Back to Journals » Therapeutics and Clinical Risk Management » Volume 20
Efficacy and Safety of Tranexamic Acid on Hidden Blood Loss in Osteoporotic Vertebral Compression Fractures Patients Treated with Percutaneous Kyphoplasty: A Prospective Randomized Controlled Trial
Authors Lou Z , Jiang K, Xia S, Chen S, Jiang Y, Zhu J, Zhu J
Received 5 September 2024
Accepted for publication 20 December 2024
Published 25 December 2024 Volume 2024:20 Pages 907—917
DOI https://doi.org/10.2147/TCRM.S494728
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor De Yun Wang
Zhenqi Lou, Kanling Jiang, Sanqiang Xia, Sihui Chen, Yi Jiang, Jinyu Zhu, Jieyang Zhu
Department of Orthopedics, Affiliated Hospital of Jiaxing University, Jiaxing, Zhejiang, People’s Republic of China
Correspondence: Jinyu Zhu; Jieyang Zhu, Department of Orthopedics, Affiliated Hospital of Jiaxing University, 1882 Zhonghuan South Road, Jiaxing, Zhejiang, People’s Republic of China, Email [email protected]; [email protected]
Purpose: To evaluate the efficacy and safety of intravenous tranexamic acid (TXA) in patients undergoing percutaneous kyphoplasty (PKP), and identify the factors influencing hidden blood loss (HBL).
Methods: This randomized, placebo-controlled trial included 146 patients undergoing PKP surgery from September 2023 to July 2024. Patients were randomly assigned into the TXA group (75 patients received 1.0 g/100mL TXA intravenously) and the placebo group (71 patients received 100mL of normal saline intravenously). Demographic and clinical characteristics were comparable between groups. HBL was calculated and compared on postoperative days 1 (POD1) and 3 (POD3). Visual analog scale (VAS) scores were also recorded preoperatively and during the follow-up. Multivariate logical regression analysis identified independent risk factors for HBL.
Results: The HBL in the TXA group was 183.78± 115.48mL on POD 1 and 240.65± 114.73mL on POD 3, which was significantly lower than the placebo group at 251.30± 235.58mL on POD1 (P=0.032) and 384.94± 223.18mL on POD3 (P< 0.001). The drop in hemoglobin in the TXA group was generally lower than that of the placebo group on POD1 (4.72± 3.54 vs 7.62± 8.38 g/L, P=0.007), but showed no significant difference on POD 3. The drop in hematocrit in the TXA group was significantly lower than that in the placebo group on POD1 (1.91± 1.21% vs 2.65± 2.42%, P=0.023) and POD3 (2.49± 1.23% vs 3.92± 2.09%, P< 0.001). Additionally, the VAS scores on POD1 (2.28± 0.88 vs 2.82± 0.98, P< 0.001) and POD3 (1.95± 0.75 vs 2.25± 0.69, P=0.011) were lower in the TXA group than in the placebo group. Multivariate logical regression analysis revealed that the use of TXA (P< 0.001), injury time (P< 0.001), number of punctures (P=0.004), cement leakage (P=0.001), and restoration of vertebral height (P=0.002) were significantly correlated with HBL.
Conclusion: A single of 1g dose of intravenous TXA reduces HBL and early postoperative pain in PKP patients without increasing the complication rate. The use of TXA, injury time, number of punctures, cement leakage, and restoration of vertebral height were risk factors for HBL in PKP surgery. (ChiCTR2300075428).
Keywords: hidden blood loss, osteoporotic vertebral compression fractures, Percutaneous kyphoplasty, tranexamic acid, multiple regression analysis
Graphical Abstract:

Introduction
Osteoporosis is a metabolic bone disease characterized by bone loss and changes in bone microarchitecture.1 Osteoporotic vertebral compression fractures (OVCFs) are common osteoporosis-related fractures in elderly patients, and often leads to severe back pain, kyphosis, and decreased mobility, which affect patients’ quality of.2 Percutaneous kyphoplasty (PKP), described by Galibert in 1997,3 is a minimally invasive surgical method for treating OVCFs. Its advantages include less trauma, kyphosis correction, rapid pain relief, and stabilization of the vertebral body.4,5 It is widely accepted by spinal surgeons and has been reported to achieve good clinical results. However, we observed that the hemoglobin (Hb) loss in patients undergoing PKP did not correspond to the small amount of visible intraoperative blood loss observed in clinical practice. The bleeding associated with this minimally invasive surgery is often overlooked, especially hidden blood loss (HBL).
The concept of HBL was first proposed by Sehat et al6 in 2000 and has received increasing attention in recent years. HBL can occur due to hemolysis, residual blood in dead space or spreading to the tissue space, so the true blood loss during the perioperative phase is always underestimated. HBL has been reported even in minimally invasive surgery with almost “zero” surgical blood loss.7 Wu et al8 observed that larger amounts of HBL may occur after PKP surgery, with a mean volume of HBL of 282±162mL and a mean Hb loss of 8.7±5.4g/L. Man et al9 found that HBL was as high as 341mL, accounting for nearly 80% of total blood loss (TBL) in percutaneous endoscopic transforaminal discectomy surgery. Excessive HBL is one of the main causes of postoperative anemia, which may affect the recovery of function, increase the risk of infection, and prolong the length of hospital stay, especially in elderly patients with poor physical condition and poor tolerance of blood loss.10,11
Tranexamic acid (TXA), a synthetic derivative of the amino acid lysine, promotes hemostasis by competitively blocking the lysine-binding site of plasminogen and is widely used as an antifibrinolytic agent to reduce blood loss and transfusion rate in orthopedic surgery.12 Several studies have confirmed the efficacy and safety of TXA for controlling blood loss in patients undergoing spinal surgery, and the application of TXA is expected to become an ideal bleeding prevention strategy.12–14 Although TXA is widely used in surgical procedures, the influence of TXA in PKP surgery due to OVCFs has not been studied.
The objective of this prospective randomized controlled study was to evaluate the efficacy and safety of administering TXA preoperatively to patients undergoing PKP, and identify the key factors causing HBL.
Materials and Methods
Study Design
This prospective, randomized, controlled trial was approved by the Institutional Review Board of our institution and registered at www.chictr.org.cn (ChiCTR2300075428). All the patients were enrolled between September 2023 and July 2024 in the Department of Orthopedics, and eligible patients were randomly assigned to either the TXA group or the control group using a computer-generated random number table, with an equal allocation ratio of 1:1. The procedures used in this study adhere to the tenets of the Declaration of Helsinki, and informed consent was obtained from each participant. All personnel involved in the operation and postoperative follow-up, including patients, evaluators and surgeons, were blinded to the study protocols.
Inclusion criteria: 1) elderly patients (age ≥60 years old); 2) low back pain due to low-energy injury and with considerable physical signs of local tenderness; 3) T score of bone mineral density (BMD)<-2.5; 4) X-ray, CT and MRI showed one or two segment fresh vertebral compression fractures, 5) no symptoms of spinal cord or nerve root compression.
Exclusion criteria: 1) allergy to TXA; 2) bleeding disorders or coagulation dysfunction, and a history of thromboembolism or antiplatelet therapy in the 3 months preceding the start of the study; 3) pathological vertebral fractures caused by spinal infection, tuberculosis, or tumors and so on; 4) unstable spinal fracture or destruction of the posterior column; 5) patients with severe liver, kidney and cardiovascular disease; and 6) incomplete follow-up data.
Sample size: We performed a power analysis to determine the required sample size with the limit for type I error set at 5% and the power set at 80%. According to the pretest results, the minimum required sample size was 64 in each group. Taking into account a potential loss of 10% to follow-up, 140 patients were randomized and distributed equally into the two groups.
A total of 150 patients were included in this study and randomized into two groups: patients in the TXA groups (n=75) received an intravenous infusion of 1g of TXA in 100mL of saline 15min prior to skin incision, while in the placebo group, the same volume of normal saline was administered without TXA. The period between symptom onset and surgical treatment ranged from 1 day to 6 weeks. The patients were placed in prone position, the fracture segments were identified by X-ray, and all the operations were performed under local/general anesthesia by the same three surgeons.
Data Collection
Demographic and clinical characteristics were collected, including sex, age, weight, height, BMD, comorbidity (hypertension, diabetes mellitus), injury time, operation duration, type of anesthesia, fracture site and level, and the length of hospital stay. Complete blood count, coagulation function, including hematocrit (Hct), hemoglobin (Hb), activated partial thromboplastin time (APTT), prothrombin time (PT), and international normalized ratio (INR) were measured before the operation and repeated on the first (POD1) and third days (POD3) postoperatively. Visual analog scale was administered to all the participants pre-operation and during the follow-up, at POD1, POD3, and 1 month after surgery.
We measured muscle thickness and subcutaneous fat thickness of each patient (Figure 1), and calculated the loss and restoration of vertebral height (VH).
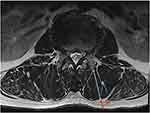 |
Figure 1 Diagram of measuring muscle thickness and subcutaneous fat thickness. (a) muscle thickness; (b) subcutaneous fat thickness. |


Where VHave was the average of the heights of the two adjacent vertebrae of the fractured vertebra. VHpre and VHpost were the preoperative and postoperative anterior or midline vertebral body heights, respectively.
There was little intraoperative blood loss during the PKP surgery and the visible blood loss was negligible, therefore the HBL was approximated to the TBL. HBL was calculated at POD 1 and POD 3 using the Nadler15 and Gross formula.16
PBV(L)= k1×height(m)3+k2×weight(kg)+k3, (male: k1=0.3669, k2=0.03219, k3=0.6041; female: k1=0.3561, k2=0.03308, k3=0.1833).
Total blood loss=HBL=PBV×(Hctpre-Hctpre)/Hctave, where Hctave=the average of Hctpre and Hctpre.
Patients were followed for 1 month postoperatively and complications such as cement leakage, wound infection, hematoma, and thromboembolic events were recorded. Deep vein thrombosis (DVT) was detected by routine ultrasound examination 1 week postoperatively and at 1 month of follow-up, or at any time during the onset of clinical symptoms. Pulmonary embolism was diagnosed based on clinical symptoms and an enhanced chest CT scan.
Statistical Analysis
All statistical analyses were performed using SPSS version 22.0 (IBM, Armonk, NY, USA). Normally distributed data were presented as mean ±standard deviation and an independent sample t-test was used. Non-normally distributed data were presented as M [P25; P75] and compared using Mann–Whitney’s U-test. Categorical variables were analyzed by using the Pearson’s chi-squared test or Fisher’s exact test. Univariate linear regression analysis was used to assess the potential risk factors, and multivariate logistic regression analysis was performed to identify risk factors associated with HBL. The level of statistical significance was set at P<0.05.
Results
Between September 2023 and July 2024, a total of 146 patients were assessed for eligibility, four patients were dropped-out owing to being lost to follow-up. The mean average age of the patients was 68.32±8.12 years, and 72.6% of the patients were females. Out of the 146 patients, 75 patients with 82 segments of vertebra fractures were in the TXA group, while the placebo group consisted of 71 patients with 88 segments of vertebra fractures (Figure 2). The fracture counts are presented in Figure 3, there was no considerable difference between the groups in demographic characteristics as shown in Table 1. The operation duration, type of anesthesia, number of punctures, bone cement volume, length of hospital stays and radiographic parameters in the TXA group were similar to those in the placebo group. The details are presented in Table 2.
 |
Table 1 Comparison of Baseline Characteristics |
 |
Table 2 Comparison of Surgical and Radiologic Characteristics, Changes in Hb and Hct Between the Groups |
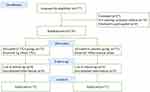 |
Figure 2 Patients flow chart. |
 |
Figure 3 Distribution of fracture level for patients in the two groups. |
Preoperative Hb level were significantly lower in the TXA compared to the placebo group (125.45±15.56 vs 130.66±13.28, P=0.032). However, the Hb levels between the two groups on POD1 and POD3 were similar. The Hb drop on POD1 (4.72±3.54 vs 7.62±8.38, P=0.007) was significantly lower in the TXA group, but showed no significant difference on POD3. Interestingly, the mean ΔHct in the TXA group was significantly lower than that in the placebo group on POD1 (1.91±1.21 vs 2.65±2.42, P=0.023) and POD3 (2.49±1.23 vs 3.92±2.09, P<0.001). The TXA group had significantly less HBL on POD1 (183.78±115.48mL VS 251.30±235.58mL, P=0.032) and POD3 (240.65±114.73mL VS 384.94 ±223.18mL, P<0.001)) than the control group (Figure 4). None of the patients in the TXA or placebo group required a postoperative blood transfusion. The VAS scores of both groups decreased significantly after the operation, with VAS scores in the TXA group being obviously better than those in the placebo group on POD1 (2.28±0.88 vs 2.82±0.98, P<0.001) and POD3 (1.95±0.75 vs 2.25±0.69, P=0.011). However, there was not significant difference between the two groups at 1 month (1.32±0.72 vs 1.35±0.64, P=0.776) after surgery (Figure 5). There were no significant differences in PT, APTT, INR, and FIB between the two groups preoperatively as well as on POD1 and POD3 (Figure 6).
 |
Figure 4 Hidden blood loss between the TXA and placebo groups. Asterisks indicate values that were significantly different between the two groups, P<0.05 indicates statistical significance. |
 |
Figure 5 VAS scores between the two groups. Asterisks indicate values that were significantly different between the two groups, P<0.05 indicates statistical significance. |
There was no significant difference in complication rates between the two groups (Table 3). Two patients in the TXA group and Three patients in the placebo group developed intramuscular venous thrombus confirmed by a routine Doppler ultrasound examination during the follow-up period. One case of DVT (peroneal vein thrombosis) was observed in the TXA group. Wound hematoma was observed in one patient in the TXA group.
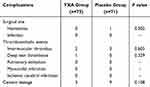 |
Table 3 Complications Following Percutaneous Kyphoplasty |
The Univariate linear regression analysis found the following parameters may be the potential factors affect HBL as shown in Table 4: the use of TXA (P<0.001), age (P=0.049), injury time (P<0.001), number of punctures (P<0.01), cement leakage (P<0.001), loss of VH (P<0.011), and restoration of VH (p<0.001). Multiple logistic regression analysis indicated that the use of TXA (P<0.001), injury time (P<0.001), number of punctures (P=0.004), cement leakage (P=0.001), and restoration of VH (p=0.002) were independent risk factors for HBL (Table 5).
 |
Table 4 Univariate Linear Regression Analysis on Potential Factors of HBL |
 |
Table 5 Multivariate Logistic Regression Analysis on Independent Risk Factors of HBL |
Discussion
Percutaneous kyphoplasty has been gradually recognized as an effective and safe surgical procedure for the treatment of patients with osteoporotic vertebral compression fractures (OVCFs) due to its minimal invasiveness, short operation time, and good postoperative function recovery. However, patients still show symptoms of anemia in the perioperative period, probably due to HBL. TXA is an ideal drug expected to reduce blood loss and, consequently, the need for blood transfusion by blocking the lysine-binding site of plasminogen and inhibiting its activation. Roman et al17 concluded that TXA significantly reduced TBL (453mL vs 701mL, P=0.03) in posterior cervical decompression and fusion surgery. Li et al18 demonstrated that TXA was effective in decreasing perioperative HBL (708.565±307.985mL vs 844.366±237.063mL, P<0.05) in percutaneous endoscopic transforaminal lumbar interbody fusion surgery. However, although the use and investigation of TXA for spinal surgeries has become widespread, there has been no evaluation of the effect of TXA on HBL in PKP surgery. To our knowledge, this is the first randomized clinical trial of intravenous use of a single dose of TXA in patients undergoing percutaneous kyphoplasty surgery.
There is no uniform standard for the optimal dose of TXA in spinal surgery. Several meta-analyses concluded that the dose of TXA for single intravenous application ranged from 15 to 30 mg/kg or 1 to 2 g in spinal surgery.13,19 A dose of 1 g appeared to be sufficient for most adults, resulting in a simple, standardized, and relatively low-cost treatment, whereas no evidence emerged to support the use of high doses of TXA, especially in elderly patients. Therefore, we chose to use 1 g of IV TXA prior to surgery as the dosing regimen protocol for this study.
In the present study, we found a notable association between OVCFs in patients undergoing PKP surgery and perioperative HBL. Owing to the completion of hemodynamic stability on the second or third days postoperatively, we calculated the HBL based on the Hct on POD1 and POD3 as 251.30±235.58mL and 384.94 ±223.18mL, respectively. The final HBL is consistent with that reported by Chai et al20 but slightly higher than that reported by Wu et al.8 Our results also showed that 1 g of TXA decreased HBL by 26.9% and 37.5% on POD1 and POD3, respectively, compared to the placebo group. Excessive HBL will cause a serious decrease in HB, and increase the postoperative incidence of anemia. Our results showed that IV TXA can significantly reduce the drop of Hb and Hct on POD1 and POD3. However, despite the minimally invasive nature of PKP procedures, perioperative HBL remains a grave concern. Early patients often have coexisting cardio-cerebral-vascular diseases and are particularly vulnerable to hemodynamic fluctuations. TXA can be protective in these patients as it helps maintain hemodynamic stability by reducing HBL during PKP surgery, and less perioperative HBL contributes to a safe surgery, quick recovery, and greater patient satisfaction.
Overall, patients in both groups showed significant improvements in VAS scores following PKP surgery. IV administration of TXA was effective in controlling pain control after arthroscopic rotator cuff repair by reducing hematoma formation.21 In our patients receiving TXA, we observed significantly lower pain (VAS) scores on POD1 and week 1, but the difference in VAS scores between the two groups at 1 postoperative month was relatively minimal. TXA acids in pain management when used in the early stages of PKP procedures because it reduces local soft tissue swelling and hematoma at the operative site caused by HBL.
Although the hemostatic mechanism of TXA relies on inhibition of the fibrinolytic system rather than directly affecting coagulation, in principle, it does not increase the risk of thrombosis.14 However, its potential to increase the risk of thrombotic events remains the greatest concerns regarding the use of TXA. Wang et al22 found that postoperative coagulation profiles including PT, APTT and INR were similar when different doses of TXA were used in patients with thoracolumbar burst fracture. Similarly, the present study found that there was no significant difference in postoperative coagulation between the two groups, indicating that the low dose of TXA used did not affect coagulation function. A recently meta-analysis of 216 studies also concluded that IV TXA was not associated with increased risk of thromboembolism.23 As continuous activation of the coagulation process after major orthopedic surgery can last up to 4 weeks,24 we performed routine ultrasound examination at 1 week postoperatively and at 1 month of follow-up. During the follow-up, the incidence of thromboembolic events in the TXA and placebo groups were 4% and 4.2% respectively, which was similar to the findings of Fan et al reported.25 Additionally, no patient presented with clinical signs of DVT during the follow-up. We also compared the incidence of wound complication and cement leakage, and found no significant difference. Intravenous administration of TXA in elderly patients undergoing PKP surgery did not significantly increase the risk of thrombotic complications and other surgery-related complications.
There are many factors affecting HBL in minimally invasive surgery performed for the treatment of spinal diseases. Hu et al9 concluded that sex, disk degeneration grade, preoperative FIB, and preoperative platelets are independent risk factors for HBL in percutaneous endoscopic transforaminal discectomy surgery. Guo et al26 determined that tissue thickness, PT, and diabetes mellitus influence HBL during unilateral biportal endoscopic surgery. In this study, multiple linear regression analysis was performed to determine the risk factors for HBL during PKP surgery. We found that the use of TXA and injury time of patients before surgery were negatively correlated with HBL, while the number of punctures, cement leakage, and recovery of VH were positively correlated with HBL. Our results were similar to those reported by Wu et al8 and Cai et al.20 During surgery for PKP, if the guide needle is not in the correct position, repeated adjustment is required. These high numbers of puncture cause damage to paravertebral muscles and soft tissues, even causing the cortical breakdown of the affected vertebra, which may result in bleeding from the paravertebral muscles and gathering blood in the tissue space, further increasing HBL. The pre-existing defect of vertebrae cortical bone is the main reason for cement leakage and persistent vertebral hemorrhage.27 Intraoperative inflation of the balloon could lead to widening of the pre-existing defect of the vertebrae, causing the intravertebral vacuum phenomenon, thereby increasing HBL. In addition, bone cement is also toxic to cells and can increase erythrocyte destruction during injection. The restoration of vertebral body height can reduce kyphosis in patients suffering from OVCFs, but the process may cause a secondary injury to the fractured vertebrae. An enhancement of vertebral body height restoration implies the formation of a larger cavity within the vertebrae, which leads to more hidden blood produced and remaining in the dead space. Several studies have shown that muscle thickness is an independent risk factor for HBL in spinal surgery.28,29 However, in our study, muscle thickness and subcutaneous fat thickness were not related to HBL, which was corroborated by Yue et al30 with minimally invasive percutaneous transpedicular screw fixation in thoracolumbar fracture. Overall, our observations are attributable to the minimally invasive nature of PKP, which involves smaller incisions with less muscle or other soft tissue damage.
There are several limitations to this study. First, this was a single-center study with relatively small sample size, and the findings might be somewhat biased. Second, the lack of longer follow-up might be a limitation as 1 postoperative month may be too short to observe complications. Third, TXA was only administered intravenously at a single dose in this study. Multi-center prospective randomized clinical trials with larger sample size will be needed to further investigate the optimal timing and dose of preoperative TXA administration.
Conclusion
This double-blind, randomized, controlled trial concluded that intravenous TXA is effective in reducing HBL and early postoperative pain in percutaneous kyphoplasty for OVCFs without increasing the risk of thrombotic events. Factors such as use of TXA, injury time, number of punctures, cement leakage, and restoration of VH were closely related to the occurrence of HBL.
Abbreviations
APTT, Activated partial thromboplastin time; DVT, Deep vein thrombosis; HBL, Hidden blood loss; INR, International normalized ratio; OVCF, Osteoporotic vertebral compression fracture; PT, Prothrombin time; TBL, Total blood loss; TXA, Vertebral height.
Data Sharing Statement
All data generated or analyzed during this study are available from the corresponding authors upon reasonable request.
Ethic Approval
This study was approved by the Ethics Committee of the Affiliated Hospital of Jiaxing University. Written informed consent was obtained from all the participants.
Acknowledgment
The authors thank all patients for their participation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Jiaxing Science and Technology Project (2021AY30020, 2021AD30165) and Jiaxing Key Discipline of Medicine-Orthopedics (2023-ZC-012).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang AQ, Lin YC, Kong MX, et al. A nomogram for predicting the risk of new vertebral compression fracture after percutaneous kyphoplasty. Eur J Med Res. 2023;28(1):280–290. doi:10.1186/s40001-023-01235-y
2. Zhan ZH, Li R, Fu DM, et al. Clinical efficacy and influencing factors of percutaneous kyphoplasty for osteoporotic vertebral compression fractures: a 10-year follow-up study. BMC Surg. 2024;24(1):29–36. doi:10.1186/s12893-024-02322-5
3. Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33(2):166–168. PubMed PMID: 3600949.
4. Matthew M, Jake M, Aisyah A, et al. Mechanical vertebral body augmentation versus conventional balloon kyphoplasty for osteoporotic thoracolumbar compression fractures: a systematic review and meta-analysis of outcomes. Global Spine J. 2024;14:21925682241261988. doi:10.1177/21925682241261988
5. Jan C, Jan S, Kadzhik P, et al. Efficacy and complication rates of percutaneous vertebroplasty and kyphoplasty in the treatment of vertebral compression fractures: a retrospective analysis of 280 patients. J Clin Med. 2024;13(5):1495–1507. doi:10.3390/jcm13051495
6. Sehat K, Evans R, Newman J. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee. 2000;7(3):151–155. doi:10.1016/s0968-0160(00)00047-8
7. Yang Z, Wang X, Xu ZY, et al. Effects and safety of traditional Chinese medicine for hidden blood loss after total Hip arthroplasty-a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2022;2022:1–8. doi:10.1155/2022/6937295
8. Wu YS, Zhang H, Zheng WH, et al. Hidden blood loss and the influential factors after percutaneous kyphoplasty surgery. Eur Spine J. 2017;26(7):1878–1883. doi:10.1007/s00586-017-4950-9
9. Man H, Zhang Y, Zhao WJ, et al. Perioperative hidden blood loss in lumbar disk herniation patients with percutaneous endoscopic transforaminal discectomy and influencing factors. Clin Spine Surg. 2022;35(5):E438–E443. doi:10.1097/bsd.0000000000001282
10. Zhang R, Yang ZQ, Lei T, et al. Effects of aminocaproic acid on perioperative hidden blood loss in elderly patients with femoral intertrochanteric fracture treated with proximal femoral nail anti-rotation. J Int Med Res. 2019;47(10):5010–5018. doi:10.1177/0300060519872037
11. Cai LY, Lu W, Hu W, et al. Comparison of intramedullary and extramedullary fixation of stable intertrochanteric fractures in the elderly: a prospective randomised controlled trial exploring hidden perioperative blood loss. BMC Musculoskelet Disord. 2016;17(1):475–482. doi:10.1186/s12891-016-1333-z
12. Setliff J, Dalton J, Sadhwani S, et al. Examining the safety profile of a standard dose tranexamic acid regimen in spine surgery. Neurosurg Focus. 2023;55(4):E16–E22. doi:10.3171/2023.7.Focus23384
13. Thomas C, Stephen P, Kristina B, et al. Efficacy of tranexamic acid on surgical bleeding in spine surgery: a meta-analysis. Spine J. 2015;15(4):752–761. doi:10.1016/j.spinee.2015.01.013
14. Hao SS, Li HK, Liu S, et al. The effect of intravenous unit-dose tranexamic acid on visible and hidden blood loss in posterior lumbar interbody fusion: a randomized clinical trial. Sci Rep. 2023;13(1):4714–4722. doi:10.1038/s41598-022-27307-3
15. Nadler S, Hidalgo J, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–232. PubMed PMID: 21936146.
16. Gross J. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58(3):277–280. doi:10.1097/00000542-198303000-00016
17. Roberto J, Julian G, Joshua D, et al. Short-term safety of tranexamic acid use in posterior cervical decompression and fusion surgery. J Clin Neurosci. 2019;66:41–44. doi:10.1016/j.jocn.2019.05.029
18. Li YL, Ge M, Tian J, et al. Effect of tranexamic acid on hidden blood loss in percutaneous endoscopic transforaminal lumbar interbody fusion: a retrospective study. Ther Clin Risk Manag. 2024;20:325–334. doi:10.2147/tcrm.S462784
19. Deng B, Li XD, Xie P, et al. Intravenous versus topical tranexamic acid in spinal surgery: a systematic review and meta-analysis. J Orthop Surg Res. 2024;19(1):512–521. doi:10.1186/s13018-024-04989-1
20. Cai TC, Wang F, Nan LP, et al. Perioperative hidden blood loss in elderly osteoporotic vertebral compression fracture patients with percutaneous vertebroplasty and influencing factors. Geriatr Orthop Surg & Rehabil. 2021;12:1–7. doi:10.1177/2151459321996178
21. Liu YF, Hong CK, Hsu KL, et al. Intravenous administration of tranexamic acid significantly improved clarity of the visual field in arthroscopic shoulder surgery. a prospective, double-blind, and randomized controlled trial. Arthroscopy. 2020;36(3):640–647. doi:10.1016/j.arthro.2019.10.020
22. Wang F, Nan LP, Feng XM, et al. The efficacy and safety of multiple-dose intravenous tranexamic acid in reducing perioperative blood loss in patients with thoracolumbar burst fracture. Clini Neurol Neurosurg. 2020;193:105766. doi:10.1016/j.clineuro.2020.105766
23. Taeuber I, Weibel S, Herrmann E, et al. Association of intravenous tranexamic acid with thromboembolic events and mortality: a systematic review, meta-analysis, and meta-regression. JAMA Surg. 2021;156(6):E1–14. doi:10.1001/jamasurg.2021.0884
24. Falck-Ytter Y, Francis C, Johanson N, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e278S–e325S. doi:10.1378/chest.11-2404
25. Fan W, Qiao T, You Y, et al. Perioperative prevalence of deep vein thrombosis in patients with percutaneous kyphoplasty: a retrospective study with routine ultrasonography. Medicine. 2020;99(10):e19402–19406. doi:10.1097/md.0000000000019402
26. Guo S, Yu Z, Wang C, et al. Risk factors of hidden bloodloss in unilateral biportal endoscopic surgery for patients with lumbar spinal stenosis. Orthop Surg. 2024;16(4):842–850. doi:10.1111/os.14009
27. Yang Y, Peng YX. Hidden blood loss and its risk factors in percutaneous vertebroplasty surgery for osteoporotic vertebral compression fractures. Arch Orthop Trauma Surg. 2023;143(9):1–7. doi:10.1007/s00402-023-04873-3
28. Zhou Y, Fu X, Yang M, et al. Hidden blood loss and its possible risk factors in minimally invasive transforaminal lumbar interbody fusion. J Orthop Surg Res. 2020;15(1):445–452. doi:10.1186/s13018-020-01971-5
29. Jiang C, Chen TH, Chen ZX, et al. Hidden blood loss and its possible risk factors in cervical open-door laminoplasty. J Int Med Res. 2019;47(8):3656–3662. doi:10.1177/0300060519856987
30. Yue X, Zhang J, Sun T, et al. Hidden blood loss and its influencing factors after minimally invasive percutaneous transpedicular screw fixation in thoracolumbar fracture. BMC Musculoskelet Disord. 2022;23(1):959–967. doi:10.1186/s12891-022-05938-x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


