pro-inflammatory factors, thereby inhibiting type 2 inflammation.3,23 In the multicenter randomized controlled trial conducted by Bhatt et al,23 researchers enrolled COPD patients aged 40–85 years who met the following criteria: (1) ≥10 pack-year smoking history, (2) ongoing triple therapy (ICS/LABA/LAMA), (3) peripheral blood eosinophil count ≥300 cells/μL, (4) symptomatic chronic obstructive pulmonary disease, and (5) ≥2 moderate or 1 severe acute exacerbation within the preceding year. Participants received subcutaneous administration of either dupilumab 300 mg or placebo every two weeks. Compared with placebo, dupilumab showed a 30% reduction in moderate-to-severe AECOPD rates and delayed time to first exacerbation. Dupilumab treatment resulted in clinically meaningful improvements in lung function, respiratory symptoms, demonstrating comprehensive therapeutic benefits. Our study findings corroborate the results reported by Bhatt et al, providing further evidence that dupilumab treatment yields clinically significant benefits for COPD patients, including reduction in disease severity and improvement in lung function parameters. It is worth noting that although changes in eosinophil count level can be observed during the treatment of asthma and atopic dermatitis with dupilumab, this has not been found in clinical studies of dupilumab treatment for COPD, which indicating that dupilumab’s therapeutic effect on COPD is not only to inhibit eosinophil inflammation.3,30
Concurrently, numerous clinical trials have investigated IL-5 pathway inhibitors for COPD treatment. Our network meta-analysis incorporated data from three publications encompassing five clinical studies. The IL-5 pathway serves as the principal mechanism for eosinophil activation in type 2 inflammation associated with COPD. As a lineage-specific cytokine for eosinophils, IL-5 binds to the IL-5 receptor complex (composed of IL-5Rα and βc subunits), triggering JAK-STAT pathway activation. This signaling cascade induces: (1) progenitor cell proliferation and differentiation, (2) inhibition of eosinophil apoptosis, (3) upregulation of adhesion molecule expression (including very late antigen-4 [VLA-4]), (4) enhanced transendothelial migration, and (5) release of cytotoxic granule proteins.3,32 Furthermore, clinical evidence demonstrates that IL-5 pathway inhibitors effectively reduce eosinophil burden. The landmark DREAM study (Pavord et al, 2012)3 reported that mepolizumab treatment decreased peripheral blood eosinophil counts by >90% in patients with severe asthma. These findings suggest that the therapeutic efficacy of IL-5 pathway inhibitors in COPD may similarly correlate with baseline eosinophil levels. For example, Pavord et al,22 demonstrated that the IL-5 monoclonal antibody mepolizumab significantly reduced the annualized rate of AECOPD in patients with an eosinophilic phenotype (defined as peripheral blood eosinophil counts ≥150 cells/mm³ at screening or ≥300 cells/mm³ during the preceding 12 months). However, the clinical trials conducted by Brightling et al,25 and Criner et al,26 demonstrated that benralizumab (an IL-5 receptor α [IL-5Rα]-targeted monoclonal antibody) failed to significantly reduce the annualized rate of AECOPD, both in the overall COPD population and in subgroups with elevated eosinophil counts (≥220 cells/μL). Although the original clinical trial results showed some inconsistencies, our NMA revealed that benralizumab demonstrated modest but significant efficacy in the high-eosinophil subgroup (peripheral blood eosinophil counts ≥200 cells/μL), particularly in reducing the annualized rate of moderate-to-severe AECOPD and improving SGRQ total scores. These findings not only highlight the methodological advantages of NMA, but also provide evidence-based support for interleukin-5 (IL-5)-targeted monoclonal antibody therapy in eosinophilic COPD. Additional studies are required to further validate the clinical efficacy and establish optimal eosinophil count thresholds for treatment response.
The important roles of IL-4/IL-13 pathway and IL-5 pathway in type 2 inflammation make it more likely for biologics targeting these two pathways to demonstrate efficacy in COPD patients with high eosinophil level. The ranking plot from our NMA subgroup analysis (peripheral blood eosinophils ≥200 cells/μL) suggests that dupilumab may demonstrate superior efficacy to benralizumab in both reducing the annualized rate of AECOPD and improving SGRQ scores. This observed therapeutic differential may be mechanistically explained by dupilumab’s unique dual-pathway inhibition. As a high-affinity IL-4Rα antagonist, dupilumab concurrently blocks both IL-4 and IL-13 downstream signaling, thereby providing broader suppression of type 2 inflammation compared to single-pathway inhibitors. Compared to IL-5 single pathway inhibitors, its inhibitory effect on type 2 inflammation is more extensive. On the other hand, blocking IL-13 pathway can reduce airway secretion and lower airway hyperresponsiveness, which not only alleviates the clinical symptoms of asthma and sputum in COPD patients, but also helps to reduce the frequency of acute exacerbations and improve lung function.33 Methodologically, our network meta-analysis subgroup was defined using a uniform eosinophil cutoff (≥200 cells/μL) to ensure comparability across studies. Notably, the original clinical trials employed different eosinophil thresholds: the pivotal dupilumab study (Bhatt et al,) enrolled patients with ≥300 eosinophils/μL, while the benralizumab investigation (Criner et al,) analyzed a high-eosinophil cohort with ≥220 cells/μL. The variability in eosinophil threshold levels across studies may influence the analytical outcomes. Moving forward, precise eosinophil cutoffs are expected to serve as key biomarkers for predicting treatment response to specific biologics in COPD.
This network meta-analysis also incorporated IL-33/ST2 pathway inhibitors, including itepekimab and astegolimab. While the clinical trial conducted by Rabe et al, reported that itepekimab reduced acute exacerbation of AECOPD incidence and improved lung function parameters in former smokers, these therapeutic benefits did not reach statistical significance in our comparative effectiveness analysis.27 In the clinical trial conducted by Yousuf et al, astegolimab failed to demonstrate a statistically significant reduction in acute exacerbation rates of COPD, which aligns with the findings of our network meta-analysis.3 In human pulmonary tissues, IL-33 expression is predominantly localized to the microvascular endothelium and distinct airway basal cell populations, with particularly prominent expression observed in regions exhibiting epithelial hyperplasia and mucosal remodeling in COPD patients.34 The cytokine primarily influences eosinophilic inflammation indirectly through IL-5 induction, while its potential direct effects on eosinophils remain controversial in current literature.3 Accumulating evidence reveals that IL-33 exhibits significant context-dependent heterogeneity, manifesting as: (1) stage-specific differential signaling in disease progression, and (2) occasionally opposing biological effects within the same pathological condition.35 Such uncharacterized molecular complexities potentially underlie the suboptimal clinical performance of current IL-33/ST2-targeted therapeutic agents.
Collectively, our findings highlight that only a limited subset of type 2 inflammation-targeting biologics have demonstrated clinical efficacy in COPD. This therapeutic limitation warrants systematic investigation to optimize biologic therapy for COPD patients. First, the inflammatory pathogenesis of COPD involves a complex interplay of multiple immune cells (including macrophages, neutrophils, and eosinophils) and inflammatory mediators (such as TNF-α, IL-1β, IL-5, IL-6, IL-8, IL-13, and IL-33). Given this complexity, single-target biologic agents may be insufficient to adequately modulate the entire inflammatory network in COPD. Secondly, COPD-associated inflammation involves not only adaptive immunity, such as Th1/Th2 responses, but also significant innate immune activation (particularly smoking- or infection-induced macrophage polarization). This dual mechanism may explain the suboptimal therapeutic response observed with biologics exclusively targeting Th1/Th2 pathways.36 Thirdly, the characteristic structural alterations in COPD - including emphysematous destruction and airway remodeling/fibrosis - are largely irreversible through anti-inflammatory interventions alone. Furthermore, the typical late-stage diagnosis of COPD raises significant concerns regarding the therapeutic window for biological agents, as irreversible structural damage may already be established by initiation of treatment. Finally, a critical consideration is that the efficacy of cytokine-targeted biologics appears restricted to specific COPD endotypes. Current clinical trial designs frequently fail to adequately stratify patients using predictive biomarkers. Future development of COPD biologic therapies should therefore incorporate early diagnostic protocols, and comprehensive biomarker profiling (including blood/sputum cytokine analysis and transcriptomic signatures) to identify dominant Th1/Th2 inflammatory subtypes. Additionally, there are many new strategies worth paying attention to, including inhibiting key receptors (PAFr, ICAM-1, and TLR), targeting key proteins and enzymes (PKC, EGFR, ST6GAL1, and Annexin-1), regulating immune cells (such as macrophages), and controlling other factors (such as oxidative stress and iron levels).3
This NMA demonstrates several methodological strengths. First, we specifically evaluated biologics targeting type 2 inflammatory pathways in COPD, with confirmed homogeneity across studies, ensuring valid NMA comparisons. Second, the exclusive inclusion of RCTs provides high-level evidence for clinical decision-making. Furthermore, our comprehensive analysis incorporated data from 6,884 participants across five distinct biologics with multiple dosage regimens, enabling both inter-agent efficacy comparisons and intra-agent dose-response evaluations.
Several limitations should be acknowledged. First, inherent heterogeneity exists across the original studies, stemming not only from conventional variations (temporal, geographic, and duration differences) but particularly from population characteristics, especially the divergent eosinophil thresholds used for inclusion. Second, several clinically relevant COPD outcome measures - including severe AECOPD rates, post-bronchodilator FEV1 (post-FEV1) changes, hospitalization metrics (frequency and duration) - could not be analyzed due to insufficient reported data. Third, our efficacy analysis did not incorporate consideration of minimal clinically important differences (MCIDs).38 These limitations may impact the clinical interpretation of our findings.
What needs to be declared is that, the types of biologics included in this study are limited, and some monoclonal antibodies under study were not included in the analysis. For example, among the current biologics targeting the IL-4/IL-13 target, there is a rapid development progress of Stapokibart and SSGJ-611, which are in Phase III and Phase II clinical trials respectively. Currently, no definitive clinical trial results have been reported for these investigational agents. Tezepelumab is currently the only TSLP monoclonal antibody that has published clinical data on COPD in January 2025, but have not yet been included in this NMA analysis. Tezepelumab is a human monoclonal antibody targeting TSLP. Clinical trial data demonstrate its efficacy in reducing the annualized rate of acute exacerbations and improving both FEV1 and SGRQ scores in COPD patients with elevated eosinophil levels (≥200 cells/μL).39 Based on this clinical evidence, tezepelumab has received Breakthrough Therapy designation from the US Food and Drug Administration (FDA) for COPD treatment. Preliminary observational data suggest omalizumab may reduce exacerbations and improve lung function in ACOS patients, though phase III RCTs are needed to confirm these therapeutic benefits.40–43
Conclusion
This NMA provides a comprehensive evaluation of the efficacy and safety profiles of type 2 inflammation-targeting biologics for chronic obstructive pulmonary disease (COPD). The results demonstrate that both dupilumab (an IL-4/IL-13 pathway inhibitor) and benralizumab (an IL-5 receptor α antagonist) show statistically significant clinical benefits in reducing acute exacerbations and improving symptoms, with enhanced therapeutic effects observed in patients exhibiting eosinophilic inflammation (defined as peripheral blood eosinophils ≥200 cells/μL in this study). The observed therapeutic effects correspond to the established pathophysiology of eosinophil-mediated inflammation in COPD, wherein dupilumab and benralizumab appear to target critical nodes in the type 2 inflammatory cascade that drive disease progression. Furthermore, the analysis demonstrates favorable safety profiles for these biological agents, supporting their clinical applicability in COPD management.
Notably, while the mepolizumab (anti-IL-5), itepekimab (anti-IL-33), and astegolimab (anti-ST2) failed to demonstrate statistically significant efficacy in this NMA, their potential therapeutic value warrants further investigation. First, large-scale multicenter trials with adequate power are needed to confirm potential clinical benefits. Second, advanced phenotyping using blood/sputum biomarkers may identify responsive subpopulations. Third, optimized dosing regimens require evaluation. Fourth, multi-target combination therapies should be explored.
Abbreviations
COPD, Chronic obstructive pulmonary disease; ICS, Inhaled corticosteroids; LABA, Long-acting Beta-agonists; LAMA, Long-acting Muscarine Anticholinergic; TSLP, Thymic stromal lymphopoietin; Th2, T helper cell-2; ILC2, Type 2 intrinsic lymphoid cells; NMA, Network meta-analysis; RCT, Randomized controlled trial; AECOPD: Acute exacerbation of COPD; SGRQ, St. George’s Respiratory Questionnaire; pre-FEV1, FEV1 value before bronchodilator use; SAE, Serious adverse events; EOS, Peripheral blood eosinophils; JAK-STAT, Janus kinase-signal transducer and activator of transcription; VLA-4, Very Late Appearing Antigen-4; PAFr, Platelet-activating Factor Receptor; ICAM-1, Intercellular Cell Adhesion Molecule-1; TLR, Toll like receptors; PKC, Protein Kinase C; EGFR, Epidermal Growth Factor Receptor; ST6GAL1, ST6 β-galactoside α-2, 6-sialyltransferase 1; ACOS, Asthma‑COPD overlap syndrome.
Data Sharing Statement
The data included in this NMA comes from previously publicly reported studies and datasets, which have been cited in the references. The analysis results of this study have been reported in this article and can be obtained from the corresponding author upon reasonable request.
Ethics Approval
This is an observational study. The Research Ethics Committee has confirmed that no ethical approval is required.
Acknowledgments
Thanks for the technical help from Clinical Research Center, Tongji Hospital, School of Medicine, Tongji University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
2. Schleich F, Bougard N, Moermans C, Sabbe M, Louis R. Cytokine-targeted therapies for asthma and COPD. Eur Respir Rev. 2023;32(168):220193. doi:10.1183/16000617.0193-2022
3. Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227–2242. doi:10.1016/s0140-6736(22)00470-6
4. Rabe KF, Rennard S, Martinez FJ, et al. Targeting type 2 inflammation and epithelial alarmins in chronic obstructive pulmonary disease: a biologics outlook. Am J Respir Crit Care Med. 2023;208(4):395–405. doi:10.1164/rccm.202303-0455CI
5. Mahler DA, Huang S, Tabrizi M, Bell GM. Efficacy and safety of a monoclonal antibody recognizing interleukin-8 in COPD: a pilot study. Chest. 2004;126(3):926–934. doi:10.1378/chest.126.3.926
6. van der Vaart H, Koëter GH, Postma DS, Kauffman HF, Ten Hacken NH. First study of infliximab treatment in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(4):465–469. doi:10.1164/rccm.200501-147OC
7. Rennard SI, Fogarty C, Kelsen S, et al. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(9):926–934. doi:10.1164/rccm.200607-995OC
8. Calverley PMA, Sethi S, Dawson M, et al. A randomised, placebo-controlled trial of anti-interleukin-1 receptor 1 monoclonal antibody MEDI8968 in chronic obstructive pulmonary disease. Respir Res. 2017;18(1):153. doi:10.1186/s12931-017-0633-7
9. Maspero J, Adir Y, Al-Ahmad M, et al. Type 2 inflammation in asthma and other airway diseases. ERJ Open Res. 2022;8(3):00576–2021. doi:10.1183/23120541.00576-2021
10. Barnes PJ. Inflammatory endotypes in COPD. Allergy. 2019;74(7):1249–1256. doi:10.1111/all.13760
11. Caramori G, Adcock IM, Di Stefano A, Chung KF. Cytokine inhibition in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:397–412. doi:10.2147/copd.S42544
12. Castaldi PJ, Dy J, Ross J, et al. Cluster analysis in the COPDGene study identifies subtypes of smokers with distinct patterns of airway disease and emphysema. Thorax. 2014;69(5):415–422. doi:10.1136/thoraxjnl-2013-203601
13. Woodruff PG, Agusti A, Roche N, Singh D, Martinez FJ. Current concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: making progress towards personalised management. Lancet. 2015;385(9979):1789–1798. doi:10.1016/s0140-6736(15)60693-6
14. Kaur R, Chupp G. Phenotypes and endotypes of adult asthma: moving toward precision medicine. J Allergy Clin Immunol. 2019;144(1):1–12. doi:10.1016/j.jaci.2019.05.031
15. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi:10.1164/rccm.200912-1843CC
16. Garudadri S, Woodruff PG. Targeting chronic obstructive pulmonary disease phenotypes, endotypes, and biomarkers. Ann Am Thorac Soc. 2018;15(Suppl 4):S234–s238. doi:10.1513/AnnalsATS.201808-533MG
17. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi:10.1016/s0140-6736(12)60988-x
18. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi:10.1056/NEJMoa1403290
19. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, Phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. doi:10.1016/s2213-2600(17)30125-x
20. Brusselle GG, Koppelman GH. Biologic Therapies for Severe Asthma. N Engl J Med. 2022;386(2):157–171. doi:10.1056/NEJMra2032506
21. Gyawali B, Georas SN, Khurana S. Biologics in severe asthma: a state-of-the-art review. Eur Respir Rev. 2025;34(175):240088. doi:10.1183/16000617.0088-2024
22. Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377(17):1613–1629. doi:10.1056/NEJMoa1708208
23. Bhatt SP, Rabe KF, Hanania NA, et al. Dupilumab for COPD with blood eosinophil evidence of type 2 inflammation. N Engl J Med. 2024;390(24):2274–2283. doi:10.1056/NEJMoa2401304
24. Xiang H, Yue L, Zhao H, et al. Implementation of Bayesian network meta-analysis with BUGSnet package in R Software. Chinese J of Evidence-Based Med. 2022;22(5):600–608. doi:10.7507/1672-2531.202111140
25. Brightling CE, Bleecker ER, Panettieri RA, et al. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir Med. 2014;2(11):891–901. doi:10.1016/s2213-2600(14)70187-0
26. Criner GJ, Celli BR, Brightling CE, et al. Benralizumab for the Prevention of COPD Exacerbations. N Engl J Med. 2019;381(11):1023–1034. doi:10.1056/NEJMoa1905248
27. Rabe KF, Celli BR, Wechsler ME, et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med. 2021;9(11):1288–1298. doi:10.1016/s2213-2600(21)00167-3
28. Yousuf AJ, Mohammed S, Carr L, et al. Astegolimab, an anti-ST2, in chronic obstructive pulmonary disease (COPD-ST2OP): a phase 2a, placebo-controlled trial. Lancet Respir Med. 2022;10(5):469–477. doi:10.1016/s2213-2600(21)00556-7
29. Wechsler ME, Klion AD, Paggiaro P, et al. Effect of dupilumab on blood eosinophil counts in patients with asthma, chronic rhinosinusitis with nasal polyps, atopic dermatitis, or eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2022;10(10):2695–2709. doi:10.1016/j.jaip.2022.05.019
30. Bhatt SP, Rabe KF, Hanania NA, et al. Dupilumab for chronic obstructive pulmonary disease with type 2 inflammation: a pooled analysis of two phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med. 2025;13(3):234–243. doi:10.1016/s2213-2600(24)00409-0
31. Barnes PJ. Cellular and molecular mechanisms of asthma and COPD. Clin Sci. 2017;131(13):1541–1558. doi:10.1042/cs20160487
32. Kandikattu HK, Upparahalli Venkateshaiah S, Mishra A. Synergy of Interleukin (IL)-5 and IL-18 in eosinophil mediated pathogenesis of allergic diseases. Cytokine Growth Factor Rev. 2019;47:83–98. doi:10.1016/j.cytogfr.2019.05.003
33. Le Floc’h A, Allinne J, Nagashima K, et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75(5):1188–1204. doi:10.1111/all.14151
34. Byers DE, Alexander-Brett J, Patel AC, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest. 2013;123(9):3967–3982. doi:10.1172/jci65570
35. Johnston LK, Bryce PJ. Understanding Interleukin 33 and its roles in eosinophil development. Front Med. 2017;4:51. doi:10.3389/fmed.2017.00051
36. Qi Y, Yan Y, Tang D, et al. Inflammatory and immune mechanisms in COPD: current status and therapeutic prospects. J Inflamm Res. 2024;17:6603–6618. doi:10.2147/jir.S478568
37. Razia DEM, Gao C, Wang C, et al. Targeting non-eosinophilic immunological pathways in COPD and AECOPD: current insights and therapeutic strategies. Int J Chron Obstruct Pulmon Dis. 2025;20:511–532. doi:10.2147/copd.S506616
38. Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189(3):250–255. doi:10.1164/rccm.201310-1863PP
39. Singh D, Brightling CE, Rabe KF, et al. Efficacy and safety of tezepelumab versus placebo in adults with moderate to very severe chronic obstructive pulmonary disease (COURSE): a randomised, placebo-controlled, phase 2a trial. Lancet Respir Med. 2025;13(1):47–58. doi:10.1016/s2213-2600(24)00324-2
40. Hanania NA, Chipps BE, Griffin NM, Yoo B, Iqbal A, Casale TB. Omalizumab effectiveness in asthma-COPD overlap: post hoc analysis of PROSPERO. J Allergy Clin Immunol. 2019;143(4):1629–1633.e1622. doi:10.1016/j.jaci.2018.11.032
41. Casale TB, Luskin AT, Busse W, et al. Omalizumab effectiveness by biomarker status in patients with Asthma: evidence from PROSPERO, A prospective real-world study. J Allergy Clin Immunol Pract. 2019;7(1):156–164.e151. doi:10.1016/j.jaip.2018.04.043
42. Yalcin AD, Celik B, Yalcin AN. Omalizumab (anti-IgE) therapy in the asthma-COPD overlap syndrome (ACOS) and its effects on circulating cytokine levels. Immunopharmacol Immunotoxicol. 2016;38(3):253–256. doi:10.3109/08923973.2016.1173057
43. Tat TS, Cilli A. Omalizumab treatment in asthma-COPD overlap syndrome. J Asthma. 2016;53(10):1048–1050. doi:10.1080/02770903.2016.1178281
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Management of Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) in the Pan-Arab Region: Consensus Recommendations from a Multidisciplinary Expert Working Group
Marglani O, Al Abri R, Al Ahmad M, Alsaleh S, Abuzakouk M, Kamel R
Journal of Asthma and Allergy 2023, 16:1055-1063
Published Date: 29 September 2023