Back to Journals » Infection and Drug Resistance » Volume 18
Efficacy and Safety of Eravacycline Combination Therapy for Carbapenem-Resistant Acinetobacter baumannii Pneumonia in ICU Patients: A Retrospective Study
Authors Guo Q , Wei Y , Zhao Q, Zhang W, Lv P, Zhou Z, Wang S
Received 30 January 2025
Accepted for publication 29 May 2025
Published 17 June 2025 Volume 2025:18 Pages 3013—3021
DOI https://doi.org/10.2147/IDR.S515207
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Qingli Guo,1,* Yifei Wei,2,* Qiongrui Zhao,3 Wenping Zhang,4 Pin Lv,5 Zhimin Zhou,1 Shanmei Wang6
1Department of Clinical Laboratory, Xuchang People’s Hospital, Xuchang, Henan, People’s Republic of China; 2Department of Clinical Laboratory, Henan University People’s Hospital, Henan Provincial People’s Hospital, Zhengzhou, Henan, People’s Republic of China; 3Research and Foreign Affairs Office, Henan Provincial People’s Hospital, Zhengzhou, Henan, People’s Republic of China; 4Department of Respiratory and Critical Care Medicine, People’s Hospital of Henan Province, People’s Hospital of Zhengzhou University, People’s Hospital of Henan University, Zhengzhou, Henan, People’s Republic of China; 5Department of Pharmacy Henan Provincial People’s Hospital, Zhengzhou, Henan, People’s Republic of China; 6Department of Clinical Microbiology, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, Henan University People’s Hospital, Zhengzhou, Henan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shanmei Wang, Department of Clinical Laboratory, Henan Provincial People’s Hospital, No. 7, Weiwu Road, Zhengzhou, Henan, 450003, People’s Republic of China, Tel +86 137 8353 0126, Email [email protected]
Introduction: The rising incidence of carbapenem-resistant Acinetobacter baumannii (CRAB) poses challenges in Intensive Care Unit (ICU) settings. Eravacycline, the first fluorocycline antibiotic introduced globally, is increasingly utilized for CRAB-related infections. This study compares eravacycline with tigecycline for CRAB pneumonia in ICU patients.
Methods: This retrospective study analyzed 65 ICU patients with CRAB pneumonia treated at Henan Provincial People’s Hospital from September 2023 to March 2024; the clinical efficacy and adverse reactions of eravacycline and tigecycline as target therapies were compared.
Results: Both CRAB pneumonia cohorts exhibited more than 50% co-infection rates, predominantly Aspergillus and Klebsiella pneumoniae. Multiple organ dysfunction syndrome (MODS) incidence was significantly higher in the eravacycline group (45.5% vs 15.5%, P< 0.05). Prior to target-drug administration, patients in the eravacycline group showed a longer duration of antibiotic therapy (19.79 ± 9.97 days vs 14.25 ± 7.98 days; P< 0.05). Similarly, the median hospital stay was longer in the eravacycline group [27 (19.50, 39.00) days] versus the tigecycline group [19 (14.00, 29.00) days]. After the index culture, the median time to antibiotic initiation was longer in the eravacycline group than in the tigecycline group (6.52 ± 5.03 days vs 4.09 ± 3.14 days; P < 0.05). The 30-day mortality rate was lower in the eravacycline group than in the tigecycline group (15.2% vs 25.0%). Notably, no deaths occurred among eravacycline-treated patients who received infectious disease specialist consultations. Infection symptom resolution and cure rates did not differ significantly between the two groups.
Conclusion: Eravacycline demonstrated non-inferior efficacy to tigecycline in CRAB pneumonia treatment, with a favorable safety profile. Prompt consultation with infectious disease specialists and timely initiation of eravacycline therapy following the index culture are crucial factors in enhancing clinical outcomes.
Keywords: CRAB, ICU, pneumonia, eravacycline, tigecycline, clinical efficacy
Introduction
Acinetobacter baumannii represents the most prevalent species within the Acinetobacter baumannii-calcoaceticus complex that is implicated in human infections. In hospital settings, ICU patients demonstrate heightened susceptibility to Acinetobacter baumannii infections, primarily attributed to therapy-induced immunosuppression, recurrent exposure to invasive iatrogenic interventions like mechanical ventilation, and persistent microbial colonization within ICU environments, typically manifesting as ventilator-associated pneumonia, catheter-related bloodstream infections, and surgical site infections. The distinctive biological traits of A. baumannii have positioned it as a pivotal pathogen in global healthcare environments.1,2 The extensive utilization of carbapenem antibiotics has resulted in a heightened detection rate of CRAB. The emergence of CRAB, characterized by its intricate resistance mechanisms, indicates resistance to the majority of other antibiotic classes. Both in China and internationally, CRAB infections are prominent among the leading causes of antibiotic resistance. Multi-drug-resistant, extensively drug-resistant, and pan-drug-resistant strains of A. baumannii have emerged as significant contributors to nosocomial infections in China. The surge of CRAB not only poses a grave threat in the form of life-threatening nosocomial infections but also stands as a prominent cause of mortality attributed to drug-resistant bacteria in China.3–5
Eravacycline, which structurally resembles tigecycline, features a tetracycline core scaffold that has been modified by the tetracycline D-ring. As the world’s first fluorocycline antibacterial agent, eravacycline exhibits broad-spectrum antibacterial activity. Compared to tigecycline, eravacycline demonstrates 2 to 8 times greater potency against Gram-negative bacilli. In 2018, the US Food and Drug Administration (FDA) approved it for the treatment of complicated intra-abdominal infections.6,7 Numerous studies have confirmed the favorable efficacy, safety, and tolerability of eravacycline in treating complex infections.8–10 Effective anti-infective therapy serves as a critical component in controlling and preventing CRAB infections. While combined therapy is recommended in both domestic and international expert consensus and guidelines for treating CRAB infections, and eravacycline is considered a viable option when minocycline and tigecycline are ineffective or intolerable.11–13 However, research on the therapeutic effects of eravacycline specifically in CRAB pneumonia, particularly in ICU patients, remains limited. Therefore, this study aims to evaluate the clinical efficacy of eravacycline in treating CRAB pneumonia in ICU patients by comparing it with tigecycline-based combination therapy.
Materials and Methods
From September 2023 to March 2024, a total of 65 patients admitted to the ICU of Henan Provincial People’s Hospital with CRAB pneumonia were enrolled in this retrospective study. Demographic data, laboratory test results, and treatment parameters of the patients were collected and analyzed. We implemented rigorous safeguards to anonymize all participant information before analytical procedures. Identifiable data remained fully inaccessible throughout the study and subsequent phases, ensuring full compliance with privacy protection standards.Based on the therapeutic agents administered, the patients were stratified into two groups: the eravacycline group (n=33) and the tigecycline group (n=32). All patients fulfilled the following inclusion criteria: admission to the ICU, respiratory tract specimens yielded Acinetobacter baumannii, and susceptibility testing demonstrated resistance to carbapenems (meropenem, imipenem) and β-lactam/β-lactamase inhibitor combinations (cefoperazone-sulbactam), with susceptibility to polymyxin B, a clinical diagnosis of CRAB pneumonia,14 and having received treatment with either eravacycline or tigecycline for at least 72 hours. Exclusion criteria encompassed: insensitive results in vitro drug sensitivity tests for eravacycline and tigecycline, individuals aged under 18 years, initiation of eravacycline or tigecycline prior to the availability of culture results, and cases with incomplete clinical data.
The primary outcome of the study was the 30-day mortality rate. Secondary outcomes encompassed the resolution or cure rate of infection symptoms, the 30-day readmission rate, and the occurrence of treatment-related adverse reactions. Resolution or improvement in infection symptoms was defined as the reduction or normalization of inflammatory markers and body temperature associated with the infection, as assessed by attending physicians based on case records.
Combination therapy involved the concurrent administration of intravenous (IV) eravacycline (1 mg/kg every 12 hours) or IV tigecycline (initial dose of 100 mg, followed by 50 mg every 12 hours), along with other regimens specifically targeting Acinetobacter baumannii, for a minimum duration of 48 hours.
Bacterial eradication was assessed based on the following criteria: 1.Complete eradication: Three consecutive negative bacterial culture results; 2.Microbial substitution: Three consecutive bacterial cultures showing the disappearance of CRAB but growth of other bacteria; 3.Non-eradication: CRAB remained present in bacterial cultures, without the emergence of other new microbial flora. The total number of cleared cases was the sum of the number of completely cleared cases and the number of cases with microbial substitution. The bacterial clearance rate (%) was calculated as the ratio of the total number of cleared cases to the total number of cases, multiplied by 100.
Data Collection and Analysis
All the data were entered and analyzed in SPSS 27.0 (IBM Corporation, USA) and GraphPad Prism 9.5. Categorical variables were reported as counts and percentages, their proportion differences were determined using Chi-square tests between groups as appropriate. Continuous variables presented as means and standard deviations and their mean difference was evaluated using Student’s t-test between groups. For data not conform to a normal distribution curve, the median (Q1, Q3) was used, and the Mann–Whitney U-test was performed. P-value <0.05 was considered significant.
Results
During the study period, a total of 250 adult ICU patients were diagnosed with CRAB pneumonia. Of these, 185 patients were excluded, and data from 65 patients were included in the final statistical analysis (Figure 1).
 |
Figure 1 Group Details. |
There was no statistically significant difference in demographics or baseline clinical characteristics between the two groups of CRAB pneumonia patients included in this study, indicating that the groups were comparable (outcomes are shown in Table 1).
 |
Table 1 Demographic and Baseline Characteristics of Two Groups of Patients with CRAB Pneumonia |
In the eravacycline group, one patient developed a CRAB bloodstream infection, and another patient experienced both a CRAB bloodstream infection and an intracranial infection. In the tigecycline group, one patient developed a CRAB intracranial infection. Among the patients with CRAB pneumonia in both groups, most had co-infections with other pathogens, with Klebsiella pneumoniae and Aspergillus being the most common (Table 2).
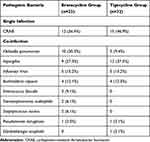 |
Table 2 Distribution of Pathogens in Two Groups of CRAB Pneumonia Patients |
All patients underwent invasive procedures. The incidence of multiple organ dysfunction syndrome (MODS) was higher in the eravacycline group compared to the tigecycline group. Additionally, the duration of antibiotic use before targeted therapy, the duration of targeted drug use after the first culture, and the total length of hospital stay were all significantly longer in the eravacycline group than in the tigecycline group, with statistically significant differences (Table 3).
 |
Table 3 Distribution of Treatment-Related Factors in Two Groups of CRAB Pneumonia Patients |
The 30-day mortality rate was lower in the eravacycline group compared to the tigecycline group, at 15.2% and 25.0%, respectively. None of the 7 patients who received an eravacycline-containing regimen after consultation with the infectious disease department died. The resolution or cure rate of infection symptoms was slightly lower in the eravacycline group compared to the tigecycline group, at 72.7% and 75.0%, respectively. In both groups, patients who received consultation within 48 hours had a higher resolution or cure rate of infection symptoms compared to those who did not, at 77.8%/76.9% and 66.7%/57.1%, respectively. The bacterial clearance rate was 54.5% in the eravacycline group, higher than the 50.0% observed in the tigecycline group (Table 4).
 |
Table 4 Efficacy of Treatment and Pathogen Clearance Rates in Two Groups of CRAB Pneumonia Patients |
The incidence of adverse reactions was lower in patients receiving eravacycline compared to those receiving tigecycline (Table 5).
 |
Table 5 Adverse Drug Reactions in Two Groups of CRAB Pneumonia Patients |
Discussion
This study presents a retrospective analysis of a combination therapy regimen based on eravacycline for CRAB pneumonia in ICU patients. Unlike previous studies that focused on CRAB-induced complex intra-abdominal infections,15–17 this research targets CRAB pneumonia, a specific and critical infection type in ICU settings. By comparing the efficacy of eravacycline with tigecycline-based combination therapy, this study provides new insights into treating CRAB pneumonia.Although early clinical experiences with Eravacycline-based combination therapy for various infections have been reported,18 data on its efficacy in CRAB pneumonia remain scarce. A recent large-scale multicenter analysis described the clinical effects of eravacycline on CRAB infections; however, the study included limited susceptibility results for isolated strains and lacked evaluations of its efficacy in pneumonia and sepsis patients.19 Given the prevalence of CRAB in ICU settings, particularly as ventilator-associated pneumonia, evaluating the clinical efficacy of eravacycline in such cases is essential.
The primary comorbidities observed in both patient groups were cerebrovascular diseases, hypertension, and respiratory system diseases. Co-infections with Aspergillus and Klebsiella pneumoniae were the most common pathogens, differing from previous findings.19 These differences underscore the importance of research tailored to specific populations and regions. Notably, ICU patients often present with multiple complications during their disease progression. The higher incidence of MODS in the eravacycline group indicates a more severe baseline condition compared to the tigecycline group. MODS is a severe condition often associated with rapid clinical deterioration and increased treatment complexity.20 Both groups received other antibiotics before initiating the target drugs, with the eravacycline group experiencing a longer duration of prior antibiotic use and a delayed initiation of eravacycline compared to tigecycline. This delayed initiation might explain the lack of significant therapeutic advantage of eravacycline over tigecycline. According to national and international guidelines, combination therapy is a cornerstone of CRAB infection management.11–13 In this study, the most common combination regimens with the target drugs were meropenem and polymyxin, with clinicians tailoring regimens based on treatment efficacy.
As a broad-spectrum antibiotic, eravacycline demonstrated superior in vitro antibacterial activity against CRAB and higher lung tissue concentrations compared to tigecycline, which likely explains the observed improvements in 30-day mortality and bacterial clearance rates in the eravacycline group in this study.8,21 Though these outcomes lower than previously reported levels in eravacycline-treated CRAB infections, but no differences were observed between the two groups in infection symptom resolution or clinical cure rates.19 Notably, infectious disease consultations improved clinical outcomes.22 Patients receiving eravacycline-based regimens after consultation experienced a significant reduction in 30-day mortality. Furthermore, timely consultation within 48 hours of the initial culture enhanced patient outcomes, highlighting the importance of early and appropriate medical intervention.Safety is another critical consideration. In this study, adverse reactions were less frequent in the eravacycline group compared to the tigecycline group. When compared with meropenem-polymyxin combinations, eravacycline combined with either meropenem or polymyxin showed a lower incidence of adverse reactions,23 underscoring its favorable safety profile.
Conclusion
This study confirms that eravacycline-based combination therapy is a clinically viable alternative to tigecycline-based regimens for CRAB pneumonia in ICU patients, with comparable efficacy outcomes. Early administration of eravacycline following culture confirmation was associated with improved clinical prognosis and reduced mortality. Additionally, outcomes in complex infections were improved by early consultation, particularly infectious disease consultation. While this study provides clinically relevant insights, its single-center retrospective design and limited sample size necessitate cautious interpretation due to potential confounding factors. To address these limitations, large-scale multicenter prospective trials are urgently needed to validate the findings.
Ethics Approval and Consent to Participate
This study was approved by the Medical Ethics Committee of Henan Provincial People’s Hospital (No. 120-2023) with a waiver of informed consent because of the retrospective nature of the study.This study complies with the Declaration of Helsinki.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Castanheira M, Mendes RE, Gales AC. Global epidemiology and mechanisms of resistance of Acinetobacter baumannii-calcoaceticus complex. Clin Infect Dis. 2023;76(Suppl 2):S166–S178. doi:10.1093/cid/ciad109
2. Liu C, Chen K, Wu Y, et al. Epidemiological and genetic characteristics of clinical carbapenem-resistant Acinetobacter baumannii strains collected countrywide from hospital intensive care units (ICUs) in China. Emerg Microbes Infect. 2022;11(1):1730–1741. doi:10.1080/22221751.2022.2093134
3. Naghavi M, Mestrovic T, Gray A. IHME Pathogen Core Group. Global burden associated with 85 pathogens in 2019: a systematic analysis for the global burden of disease study 2019. Lancet Infect Dis. 2024;24(8):868–895. doi:10.1016/S1473-3099(24)00158-0
4. Zhang C, Fu X, Liu Y, et al. Burden of infectious diseases and bacterial antimicrobial resistance in China: a systematic analysis for the global burden of disease study 2019. Lancet Reg Health West Pac. 2024;43:100972. doi:10.1016/j.lanwpc.2023.100972
5. Bartal C, Rolston KVI, Nesher L. Carbapenem-resistant Acinetobacter baumannii: colonization, infection and current treatment options. Infect Dis Ther. 2022;11(2):683–694. doi:10.1007/s40121-022-00597-w
6. Zhanel GG, Cheung D, Adam H, et al. Review of eravacycline, a novel fluorocycline antibacterial agent. Drugs. 2016;76(5):567–588. doi:10.1007/s40265-016-0545-8
7. XERAVA® (eravacycline) for injection, for intravenous use Initial U.S. Approval: 2018. Available from: https://xerava-assets.s3.amazonaws.com/prescribing-information.pdf.
8. Kunz Coyne AJ, Alosaimy S, Lucas K, et al. Eravacycline, the first four years: health outcomes and tolerability data for 19 hospitals in 5 U.S. regions from 2018 to 2022. Microbiol Spectr. 2024;12(1):e0235123. doi:10.1128/spectrum.02351-23
9. Huang PY, Hsu CK, Tang HJ, et al. Eravacycline: a comprehensive review of in vitro activity, clinical efficacy, and real-world applications. Expert Rev Anti Infect Ther. 2024;22(6):387–398. doi:10.1080/14787210.2024.2351552
10. Solomkin JS, Sway A, Lawrence K, et al. Eravacycline: a new treatment option for complicated intra-abdominal infections in the age of multidrug resistance. Future Microbiol. 2019;14:1293–1308. doi:10.2217/fmb-2019-0135
11. Tamma PD, Heil EL, Justo JA, et al. Infectious diseases society of America 2024 guidance on the treatment of antimicrobial-resistant gram-negative infections. Clin Infect Dis. doi:10.1093/cid/ciae403
12. Liu H, Vohra N, Bailey K, El-Shenawee M, Nelson A. Visual Enhancement and Semantic Segmentation of Murine Tissue Scans with Pulsed THz Spectroscopy. Proceedings. 2023;2023(23):80–90. doi:10.1109/ICSC56153.2023.00018
13. Zeng M, Xia J, Zong Z, et al. Guidelines for the diagnosis, treatment, prevention and control of infections caused by carbapenem-resistant gram-negative bacilli. J Microbiol Immunol Infect. 2023;56(4):653–671. doi:10.1016/j.jmii.2023.01.017
14. Ministry of Health of the People’s Republic of China. 医院感染诊断标准(试行) [Diagnostic Criteria for Nosocomial Infection (Trial)]. Chin Med J. 2001;81(05):314–320. Chinese.
15. Alosaimy S, Abdul-Mutakabbir JC, Kebriaei R, et al. Evaluation of eravacycline: a novel fluorocycline. Pharmacotherapy. 2020;40(3):221–238. doi:10.1002/phar.2366
16. Solomkin J, Evans D, Slepavicius A, et al. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the investigating gram-negative infections treated with eravacycline (IGNITE 1) trial: a randomized clinical trial. JAMA Surg. 2017;152(3):224–232. doi:10.1001/jamasurg.2016.4237
17. Solomkin JS, Gardovskis J, Lawrence K, et al. IGNITE4: results of a Phase 3, randomized, multicenter, prospective trial of eravacycline vs meropenem in the treatment of complicated intraabdominal infections. Clin Infect Dis. 2019;69(6):921–929. doi:10.1093/cid/ciy1029
18. Alosaimy S, Molina KC, Claeys KC, et al. Early experience with eravacycline for complicated infections. Open Forum Infect Dis. 2020;7(5):ofaa071. doi:10.1093/ofid/ofaa071
19. Alosaimy S, Morrisette T, Lagnf AM, et al. Clinical outcomes of eravacycline in patients treated predominately for carbapenem-resistant Acinetobacter baumannii. Microbiol Spectr. 2022;10(5):e0047922. doi:10.1128/spectrum.00479-22
20. Hu GH, Bao HY. 多器官功能障碍综合征的研究进展 [Research Progress in Multiple Organ Dysfunction Syndrome]. Chin J Clin Res. 2014;27(12):1542–1544. Chinese. doi:10.13429/j.cnki.cjcr.2014.12.040
21. Zeng M, Xia J, Zong ZY, et al. 碳青霉烯类耐药革兰阴性菌感染的诊断、治疗及防控指南 [Diagnosis, Treatment and Prevention Guidelines for Carbapenem-Resistant Gram-Negative Bacterial Infections]. Chin J Infect Chemother. 2024;24(2):135–151. Chinese. doi:10.16718/j.1009-7708.2024.02.002
22. Burnham JP, Olsen MA, Stwalley D, et al. Infectious diseases consultation reduces 30-day and 1-year all-cause mortality for multidrug-resistant organism infections. Open Forum Infect Dis. 2018;5(3):ofy026. doi:10.1093/ofid/ofy026
23. Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391–400. doi:10.1016/S1473-3099(18)30099-9
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

