Back to Journals » Journal of Pain Research » Volume 18
Efficacy and Safety of Kilohertz Frequency Alternating Current with Non-Invasive Electrodes for Treatment of Neuropathic Pain After Spinal Cord Injury: A Randomized, Single-Blind, Sham-Controlled Study
Authors Shuai N, Zhu Y, Xiao Y, Yu B
Received 9 April 2025
Accepted for publication 16 June 2025
Published 1 July 2025 Volume 2025:18 Pages 3321—3330
DOI https://doi.org/10.2147/JPR.S528057
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Andrea Tinnirello
Niannian Shuai,1,* Yuyao Zhu,2,* Yao Xiao,3,* Bin Yu4
1Department of Anesthesiology, Ningbo University Affiliated People’s Hospital, Zhejiang, People’s Republic of China; 2Department of Anesthesiology, Shanghai Tongji Hospital, Shanghai, People’s Republic of China; 3Department of Rehabilitation, Shanghai Tongji Hospital, Shanghai, People’s Republic of China; 4Department of Pain Rehabilitation, Yangzhi Rehabilitation Hospital, Affiliated with Tongji University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bin Yu, Department of Pain Rehabilitation, Yangzhi Rehabilitation Hospital, Affiliated with Tongji University, 2209 Guangxing Road, Songjiang District, Shanghai, 201613, People’s Republic of China, Tel +86 139 1810 8880, Email [email protected]
Objective: To evaluate the effectiveness and safety of noninvasive kilohertz frequency alternating current (KHFAC) therapy for neuropathic pain in patients following spinal cord injury.
Methods: In this randomized, single-blind, sham-controlled trial conducted from June 1, 2023, to January 31, 2024, 50 patients suffering from neuropathic pain post-spinal cord injury were assigned to receive either KHFAC or sham stimulation for 7 days, complemented by twice-daily oral administration of 75 mg pregabalin. Outcomes were assessed using the Visual Analog Scale (VAS), Pittsburgh Sleep Quality Index (PSQI), and Brief Pain Inventory (BPI) at baseline, at the end of treatment, and 30 days post-treatment. The primary outcome was the effective rate at the end of treatment, defined as a decrease in VAS score of 30% or more.
Results: At the end of the 7-day treatment period, the experimental group demonstrated a significantly higher response rate, with 60% of participants achieving a 30% or greater reduction in VAS scores compared to 28% in the control group (X²=5.195, P< 0.05). During this initial period, KHFAC effectively decreased pain intensity, reduced the frequency of paroxysmal pain, and lessened the need for additional analgesics. It also improved sleep quality and overall quality of life relative to sham stimulation. However, at the 30-day follow-up, no statistically significant differences were observed between the groups.
Conclusion: KHFAC is effective and safe for short-term neuropathic pain relief and quality of life enhancement in spinal cord injury patients, without an increase in adverse events versus sham stimulation. However, while reductions in analgesic use suggest potential lasting benefits, the long-term effectiveness remains uncertain. Further studies are required to assess the persistence of these effects.
Trial Registration: Chinese Clinical Trial Registry, ChiCTR2300068114.
Keywords: neuropathic pain, kilohertz electrical stimulation, patch electrode, spinal cord injury, visual analog scale
Introduction
Neuropathic pain (NP), mainly caused by spinal cord injury (SCI), impacts 50%–60% of SCI sufferers with severe pain that disrupts daily life.1–3 Treatment globally includes various pharmacological options; however, only pregabalin is FDA-approved for SCI-related NP, highlighting a significant gap in standardized treatments and emphasizing the need for more universal therapeutic standards and clinical research.
Spinal cord stimulation (SCS) using kilohertz frequency alternating current (KHFAC), ranging from 1 kHz to 100 kHz, has proven effective in NP management by blocking nerve conduction, demonstrated in both animal studies and early clinical trials.3–5 Nevertheless, the clinical adoption of SCS, including invasive dorsal root ganglion (DRG) stimulation, is restricted due to associated surgical risks.6,7
Addressing these limitations, our clinical trial investigates the efficacy and safety of a noninvasive 10 kHz KHFAC DRG stimulation via patch electrodes, compared with sham stimulation alongside medication. This pioneering study is the first to explore noninvasive KHFAC for NP treatment post-SCI, aiming to enhance noninvasive neuromodulation technologies and potentially set new standards in NP care. This trial marks a critical step towards improving clinical outcomes and broadening treatment accessibility for NP patients.
Methods
This was a single-center, randomized, single-blind, sham-controlled trial designed to assess the efficacy and safety of KHFAC with patch electrodes for the treatment of NP following SCI in adult patients. This investigation was carried out from June 1, 2023, to January 31, 2024.
Experiment Instrument
This test uses the electromyographic biofeedback instrument produced by Shanghai Nuocheng Electric Co., Ltd. (Registered in Shanghai with the medical device registration number 20162210785).
Research Participants
Adults (aged between 18 and 60 years) with a clear history of thoracolumbar SCI and symptoms of NP were included in this study. NP had to be diagnosed according to the Douleur Neuropathique 4 Questions (DN4) scale recommended by the China Guidelines for the Evaluation and Management of Neuropathic Pain (2024). Participants were expected to comprehend and be willing to follow the research methods and requirements, as well as offer informed permission. Women were eligible for participation if they were not pregnant or breastfeeding and committed to avoiding pregnancy throughout the trial. Patients who were in the acute or shock stage after SCI and those with non-thoracolumbar SCI, soft tissue injury, infection, or unhealed fracture were excluded. Patients with pacemakers, renal insufficiency (creatinine clearance rate <60 mL/min), severe liver and kidney dysfunction, and poor blood glucose control (fasting blood glucose level >11.1 mmol/L) were excluded to prevent potential skin reactions and infections from stimulation. People with mental illness or mental retardation who could not cooperate with the treatment and researchers who believed were not suitable for other reasons were also excluded.
Randomization, Blinding, and Study Treatment
Before therapy, participants were assessed and educated on evaluation tools, requiring a minimum pain score of 5 for eligibility. Using a computer-generated method, participants were divided into two groups in a 1:1 ratio: experimental and control, both receiving 75 mg of pregabalin after breakfast and lunch daily. The experimental group underwent KHFAC therapy, with patch electrodes placed over dermatomes linked to injured spinal segments, using 10 kHz biphasic rectangular waves. The intensity, adjustable from 1–7 mA based on tolerance, aimed to achieve numbness without pain, for 30-minute sessions twice daily for 7 days (Figure 1). In the control group, electrodes were placed but no current was applied. Patients experiencing severe pain could take additional analgesics, with the option to increase pregabalin to 150 mg twice daily. The effectiveness and safety of the treatments were assessed 30 days after therapy.
 |
Figure 1 Kilohertz electrical stimulator (left) and patch electrode (right). |
Study Assessments
Pain was assessed using the Visual Analog Scale (VAS) before therapy, after treatment, and 30 days later. The VAS, a 100-mm line, gauges pain intensity from no pain to extreme pain. The Brief Pain Inventory (BPI) evaluated pain’s impact on life across seven areas, with scores ranging from 0 (no impact) to 10 (severe impact), totaling up to 70. Sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI), which scores from 0 (excellent) to 21 (poor) across several sleep-related metrics. Improvements were noted by grade changes, with a one-grade increase suggesting remission.
Participant withdrawals and reasons were documented, excluding their data from final analysis. Safety was monitored by recording adverse events and changes in blood pressure and heart rate, including serious adverse events and local skin reactions like pain and erythema.
Endpoints
The main purpose of this study was to determine the effectiveness of treatment in the two groups of participants according to the VAS score at different time points, that is, the effectiveness rate. The specific calculation method used was as follows: curative effect = (before treatment-after treatment)/before treatment ×100%. According to previous clinical research, an effective curative effect is ≥30%, and < 30% indicates that the treatment is ineffective. Effective rate = effective number/total number of people ×100%.8
Other indicators include the statistics at d0, d7, and d30: (1) the degree of pain indicated by the VAS score; (2) the frequency of paroxysmal pain per week; (3) the use of additional analgesic drugs; (4) the PSQI score; and (5) the BPI score.
Adverse events were recorded in detail at any time during the test.
Statistical Analysis
In the primary outcome analysis, we presented the pre- and post-treatment differences between the two groups as percentages and used Pearson’s chi-square test for statistical evaluation. When the data did not meet the conditions for chi-square testing, we applied the continuity-corrected chi-square test or Fisher’s exact test as appropriate. For secondary outcomes, such as the proportion of patients using additional analgesics, we also employed chi-square tests.
We conducted two-way repeated measures ANOVA on data collected at d0, d7, and d30 to assess the effects of time, treatment group, and their interaction on pain intensity (VAS score), weekly frequency of paroxysmal pain, sleep quality (PSQI score), and pain interference (BPI score).
At the end of the treatment period, we utilized binary logistic regression analysis to investigate factors associated with treatment efficacy (effective/ineffective), including treatment method, duration of illness, and gender. All statistical tests were considered significant at a threshold of P<0.05, ensuring the scientific rigor and reliability of the results.
Compliance with Ethical Standards
This study was conducted at Yangzhi Rehabilitation Hospital (Shanghai Sunshine Rehabilitation Center) in accordance with the Declaration of Helsinki and relevant national/international guidelines and regulations. The study protocol, informed consent form and all supporting documents were reviewed and approved by the Medical Ethics Committee of Shanghai Yangzhi Rehabilitation Hospital (Approval No. Y.Z.L. Shen Zi (2023) No.003). All participants were adult subjects capable of giving written informed consent; no consent from a legal guardian or surrogate was required. The trial was registered with the China Clinical Trials Registry (ChiCTR2300068114) on July 2, 2023.
Results
Demographic Data
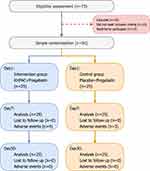
From February 2023 to January 2024, patients who were hospitalized at Yangzhi Rehabilitation Hospital, which is affiliated with Tongji University, and were clinically diagnosed with SCI with NP using a professional scale were included. A total of 73 persons were screened; 10 (13.7%) did not match the inclusion requirements, and 13 (17.8%) declined to participate in the study for personal reasons. 50 participants successfully participated in the study and were randomly separated into two groups, with 25 in the experimental group and 25 in the control group (Figure 2).
All the participants accepted the specified treatment plan and completed all the evaluation items before and after treatment. Therefore, the data sets of the treatment analysis and safety analysis were the same, consisting of all 50 participants. Table 1 summarizes the demographic and baseline characteristics of the participants.
 |
Table 1 Comparison of Baseline Characteristics Between the KHFAC Group and Sham Group |
The demographic features of the two groups were comparable, with no significant variations (P>0.05). The majority of participants (70.0%) were male. The median ages of the experimental and control groups were 47 (38.50–52.00) and 45 (35.00–50.00) years, respectively. The experimental group had a median illness duration of 8 (3.42–17.00) months, while the control group had a median of 7 (3.17–11.75) months. More than half of the participants (60.0%) had a thoracic SCI. Hypertension and diabetes were the main comorbid chronic diseases, and no patients in the two groups had heart disease or severe liver and kidney diseases.
The baseline characteristics were not significantly different between the two groups. The plan said that all participants experienced moderate to severe pain at baseline, with average VAS values of 7.46±0.75 and 7.25±1.02, respectively. The median (interquartile interval) frequency of paroxysmal pain per week in the experimental group was 2.0 (1.0–3.0), whereas that in the control group was 1.0 (0.5–2.0). The number of all participants who used additional painkillers was 11 (22.0%). At baseline, the sleep quality was mostly “grade B” (74.0%), and no participants with poor sleep quality were found during the whole process. At baseline, the two groups had mean BPI scores of 42.08 ± 6.51 and 38.92 ± 6.26.
Main Results
The indicators for the main results are shown in Figure 3A. At the end of treatment (d7), the experimental group had 15 patients who responded positively to the treatment, while the control group had 7. The two groups had effective rates of 60.0% and 28.0%, respectively, for a difference of 32.0%. The X2 test showed a statistically significant distinction between the two groups (X2 = 5.195; P <0.05).
On the 30th day after treatment (d30), compared with sham stimulation, KHFAC was no more effective at treating pain (32.0% vs 28.0%, 4.0% difference; X2=0.095; P> 0.05 indicates that there was no statistical significance).
The degree of pain was expressed by the specific VAS score of each subject. Figure 3B shows the changes in the VAS score before and after treatment. The degree of pain was obviously lower in the experimental group (7.46±0.75 vs 5.19±0.96; P<0.001) than in the control group (7.25±1.02 vs 5.36±1.03; P<0.001). There was no significant difference between the time points of 30 days after therapy and the completion of treatment, however, the degree of pain was much less in both groups after treatment than before treatment. (P<0.001).
Figure 3C depicts the absolute value of the drop in VAS score after treatment compared to pre-treatment in the two groups. The experimental group experienced more pain alleviation than the control group (2.26±0.80 vs 1.89±0.87), albeit the difference was not statistically significant (P>0.05). Notably, only the average reduction in the VAS score in the experimental group met the clinically significant difference (MCID) threshold (more than or equal to 2 points on the numerical scale score). There was no significant difference in the improvement in VAS score between the two groups 30 days after therapy.
Other Secondary Results
In the secondary outcome analysis, all indicators demonstrated a significant main effect of time (p < 0.01), indicating notable improvements in VAS, BPI, PSQI, and Paroxysmal Pain scores across different time points after treatment, regardless of group (Figure 4A–D). This finding highlights the significant impact of time on treatment efficacy, suggesting that both groups experienced meaningful improvements in pain relief and sleep quality over time, particularly at Day 7 and Day 30. This pattern indicates that as treatment progresses, patients generally benefit from enhanced therapeutic effects on both pain and sleep.
Although the main effect of group was not significant for any of the indicators (VAS: p = 0.55, BPI: p = 0.18, PSQI: p = 0.90, Paroxysmal Pain: p = 0.23), which implies that there was no substantial difference between the KHFAC and sham groups when considering group effects alone, a significant group x time interaction effect was observed for VAS (Figure 4A, p = 0.04), PSQI (Figure 4C, p < 0.01), and Paroxysmal Pain (Figure 4D, p = 0.03). This interaction suggests that the improvement trends across time points varied significantly between the groups. Specifically, the KHFAC group showed more pronounced improvements in pain relief and sleep quality at the early stage (Day 7) compared to the sham group. This interaction effect indicates that the KHFAC treatment may have provided earlier benefits, with patients in the KHFAC group responding to treatment faster than those in the sham group. Although the interaction effect for BPI (Figure 4B) was not significant (p = 0.12), both groups showed improvement trends in the initial phase of treatment, indicating that both interventions may provide early relief, albeit without significant differentiation between the two groups for this specific measure.
In terms of analgesic use, 5, 2, and 4 patients in the experimental group used additional painkillers before treatment, at the end of treatment, and on Day 30 after treatment, respectively; in the control group, 6, 8, and 8 patients used additional painkillers at the corresponding time points (Figure 4B). At the end of treatment, the proportion of additional analgesic use in the experimental group was significantly lower than that in the control group (8.0% vs 32.0%, X² = 4.50, p = 0.03), suggesting that KHFAC may reduce the need for additional pain medication in the short term, which could be beneficial for pain management. However, there was no statistically significant difference in the proportion of additional analgesic use between the two groups before treatment and on Day 30 after treatment. This lack of significant difference at Day 30 suggests that while KHFAC may provide early benefits in reducing additional analgesic needs, this effect does not appear to persist over a longer period.
Adverse Events
No serious side effects such as drowsiness, dizziness, headache, or infections were reported in any participants. In the experimental group, one patient’s patch electrode fell off due to rubbing, causing a brief tingling and temporary redness about 1 cm in diameter, which subsided without pain or itching after about 5 minutes. The main adverse events observed were electrode displacement and skin irritation.
Regression Analysis
In this study of 50 patients, effectiveness at treatment end was analyzed using binary logistic regression, considering various factors such as treatment technique, disease progression, age, gender, pain severity, and use of extra painkillers. The analysis revealed that the treatment group variable was the only statistically significant factor, indicating the experimental group was 5.8 times more likely to experience effective treatment compared to the control group (p=0.02, Exp(B)=5.80, CI: 1.32–25.4). Other factors, including disease duration, age, gender, pain severity (VAS), and additional painkillers, did not significantly affect treatment outcomes, as their p-values were above 0.05 and confidence intervals broadly included 1. This confirms KHFAC’s efficacy as the primary determinant of therapeutic effectiveness in this cohort, independent of other potential influencing factors.
Discussion
This study evaluated the effectiveness of combining kilohertz frequency alternating current (KHFAC) stimulation with drug therapy in treating neuropathic pain (NP) among patients with spinal cord injury (SCI). The primary finding was that the experimental group receiving KHFAC exhibited a significantly higher rate of effective pain relief at the end of treatment compared to the control group receiving only drug therapy. Specifically, the experimental group achieved an average reduction of 2.26 points in pain scores, reaching the minimal clinically important difference (MCID) for chronic pain reduction, whereas the control group did not. However, this significant difference was not sustained at the 30-day follow-up.
The initial efficacy of KHFAC may be attributed to its ability to influence pain conduction pathways without inducing abnormal sensations, unlike traditional low-frequency stimulation.9 KHFAC may directly block nerve conduction by affecting sodium and potassium ion currents10,11 or inhibit pain signals downstream of the injury site.12 Additionally, its mechanism might involve regulating interactions between neurons and glial cells in the dorsal root ganglion (DRG) and spinal dorsal horn.13–15 Despite these potential advantages, the absence of sustained long-term effects suggests incomplete inhibition of central sensitization and glial cell activity. The “carry-over” effect—where nerve conduction remains inhibited for a period after stimulation ceases—may also contribute.16–18 Indicating that longer stimulation durations might be necessary to achieve prolonged benefits.
At the conclusion of treatment, the experimental group required fewer supplemental painkillers and reported improved sleep quality and overall quality of life compared to the control group. Nonetheless, these benefits were not significant at the 30-day follow-up, underscoring the need for further investigation into the long-term efficacy of KHFAC therapy.
A notable aspect of this study is the use of noninvasive patch electrodes for KHFAC delivery, thereby reducing risks associated with invasive implantation methods employed in previous research. While prior studies have reported high efficacy rates with spinal cord stimulation systems.2,19–23 The noninvasive and shorter-duration treatment used in this study offers a practical alternative, although direct comparisons are challenging due to differences in treatment protocols.
Limitations of the study include its single-blind design and reliance on subjective measures such as the Visual Analog Scale (VAS). Masking participants was impractical due to the sensations caused by KHFAC stimulation. Although measures were taken to reduce bias, the absence of full masking may have influenced the results. Furthermore, patients with refractory pain may have been more inclined to participate, potentially affecting the generalizability of the findings. Nonetheless, the study was designed based on realistic criteria for comparative efficacy trials.21,24,25
In summary, combining KHFAC with drug therapy provides significant short-term relief for NP in SCI patients, improving pain levels, reducing the need for additional painkillers, and enhancing sleep quality and overall quality of life immediately after treatment. However, these therapeutic effects were not sustained over the long term. Further research with larger sample sizes and extended stimulation durations is necessary to confirm these findings and to explore the long-term efficacy and safety of KHFAC as a treatment option for SCI-induced neuropathic pain.
Data Sharing Statement
The data used in this study are available from the corresponding author upon reasonable request. All data have been anonymized in compliance with applicable privacy and ethical guidelines to protect the participants’ privacy. For further information about the dataset or access requests, please contact the corresponding author.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
There are no conflicts of interest to declare.
References
1. Cruz-Almeida Y, Martinez-Arizala A, Widerström-Noga EG. Chronicity of pain associated with spinal cord injury: a longitudinal analysis. J Rehabil Res Dev. 2005;42(5):585–594. doi:10.1682/JRRD.2005.02.0045
2. Norrbrink Budh C, Lund I, Ertzgaard P, et al. Pain in a Swedish spinal cord injury population. Clin Rehabil. 2003;17(6):685–690. doi:10.1191/0269215503cr664oa
3. Ling D, Luo J, Wang M, et al. Kilohertz high-frequency alternating current blocks nerve conduction without causing nerve damage in rats. Ann Transl Med. 2019;7(22):661. doi:10.21037/atm.2019.10.36
4. Kapural L, Yu C, Doust MW, et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: the SENZA-RCT Randomized Controlled Trial. Anesthesiology. 2015;123(4):851–860. doi:10.1097/ALN.0000000000000774
5. Kilgore KL, Bhadra N. Reversible nerve conduction block using kilohertz frequency alternating current. Neuromodulation. 2014;17(3):242–254. doi:10.1111/ner.12100
6. Bonezzi C, Fornasari D, Cricelli C, Magni A, Ventriglia G. Pharmacological Management of Adults with Chronic Non-Cancer Pain in General Practice. Pain Ther. 2020;9(S1):17–28. doi:10.1007/s40122-020-00218-9
7. Lamer TJ, Moeschler SM, Gazelka HM, et al. Spinal Stimulation for the Treatment of Intractable Spine and Limb Pain: a Systematic Review of RCTs and Meta-Analysis. Mayo Clin Proc. 2019;94(8):1475–1487. doi:10.1016/j.mayocp.2018.12.037
8. Hongqiao W, Xue Z. Efficacy of paravertebral nerve block with interferon alpha on the treatment of postherpetic neuralgia. Chin J Pain Med. 2020;26:191–195.
9. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979. doi:10.1126/science.150.3699.971
10. Tai C, de Groat WC, Roppolo JR. Simulation of nerve block by high-frequency sinusoidal electrical current based on the Hodgkin-Huxley model. IEEE Trans Neural Syst Rehabil Eng. 2005;13(3):415–422. doi:10.1109/TNSRE.2005.847356
11. Ackermann DM, Bhadra N, Gerges M, Thomas PJ. Dynamics and sensitivity analysis of high-frequency conduction block. J Neural Eng. 2011;8(6):065007. doi:10.1088/1741-2560/8/6/065007
12. Zhang X, Roppolo JR, de Groat WC, Tai C. Simulation analysis of conduction block in myelinated axons induced by high-frequency biphasic rectangular pulses. IEEE Trans Biomed Eng. 2006;53(7):1433–1436. doi:10.1109/TBME.2006.873689
13. Wei X-H, Na X-D, Liao G-J, et al. The up-regulation of IL-6 in DRG and spinal dorsal horn contributes to neuropathic pain following L5 ventral root transection. Exp Neurol. 2013;241:159–168. doi:10.1016/j.expneurol.2012.12.007
14. Chen Y-W, Tzeng J-I, Lin M-F, et al. High-frequency transcutaneous electrical nerve stimulation attenuates postsurgical pain and inhibits excess substance P in rat dorsal root ganglion. Reg Anesth Pain Med. 2014;39(4):322–328. doi:10.1097/AAP.0000000000000091
15. Finnerup NB. Pain in patients with spinal cord injury. Pain. 2013;154 Suppl 1(Supplement 1):S71–S76. doi:10.1016/j.pain.2012.12.007
16. Yang G, Xiao Z, Wang J, et al. Post-stimulation block of frog sciatic nerve by high-frequency (kHz) biphasic stimulation. Med Biol Eng Comput. 2017;55(4):585–593. doi:10.1007/s11517-016-1539-0
17. Waataja JJ, Tweden KS, Honda CN. Effects of high-frequency alternating current on axonal conduction through the vagus nerve. J Neural Eng. 2011;8(5):056013. doi:10.1088/1741-2560/8/5/056013
18. De Groote S, Goudman L, Linderoth B, et al. A Regions of Interest Voxel-Based Morphometry Study of the Human Brain During High-Frequency Spinal Cord Stimulation in Patients With Failed Back Surgery Syndrome. Pain Pract. 2020;20(8):878–888. doi:10.1111/papr.12922
19. Kapural L, Yu C, Doust MW, et al. Comparison of 10-kHz High-Frequency and Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: 24-Month Results From a Multicenter, Randomized, Controlled Pivotal Trial. Neurosurgery. 2016;79(5):667–677. doi:10.1227/NEU.0000000000001418
20. Naples GG, Mortimer JT, Scheiner A, Sweeney JD. A spiral nerve cuff electrode for peripheral nerve stimulation. IEEE Trans Biomed Eng. 1988;35(11):905–916. doi:10.1109/10.8670
21. Petersen EA, Stauss TG, Scowcroft JA, et al. Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients With Painful Diabetic Neuropathy: a Randomized Clinical Trial. JAMA Neurol. 2021;78(6):687–698. doi:10.1001/jamaneurol.2021.0538
22. Al-Kaisy A, Palmisani S, Smith TE, et al. Long-Term Improvements in Chronic Axial Low Back Pain Patients Without Previous Spinal Surgery: a Cohort Analysis of 10-kHz High-Frequency Spinal Cord Stimulation over 36 Months. Pain Med. 2018;19(6):1219–1226. doi:10.1093/pm/pnx237
23. Al-Kaisy A, Palmisani S, Smith TE, et al. 10 kHz High-Frequency Spinal Cord Stimulation for Chronic Axial Low Back Pain in Patients With No History of Spinal Surgery: a Preliminary, Prospective, Open Label and Proof-of-Concept Study. Neuromodulation. 2017;20(1):63–70. doi:10.1111/ner.12563
24. Piaggio G, Elbourne DR, Pocock SJ, et al. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308(24):2594–2604. doi:10.1001/jama.2012.87802
25. Zabor EC, Kaizer AM, Hobbs BP. Randomized Controlled Trials. Chest. 2020;158(1):S79–S87. doi:10.1016/j.chest.2020.03.013
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.