Back to Journals » Journal of Pain Research » Volume 18
Efficacy and Safety of Unilateral Biportal Endoscopy Compared With Transforaminal Route Percutaneous Endoscopic Lumbar Decompression in the Treatment of Lumbar Spinal stenosis: Minimum 1-year Follow-up
Authors Sun W, Wang J, Hu Y, Tao J, Yang C
Received 1 October 2024
Accepted for publication 23 February 2025
Published 4 March 2025 Volume 2025:18 Pages 1071—1080
DOI https://doi.org/10.2147/JPR.S493602
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Krishnan Chakravarthy
Weixiang Sun, Jie Wang, Yafei Hu, Jinzhuo Tao, Chengwu Yang
Pain Department, the Eighth People’s Hospital of Hefei, Hefei, People’s Republic of China
Correspondence: Chengwu Yang, The Eighth People’s Hospital of Hefei, No. 319, Renmin Road, Chaohu City, Hefei City, Anhui Provience, 238000, People’s Republic of China, Tel +86 15256298182, Email [email protected]
Objective: This study compared the efficacy and safety of unilateral biportal endoscopic (UBE) decompression and transforaminal route percutaneous endoscopic lumbar decompression (PELD) for lumbar spinal stenosis (LSS), assessing 1-year clinical outcomes.
Methods: A total of 120 patients (64 UBE, 56 PELD) diagnosed with LSS in 2021 were evaluated. Perioperative outcomes included overall operation time, extracanal operative time, intracanal decompression time, incision length, fluoroscopy time, estimated blood loss, preoperative and postoperative day 3 hb levels, length of post-operative hospital stays, total expenses, postoperative complications. Clinical outcomes were measured using visual analog scale (VAS) for back and leg pain, Oswestry disability index (ODI) for physical impairment and the modified MacNab criteria.
Results: Results showed no demographic differences between groups. UBE had shorter total operation and intracanal decompression times but longer extracanal operative time than PELD (all P< 0.01). Fluoroscopy time was significantly lower in UBE (P< 0.01). While UBE had longer incisions, greater blood loss, and higher costs, hemoglobin level changes and hospital stays were similar between groups. Postoperatively, UBE resulted in lower VAS-leg pain scores (P< 0.01) and had a higher excellent/good rate (93.75% vs 85.71%, P< 0.05). Moreover, there were 3 patients in PELD group who needed a revision surgery at the same level within postoperative 1-year follow-up due to the unrelieved symptoms.
Conclusion: Both techniques were safe, but PELD posed a higher risk of reoperation due to decompression failure. UBE demonstrated advantages in decompression efficiency and clinical outcomes despite longer incision length and greater blood loss.
Keywords: unilateral biportal endoscopic, percutaneous endoscopic lumbar discectomy, lumbar spinal stenosis, decompression
Introduction
Lumbar spinal stenosis (LSS) is a common age-related degenerative disease, caused by intervertebral disc herniation, facet joint bone hyperplasia, ligamentum flavum hypertrophy, degenerative slippage, and other factors.1,2 Patients with LSS often suffer low back and leg pains and neurogenic claudication, which significantly impacts overall quality of life.3,4 Previous studies have reported that the effective treatment for symptomatic LSS is spinal decompression.5,6
Traditionally, open decompression is the commonly used form of nerve decompression. With the continuous development of spinal endoscopic techniques, the minimally invasive surgical technique provides more benefits, such as less trauma, reduced pain, and rapid recovery. As a representative of single-channel spinal endoscopic technology, percutaneous endoscopic lumbar discectomy or decompression (PELD) is a classic minimally invasive spine procedure. Previously, PELD is a routine minimally invasive spinal procedure for the treatment of lumbar disc herniation (LDH).7 However, with the development of surgical methods, surgical instruments and optical systems, percutaneous endoscopic lumbar decompression (PELD) has been applied to LSS in recent years.8,9
Several studies have reported that the satisfaction rates of PELD in the treatment of lateral LSS vary from 82% to 92%.10 It is widely accepted that PELD have several advantages in treatment of LSS, including less trauma, faster recovery time and more effective results.10,11 However, the relatively fixed working channel and limited field of view in this approach may lead to insufficient decompression.12 Moreover, some patients still have a poor postoperative prognosis and require reoperation. In recent years, unilateral biportal endoscopic decompression (UBE) has provided a novel minimally invasive option for LSS. It is characterized by independent observation and operation channels, which can provide more flexible operating space and achieve a wider range of decompression.
Presently, few studies have compared PELD and UBE techniques in patients with LSS of single segment. Therefore, to explore the differences between the two surgical techniques, this study examined and compared the clinical efficacy and safety of UBE with that of PELD in the treatment of LSS.
Materials and Methods
Patient Selection
This study was approved by The Ethics Committee of The Eighth People’s Hospital of Hefei (ethical approval number: 2020120201), which complied with the Declaration of Helsinki. All patients signed the informed consent. The clinical data of patients with single-segment lumbar spinal stenosis treated at our hospital from January 2021 to December 2021 were collected. The patients were divided into the UBE group and the PELD group based on the surgical approach. Random selection of the surgical method was performed using a random number table. All patients were performed with preoperative radiography, computed tomography (CT), and magnetic resonance imaging (MRI). The inclusion criteria were as follows: i) diagnosis of lumbar spinal stenosis (included central or lateral recess stenosis) based on physical examination, clinical symptoms, and imaging studies; ii) symptoms with no relief after at least 3 months of conservative treatment. The exclusion criteria were as follows: i) symptoms caused only by LDH; ii) instability at the responsible level or more than grade-I spondylolisthesis; iii) more than one surgical level; iv) patients followed up for less than 1 year; v) history of lumbar surgery; and vi) concomitant conditions affecting the lumbar spine (fracture, tuberculosis, and tumor).
Surgical Methods
The Surgical Procedure of UBE Decompression
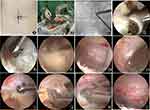
Using the left L4/5 as an example, after general anesthesia, the patient was positioned prone with a U-shaped pillow under the abdomen to fully expose the target gap. The incision site was marked under “C”-arm fluoroscopy, with horizontal incision lines drawn 1 cm above and below the L4/5 intervertebral space, intersecting the inner edge of the vertebral arch. The upper incision served as the observation channel, while the lower incision was for the operative channel. After sequential incision of the skin, subcutaneous tissue, and fascia, the primary dilator was passed through both incisions, piercing the paravertebral muscles to reach the vertebral lamina surface. Once confirmed under fluoroscopy, 4-level cannulas were inserted for observation and operative channels. A 30-degree arthroscope and sheath were inserted into the observation channel. The surgeon held the scope with the left hand, connected the irrigation system, and used isotonic saline as the flushing solution, maintaining the fluid 50 cm above the operating plane. A plasma radiofrequency electrode was inserted into the operative channel, ablating soft tissue on the vertebral lamina to achieve hemostasis. Sequential exposure revealed the lower edge of the L4 lamina, ligamentum flavum in the interlaminar space, inner edge of the facet joint, and upper edge of the L5 lamina, establishing the operative space. A dynamic drill and rongeurs removed part of the bone from the target intervertebral space, exposing the ligamentum flavum. A nerve dissector separated adhesions between the dura mater and ligamentum flavum. Instruments like rongeurs and small curved forceps removed the ligamentum flavum, exposing the dura mater and nerve roots. The nerve root was separated with a nerve hook, and plasma radiofrequency was applied for hemostasis. Under the scope, pulsations of the dura mater and nerve roots were visible, with no residual bone or significant bleeding. The arthroscope and instruments were withdrawn, residual fluid removed, and the incision sutured (Figure 1).
The Surgical Procedure of PELD
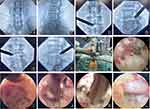
PELD was performed under local anesthesia with patients in the lateral position, allowing real-time communication to prevent nerve root injury. Fluoroscopy guided the puncture path, with the entry point typically 10–12 cm lateral to the spinal midline at the target level. After local anesthesia with 0.5% lidocaine, a puncture needle was inserted into the superior articular process (SAP) under fluoroscopic guidance. An 8 mm incision was made at the puncture site, followed by guide wire placement and sequential dilation. A hollow tapered cannula (8.5 mm) was advanced, and a trephine was used for foraminoplasty. The working cannula and endoscope were then inserted, with fluoroscopy confirming placement. If needed, a visual trephine further enlarged the foramen. Endoscopic visualization exposed the dorsal ligamentum flavum, ventral intervertebral disc, and dural sac. Hypertrophic ligamentum flavum and SAP ventral elements were removed using rongeurs, endoscopic forceps, a bone knife, or a high-speed drill until the ipsilateral nerve root was fully exposed. If necessary, disc protrusions were resected for ventral decompression. The working channel was adjusted contralaterally to decompress the contralateral nerve root by removing the ligamentum flavum. Final decompression was confirmed by adequate dural sac and nerve root mobility with good pulsation. Hemostasis was achieved, the area was irrigated, and surgical wounds were sutured (Figure 2).
Both groups of patients received antibiotic prophylaxis for infection within 24 hours after surgery and were administered 125mL of mannitol intravenously twice a day for dehydration. Vital signs were monitored, and the sensation, movement, and urination of both lower limbs were observed for any abnormalities. In both groups, patients began ambulation with a waistband 24 hours after lying flat for surgery. Bending and heavy lifting were avoided, and appropriate lumbar and back muscle exercises were performed.
Data Collection and Assessment
Demographic parameters, such as age, sex, BMI, symptom duration and surgical level, were collected.
Perioperative outcomes of the patients were assessed with overall operation time, extracanal operative time (time from skin incision to entry into the spinal canal), intracanal decompression time (time from entry into the spinal canal to the end of the operation), incision length, fluoroscopy time, estimated blood loss (mL), preoperative and postoperative day 3 hb levels, length of post-operative hospital stays.
Postoperative complications (including Dural tear, Nerve root injury, Wound hematoma, Wound infection, Transient dysesthesia, Motor deficit, Reoperation within 1 y) were recorded. The total expenses of the two groups were also collected and assessed.
Clinical outcomes were evaluated using the visual analogue scale (VAS) for low back and leg pain, the Oswestry disability index (ODI) for physical impairment related to pain at different time points (preoperatively and 3 days postoperatively, 1 month, 6 months, and 1 year). VAS was an 11-graded (0 to 10) visual scale to measure pain intensity, where 0 corresponded to no pain and 10 indicated the worst pain. ODI (0 to 100) assessed physical impairment related to pain, and a higher score indicated worse function. Clinical efficacy was evaluated using the modified MacNab criteria at the last follow-up: Excellent indicated complete disappearance of symptoms, return to original work and lifestyle; Good indicated occasional pain with the ability to perform light work; Fair indicated alleviated symptoms with persistent pain, inability to work; Poor indicated signs of compressed nerve roots, requiring further surgical treatment.
Statistical Analyses
All statistical analyses were performed using SPSS (version 24.0; IBM, USA). For continuous variables, data are presented as mean ± standard deviation. Univariate analysis was performed using independent samples t-test and chi-squared test for clinical and radiological parameters. Statistical significance was set at P < 0.05.
Results
Patients’ Demographic Characteristics
A total of 120 cases were included in the study. Among them, the UBE group comprised 64 cases, with 31 males and 33 females, aged from 54 to 86 years old, with an average of 67.83 ± 8.29 years. The average BMI was 23.71 ± 1.12 kg/m². The symptoms duration was from 3 to 7.5 months, with an average of 5.56 ± 2.43 months. There was 1 case operated at L3/4, 36 cases at L4/5 and 27 cases at L5/S1. The PELD group consisted of 56 cases, including 31 males and 25 females, aged from 55 to 88 years old, with an average of 68.66 ± 9.89 years. The average BMI was 23.09 ± 2.96 kg/m². The symptoms duration was from 2.5 to 7 months, with an average of 5.12 ± 3.26 months. The distribution of operated levels was as follows: L3-4 in 3 cases, L4-5 in 32 cases and L5-S1 in 21 cases. There were no statistically differences in demographic characteristics between the two groups (all P > 0.05) (Table 1).
 |
Table 1 Baseline Characteristics of Patients in Both Groups |
Perioperative Outcomes and Complications
The perioperative outcomes and complications are shown in Table 2. The overall operation time in the UBE group was 48.6 ± 3.03 minutes, which was significantly lower than that in the PELD group (53.6 ± 5.04 minutes) (P<0.01). At the same time, there was a significant difference in the exact operating time between two groups. The UBE group required more time for extra-canal manipulation, with an average of 24.35 ± 2.18 minutes, and less time for intra-canal decompression, with an average of 24.25 ± 2.11 minutes (P<0.01). However, the extraspinal operation time in the PELD group was relatively less, with an average of 19.7 ± 2.47 minutes, and the intra-canal decompression time was more, with an average of 33.9 ± 3.98 minutes (P<0.01). The estimated blood loss in the UBE group was significantly more than in the PELD group (37.65 ± 13.01 mL vs 16.18 ± 5.16 mL, P < 0.01). However, there was no significant difference in the change of preoperative and postoperative haemoglobin content between the two groups (details are shown in Table 2). The total incision length of patients in the UBE group was longer than that in the PELD group (18.33 ± 1.87 mm vs 7.61 ± 0.59 mm, P < 0.01). The intraoperative fluoroscopy time was only 5.45 ± 1.09 seconds in the UBE group, which was significantly lower than that in the PELD group (35.25 ± 5.58 seconds) (P<0.01). There was no significant difference of the length of post-operative hospital stays between the two groups (5.42±1.12 days vs 5.26±1.1 days, P>0.05). The average total hospitalization cost of patients in the UBE group was 18261.18 ± 327.44Ұ, which was higher than the average of 16111.44 ± 305.35 Ұ in PELD (P<0.01).
 |
Table 2 Perioperative Outcomes and Complications in Both Groups |
The complications are shown in Table 2. Dural tear occurred in 1 patient, wound hematoma in 1 patient, motor deficit in 1 patient, and 2 patients complained of transient dysesthesia after the operation in UBE group. There were 2 patients who experienced Dural tear and 3 patients who suffered transient dysesthesia after the operation in PELD group. Another 1 patient sustained motor deficit. All of these patients recovered well after conservative treatment. Moreover, there were 3 patients in PELD group who needed a revision surgery at the same level within postoperative 1-year follow-up due to the unrelieved symptoms, and no nerve root injury, wound hematoma, wound infection was found. No significant difference of the total complication rate was found between two groups (P>0.05).
Clinical Outcomes
Postoperative VAS scores of low back and leg pain and ODI decreased significantly in the two groups compared with preoperative scores (P < 0.05) (Table 3). No significant differences existed in VAS of low back pain and ODI scores between the two groups preoperatively, 3 days, 1 month, 6 months and 1 year after operation (P > 0.05) (Table 3). However, the VAS of leg pain in UBE group was significantly lower than that in PELD group respectively in 3 days, 1 month, 6 months and 1 year after operation (P<0.01) (Table 3). According to MacNab criteria, patient satisfaction rates were 93.75% and 85.71% in UBE group and PELD group, and significant difference was observed in patient satisfaction rates between both groups (P<0.05) (Table 3).
 |
Table 3 The Clinical Outcomes in Both Groups |
Discussion
With the development of minimally invasive techniques, percutaneous single-channel endoscopic decompression has gradually replaced open surgery due to its advantages of minimal trauma and rapid recovery. It has become the preferred surgical option for treating lumbar spinal stenosis (LSS).13 However, this technique has limitations such as a narrow field of view, delicate instruments, and a steep learning curve for puncture techniques.14 It often faces challenges in complex cases, such as calcification of the flavum ligamentum, facet joint hyperplasia, narrowing of the intervertebral space and so on, leading to insufficient decompression. Unilateral biportal endoscopy employs two separate channels for observation and operation, offering technical advantages such as flexibility in manipulation and a wider field of view. It allows the use of conventional open instruments for surgery. In recent years, this approach yields excellent clinical outcomes and has gained popularity as a new technique for treating LSS.15,16
In this study, a comparison of the clinical outcomes between UBE and PELD for treating LSS was conducted. The results demonstrated that the significant improvements in pain score and functional status observed in both groups at 3 days, 1 month, 6 months and 1 year follow-ups were consistent with prior findings.17–19 The modified MacNab criteria indicated satisfactory outcomes in both UBE and PELD groups, confirming the effectiveness of both techniques for treating LSS. However, UBE was associated with longer incision lengths, greater blood loss, more extra-canal manipulation, and higher hospitalization costs. Despite these factors, UBE demonstrated several advantages. It significantly reduced intraoperative fluoroscopy time, minimizing radiation exposure for patients and staff. UBE also had shorter total operation and intra-canal decompression times, reflecting greater surgical efficiency. Additionally, patients in the UBE group experienced better relief of lower extremity symptoms, resulting in a higher excellent/good rate compared to PELD. Although UBE involved more blood loss, there were no significant differences in hemoglobin changes postoperatively, suggesting this was not a clinical disadvantage. Overall, UBE offers superior decompression effectiveness and surgical efficiency, though challenges like bleeding control remain.
Although PELD was a minimally invasive surgical alternative to open decompression for the treatment of LSS, some patients still have a poor postoperative prognosis and require reoperation.20 Research conducted by Gao et al reported that the recurrence rate of LSS after PELD was 3.19%.21 The main reasons for revision included preoperative combined stenosis of the intervertebral space, poor intraoperative catheter placement and excessive postoperative movement. A study by Wang et al demonstrated that the revision rate after PELD for LSS was 6.7%, for which the same-segment revision rate was 4.4%.22 In our study, 3 cases who experienced PELD needed reoperation within 1 y, and the revision rate after PELD for LSS was 5.36%. The main reason was inadequate decompression of the nerve roots, especially those on the opposite side of the channel. While patients with UBE did not have this problem because of its adequate decompression effect.
Why is bi-portal endoscopy more effective than single-channel endoscopy for spinal decompression?
The principle of Unilateral Bi-portal Endoscopy (UBE) technique is similar to traditional posterior vertebral lamina fenestration surgery. It utilizes a posterior approach through the vertebral lamina to achieve adequate decompression of the neural roots and spinal cord by removing the compressive material. The key difference is that this technique employs two separate channels for surgery: an observation channel with a continuous irrigation endoscope and a working channel for decompression instruments. This provides a dual advantage of endoscopic visualization and flexible manipulation. During the procedure, a 30-degree arthroscope is used for observation, and decompression is performed using open surgical instruments. There is no need to purchase special single-channel endoscopic systems or matching surgical instruments.
Since the observation and working channels are independent, UBE provides a clear, unobstructed visual field for the surgical procedure. The scope can be adjusted as necessary to visualize key structures like nerve roots, the dural sac, surrounding bones, and vascular clusters. This flexibility allows for thorough exploration in various directions within the spinal canal, aiding in complete removal of the ligamentum flavum, hypertrophic bone, and full decompression of the nerve roots. Additionally, instruments used in open surgery, such as forceps and bone knives, can be maneuvered through the working channel, enabling direct operation under the endoscope’s clear view. This improves decompression efficiency while minimizing damage to nerve roots, the dural sac, and blood vessels, thereby reducing the risk of complications.
The results of this study suggested that UBE had an advantage in intracanal decompression and overall operative time. Due to the need for adequate ablation of extra-laminar soft tissues and exposing the associated anatomy structures, there was more time required for extracanal manipulation during UBE procedure. The results of this study also showed that UBE had more time to perform extracanal procedures than PELD. However, with the application of dual channels and open surgical instruments, the time of intracanal operation in UBE had been significantly shortened. The extracanal operation time of single-channel endoscopic was mainly used to establish the channel, then the endoscopic intracanal decompression would be started. For LSS patients, the decompression from ipsilateral to contralateral side was needed. However, with the limited access flexibility and the use of dedicated minimally invasive instruments under the scope, the PELD technique took longer time to complete intracanal decompression procedures.
Compared with PELD, another advantage of UBE technique was the significantly shortened fluoroscopy time, which greatly reduced the radiation injury of patients. With the application of the positioner, only one fluoroscopy was required generally for preoperative incision positioning, which greatly shortened the fluoroscopy time. At the same time, skilled microscopic operation could greatly reduce the fluoroscopy frequencies during the surgical procedure. However, it was important to establish a suitable position of the channel for PELD procedure, and each step such as puncture, the use of trepan, and the placement of the working sleeve needed to be confirmed under fluoroscopy, which greatly increased the time of intraoperative fluoroscopy.
Although the total incision length was longer in the UBE group compared to single-channel endoscopy, no significant difference in postoperative low back pain was observed between the two groups, indicating that the additional incision in UBE did not significantly increase patient discomfort compared to PELD. The UBE technique, which follows a route through the muscle interspace, avoids extensive detachment and disruption of paravertebral muscles, preserving muscle integrity and reducing the risk of muscle stretching and damage. Continuous irrigation under endoscopic visualization helps reduce residual inflammatory factors, maintain a clear surgical field, prevent bleeding, and minimize postoperative pain from aseptic inflammation and retroperitoneal hematoma. As a result, this approach lowers the incidence of complications and improves patient satisfaction. Although UBE required general anesthesia, leading to higher surgery costs compared to PELD (which used local anesthesia), there was no significant difference in postoperative hospital stay length between the two groups, and no complications related to anesthesia were observed. This suggests that the use of general anesthesia did not impose a significant additional financial burden on patients.
This study revealed that while UBE had higher estimated blood loss, no significant difference in hemoglobin levels before and after surgery indicated that the additional bleeding did not negatively affect patients compared to PELD. However, one patient experienced postoperative wound hematoma, highlighting bleeding as an issue that still needs to be addressed in UBE procedures. To minimize bleeding, several factors should be considered. First, maintaining water pressure during UBE surgery, with the water bag level 50–60 cm above the operating plane, ensures proper irrigation and circulation between the observation and operation channels. Second, controlling blood pressure through communication with the anesthetist is critical, with a target range of 100–110/80-90 mmHg. Finally, effective hemostasis is necessary to maintain a clear surgical field, addressing bleeding sources like annular fibrous tissue and intraspinal venous plexus. Using low-temperature plasma radiofrequency for hemostasis can help prevent excessive bleeding and improve surgical outcomes.
While UBE significantly reduces complications due to its flexible operating space and clear visualization, one patient experienced a dural tear, two had transient dysesthesia, and one developed motor deficits postoperatively. To prevent these complications, precise exposure of anatomical structures is essential. Thorough ablation of soft tissues and clear identification of landmarks—including the lower border of the upper vertebral body, facet joints, and upper border of the lower vertebral body—should be ensured before proceeding with decompression. Precise tissue separation and an understanding of the spatial relationship between the dura mater, nerve roots, and intervertebral disc are crucial. Rough handling must be avoided to prevent tearing. When addressing the posterior longitudinal ligament, a nerve dissector should lift it away from the dura mater before removal to prevent injury. Patients with prior minimally invasive spinal procedures or intraspinal drug injections may have severe adhesions, requiring careful dissection. Unlike open surgery, which often damages facet joints, UBE’s enhanced endoscopic view enables precise decompression while minimizing bone loss to maintain lumbar stability. Although UBE has a shorter learning curve than single-channel endoscopy, beginners must familiarize themselves with spinal anatomy and spatial orientation. When necessary, guidance from experienced surgeons is recommended.
This study had several limitations. First, it was a single-center retrospective study a small sample size. Second, it was a short follow-up period. Third, PELD in this study was operated by lateral foraminal approach instead of posterior interlaminar approach, which was also an effective, minimally invasive method for the treatment of lumbar spinal stenosis. Therefore, more techniques should be discussed together in future studies.
Conclusions
PELD and UBE decompression yield similar results for treating LSS concerning low back pain relief and functional outcomes at 1 year postoperatively. However, UBE was linked to significant leg pain relief and more excellent-good rate, which demonstrated that it was more effective in spinal decompression. The less overall operation time and intracanal decompression time indicated that it was a more efficient minimally invasive technique for LSS. UBE offered advantage in significantly shortened fluoroscopy time, but at the cost of higher expenses. Although more estimated blood loss and longer incision length, the similar change of preoperative and postoperative hemoglobin level and length of post-operative hospital stays compared with PELD suggested that UBE did not impose adverse effects on patients.
Acknowledgment
This work was supported by the Hefei Health Research Project (Grant No. Hwk2022yb036).
Disclosure
The authors have no conflict of interest to declare.
References
1. Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ. 2016;352:h6234. doi:10.1136/bmj.h62342
2. Zhou Z, Jin Z, Zhang P, et al. Correlation between dural sac size in dynamic magnetic resonance imaging and clinical symptoms in patients with lumbar spinal stenosis. World Neurosurg. 2020;134:e866–e873. doi:10.1016/j.wneu.2019.11.011
3. Djurasovic M, Glassman SD, Carreon LY, Dimar JR. Contemporary management of symptomatic lumbar spinal stenosis. Orthop Clin North Am. 2010;41(2):183–191. doi:10.1016/j.ocl.2009.12.0034
4. Siebert E, Pruss H, Klingebiel R, Failli V, Einhaupl KM, Schwab JM. Lumbar spinal stenosis: syndrome, diagnostics and treatment. Nat Rev Neurol. 2009;5(7):392–403. doi:10.1038/nrneurol.2009.905
5. Grotle M, Smastuen MC, Fjeld O, et al. Lumbar spine surgery across 15 years: trends, complications and reoperations in a longitudinal observational study from Norway. BMJ Open. 2019;9(8):e028743. doi:10.1136/bmjopen-2018-028743
6. Minamide A, Yoshida M, Maio K. The natural clinical course of lumbar spinal stenosis: a longitudinal cohort study over a minimum of 10 years. J Orthop Sci. 2013;18(5):693–698. doi:10.1007/s00776-013-0435-97
7. Pan M, Li Q, Li S, et al. Percutaneous endoscopic lumbar discectomy: indications and complications. Pain Physician. 2020;23(1):49–56.
8. Binbin W, Tian X, Shi C, et al. Clinical outcomes of “U” route transforaminal percutaneous endoscopic lumbar discectomy in chronic pain patients with lumbar spinal stenosis combined with disc herniation. Pain Res Manag. 2021;2021:6657463. doi:10.1155/2021/6657463
9. Hua L, Yufu O, Xie F, et al. Clinical efficacy of percutaneous endoscopic lumbar discectomy for the treatment of lumbar spinal stenosis in elderly patients: a retrospective study. J Orthopaedic Surg Res. 2020:
10. Ahn Y. Percutaneous endoscopic decompression for lumbar spinal stenosis. Expert Rev Med Devices. 2014;11(6):605–616. doi:10.1586/17434440.2014.940314
11. Ahn Y. Transforaminal percutaneous endoscopic lumbar discectomy: technical tips to prevent complications. Expert Rev Med Devices. 2012;9(4):361–366. doi:10.1586/erd.12.23
12. Wang L, He T, Liu J, et al. Revealing the immune infiltration landscape and identifying diagnostic biomarkers for lumbar disc herniation. Front Immunol. 2021;12:666355. doi:10.3389/fimmu.2021.666355
13. Lin Y-P, Wang S-L, Hu W-X, et al. Percutaneous full-endoscopic lumbar foraminoplasty and decompression by using a visualization reamer for lumbar lateral recess and foraminal stenosis in elderly patients. World Neurosurg. 2020;136:e83–e89. doi:10.1016/j.wneu.2019.10.123
14. Tang S, Jin S, Liao X, et al. Transforaminal percutaneous endoscopic lumbar decompression by using rigid bendable burr for lumbar lateral recess stenosis: technique and clinical outcome. Biomed Res Int. 2018;2018:2601232. doi:10.1155/2018/2601232
15. Kim J-E, Choi D-J, Park EJ. Clinical and radiological outcomes of foraminal decompression using unilateral biportal endoscopic spine surgery for lumbar foraminal stenosis. Clin Orthopedic Surg. 2018;10:439–447. doi:10.4055/cios.2018.10.4.439
16. Li C, Ju F, Li W, et al. Efficacy and safety of unilateral biportal endoscopy compared with microscopic decompression in the treatment of lumbar spinal stenosis: a protocol for systematic review and meta-analysis. Medicine. 2021;100(50):e27970. doi:10.1097/MD.0000000000027970
17. Hua W, Liao Z, Chen C, et al. Clinical outcomes of uniportal and biportal lumbar endoscopic unilateral laminotomy for bilateral decompression in patients with lumbar spinal stenosis: a retrospective pair-matched case-control study. World Neurosurg. 2022;161:e134–e145. doi:10.1016/j.wneu.2022.01.079
18. Heo DH, Quillo-Olvera J, Park CK. Can percutaneous biportal endoscopic surgery achieve enough canal decompression for degenerative lumbar stenosis? Prospective case-control study. World Neurosurg. 2018;120:e684–e9. doi:10.1016/j.wneu.2018.08.144
19. Aygun H, Abdulshafi K. Unilateral biportal endoscopy versus tubular microendoscopy in management of single level degenerative lumbar canal stenosis: a prospective study. Clin Spine Surg. 2021;34(6):E323–e8. doi:10.1097/BSD.0000000000001122
20. Fan N, Yuan S, Du P, et al. Complications and risk factors of percutaneous endoscopic transforaminal discectomy in the treatment of lumbar spinal stenosis. BMC Musculoskelet Disord. 2021;22(1):1041. doi:10.1186/s12891-021-04940-z
21. Gao Z, Jia T, Chang C, Chen B. Analysis of the efficacy of intervertebral foramen in the treatment of degenerative lumbar spinal stenosis and analysis of postoperative recurrence factors. Med Inform. 2019;32(1):115–118.
22. Wang T, Wang A, Zang L, et al. Reoperations after percutaneous endoscopic transforaminal decompression for treating lumbar spinal stenosis: incidence and predictors. Global Spine J. 2022:21925682221081030. doi:10.1177/2192568222108103017
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.